Abstract
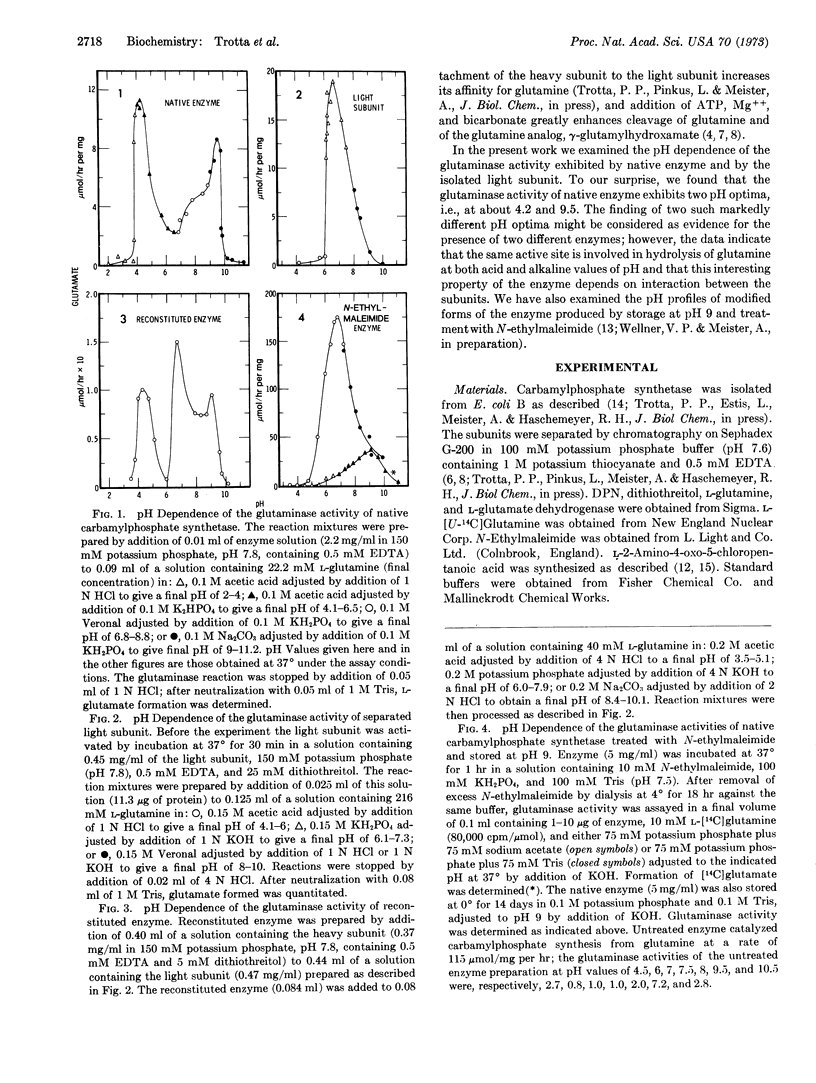
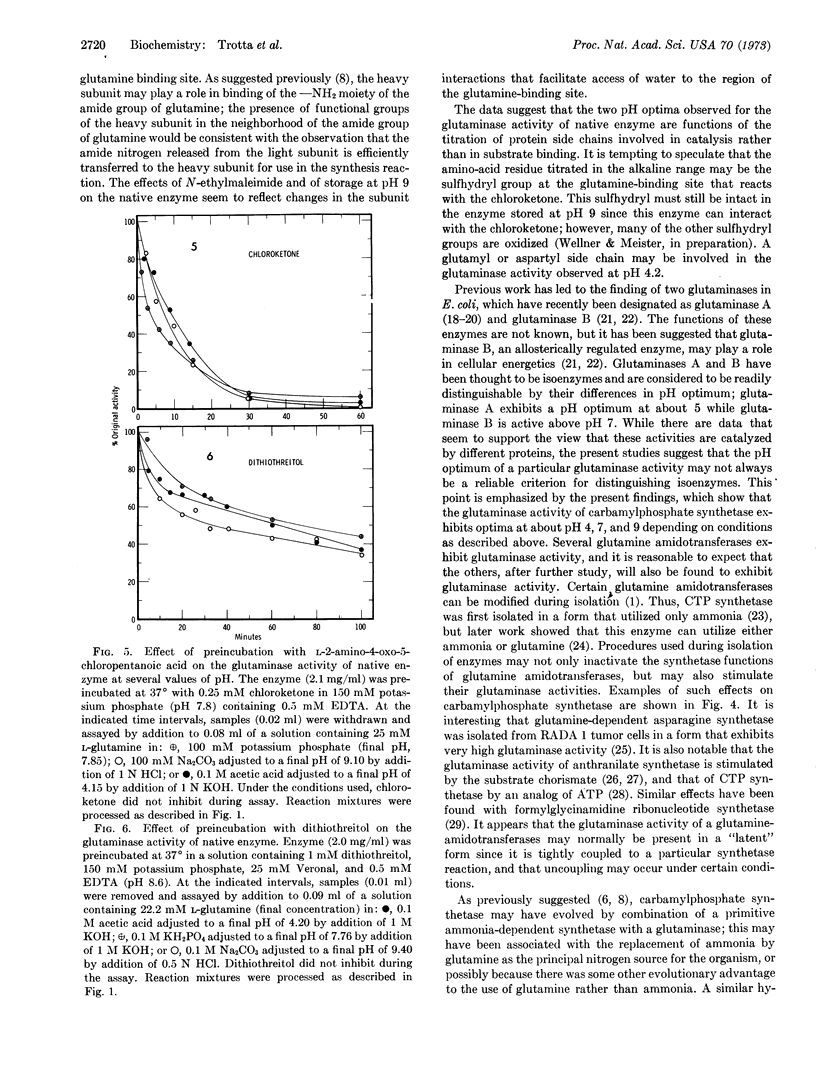
Glutamine-dependent carbamylphosphate synthetase (Escherichia coli) is composed of a heavy subunit (molecular weight about 130,000) and a light subunit (molecular weight about 42,000), which can be separated with retention of catalytic activities. The separated heavy subunit can catalyze activation of CO2 by ATP and synthesis of carbamylphosphate from ammonia (but not from glutamine). The only catalytic activity exhibited by the separated light subunit is the ability to hydrolyze glutamine; the separated heavy subunit does not exhibit glutaminase activity. The pH-activity curve of the glutaminase activity of native carbamylphosphate synthetase exhibits maxima at about pH 4.2 and 9.5, while the glutaminase activity of the separated light subunit exhibits only a single optimum at about pH 6.7. When the light and heavy subunits are recombined, the two pH optima characteristic of native enzyme are restored. Glutaminase activities of native enzyme at both pH optima are similarly inhibited by the glutamine analog, L-2-amino-4-oxo-5-chloropentanoic acid, and also by dithiothreitol. Storage of native enzyme at pH 9 abolishes the glutaminase optimum at acid pH, but greatly increases the activity at alkaline pH. Treatment of native enzyme with N-ethylmaleimide increases the glutaminase activity dramatically and converts the pH profile to one that closely resembles that of the isolated light subunit. The data indicate that the same active site is involved in hydrolysis of glutamine at both acid and alkaline values of pH, and that this property of the enzyme depends upon interactions between the heavy and light subunits of native enzyme. The double-optima behavior of native enzyme seems to be related to participation of different catalytic groups of the enzyme which affect the maximum velocity rather than the binding of substrate. The findings offer additional evidence for occurrence of significant interactions between the subunits of carbamylphosphate synthetase, and may have significance in relation to other glutamine amidotransferases, including glutaminases.
Keywords: subunits, enzyme, dissociation, E. coli
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Marvin S. V. Effect of allosteric effectors and adenosine triphosphate on the aggregation and rate of inhibition by N-ethylmaleimide of carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1970 Jan 6;9(1):171–178. doi: 10.1021/bi00803a022. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Marvin S. V. Effect of ornithine, IMP, and UMP on carbamyl phosphate synthetase from Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):928–934. doi: 10.1016/0006-291x(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Evidence for an activated form of carbon dioxide in the reaction catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1965 Dec;4(12):2803–2809. doi: 10.1021/bi00888a034. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Schroeder D. D., Buchanan J. M. Biosynthesis of the purines. XXXV. Reversible polymerization of formylglycinamide ribonucleotide amidotransferase. J Biol Chem. 1971 Aug 10;246(15):4727–4730. [PubMed] [Google Scholar]
- Hartman S. C. Glutaminase of Escherichia coli. I. Purification and general catalytic properties. J Biol Chem. 1968 Mar 10;243(5):853–863. [PubMed] [Google Scholar]
- Henderson E. J., Nagano H., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Purification of the aggregate and regulatory properties of anthranilate synthetase. J Biol Chem. 1970 Mar 25;245(6):1416–1423. [PubMed] [Google Scholar]
- Horowitz B., Meister A. Glutamine-dependent asparagine synthetase from leukemia cells. Chloride dependence, mechanism of action, and inhibition. J Biol Chem. 1972 Oct 25;247(20):6708–6719. [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Kane J. F., Homes W. M., Smiley K. L., Jr, Jensen R. A. Rapid regulation of an anthranilate synthase aggregate by hysteresis. J Bacteriol. 1973 Jan;113(1):224–232. doi: 10.1128/jb.113.1.224-232.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedouri E., Anderson P. M., Meister A. Selective inactivation of the glutamine binding site of Escherichia coli carbamyl phosphate synthetase by 2-amino-4-oxo-5-chloropentanoic acid. Biochemistry. 1966 Nov;5(11):3552–3557. doi: 10.1021/bi00875a024. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I. Enzymatic amination of uridine triphosphate to cytidine triphosphate. J Biol Chem. 1956 Oct;222(2):765–775. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry. 1971 Aug 31;10(18):3365–3371. doi: 10.1021/bi00794a008. [DOI] [PubMed] [Google Scholar]
- Li H. C., Buchanan J. M. Biosynthesis of the purines. 33. Catalytic properties of the glutamine site of formylglycinamide ribonucleotide amidotransferase from chicken liver. J Biol Chem. 1971 Aug 10;246(15):4713–4719. [PubMed] [Google Scholar]
- Long C. W., Levitzki A., Koshland D. E., Jr The subunit structure and subunit interactions of cytidine triphosphate synthetase. J Biol Chem. 1970 Jan 10;245(1):80–87. [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- MEISTER A., LEVINTOW L., GREENFIELD R. E., ABENDSCHEIN P. A. Hydrolysis and transfer reactions catalyzed by omega-amidase preparations. J Biol Chem. 1955 Jul;215(1):441–460. [PubMed] [Google Scholar]
- Nagano H., Zalkin H., Henderson E. J. The anthranilate synthetase-anthranilate-5-phosphorribosylpyrophosphate phosphoribosyltransferase aggregate. On the reaction mechanism of anthranilate synthetase from Salmonella typhimurium. J Biol Chem. 1970 Aug 10;245(15):3810–3820. [PubMed] [Google Scholar]
- Pinkus L. M., Meister A. Identification of a reactive cysteine residue at the glutamine binding site of carbamyl phosphate synthetase. J Biol Chem. 1972 Oct 10;247(19):6119–6127. [PubMed] [Google Scholar]
- Piérard A. Control of the activity of Escherichia coli carbamoyl phosphate synthetase by antagonistic allosteric effectors. Science. 1966 Dec 23;154(3756):1572–1573. doi: 10.1126/science.154.3756.1572. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Stadtman E. R. On the regulation of glutaminase in E. coli: metabolite control. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1474–1481. doi: 10.1016/0006-291x(71)90186-0. [DOI] [PubMed] [Google Scholar]
- Trotta P. P., Burt M. E., Haschemeyer R. H., Meister A. Reversible dissociation of carbamyl phosphate synthetase into a regulated synthesis subunit and a subunit required for glutamine utilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2599–2603. doi: 10.1073/pnas.68.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner V. P., Anderson P. M., Meister A. Interaction of Escherichia coli carbamyl phosphate synthetase with glutamine. Biochemistry. 1973 May 22;12(11):2061–2066. doi: 10.1021/bi00735a006. [DOI] [PubMed] [Google Scholar]



