Abstract
INTRODUCTION
Congenital fibrosis of the extraocular muscles type 2 (CFEOM2) is a distinct non-syndromic form of congenital incomitant strabismus secondary to orbital dysinnervation from recessive mutations in the gene PHOX2A. The phenotype includes bilateral ptosis, very large angle exotropia, ophthalmoplegia, and poorly-reactive pupils. Other than amblyopia, afferent visual dysfunction has not been considered part of CFEOM2; however, we have repeatedly observed non-amblyopic subnormal vision in affected patients. The purpose of this study is to document this recurrent feature of the phenotype.
METHODS
Retrospective case series (2002–2012).
RESULTS
Eighteen patients (four families) were identified; all affected individuals had confirmed homozygous recessive PHOX2A mutations except one individual for whom genetic testing was not done because of multiple genetically confirmed family members. Age at assessment ranged from 5–62 years old (median 10 years old). All patients had decreased best-corrected visual acuity not completely explainable by amblyopia in both the preferred and non-preferred eye. In those patients who had further ancillary testing, visual fields (five patients) and electroretinography (10 patients) confirmed abnormalities not ascribable to amblyopia.
CONCLUSIONS
In addition to a distinct from of congenital incomitant strabismus, the phenotype of CFEOM2 includes subnormal vision consistent with retinal dysfunction. This could be the direct result of PHOX2A mutations or a secondary effect of orbital dysinnervation.
INTRODUCTION
Although congenital fibrosis of the extraocular muscles (CFEOM) was originally considered a primary disorder of extraocular muscle formation, CFEOM is now recognized as one of several rare forms of congenital incomitant strabismus secondary to orbital dysinnervation that are collectively known as congenital cranial disinnervation disorders (CCDDs).(1) The main clinical features of CFEOM type 2 (CFEOM2; Mendelian Inheritance in Man [MIM] #602078) are bilateral ptosis and absent adduction, supraduction, and infraduction, creating the appearance of bilateral oculomotor nerve palsies.(2) Abduction is present although often incomplete, and pupils generally are variable in size and shape and non-reactive to light and accommodative targets although they respond appropriately to pupillary pharmacologic agents and accommodative ability seems intact.(3) Neuroimaging shows absent oculomotor nerves bilaterally.(3)
CFEOM2 is caused by bi-allelic mutations in the gene PHOX2A,(2) which encodes a homeodomain transcription factor prominently expressed in developing oculomotor and trochlear motor neurons and is essential to their survival. In mice, phox2a also regulates the expression of two catecholaminergic biosynthetic enzymes essential for the differentiation and maintenance of the noradrenergic neurotransmitter phenotype.(4–6)
Subnormal visual acuity has sometimes been documented in CFEOM2 patients and has often been attributed to amblyopia(3, 7, 8); however, with longer follow-up and further investigations of affected patients, we have repeatedly observed subnormal vision that is not ascribable to amblyopia. The purpose of this study is to document this as a recurrent feature of the phenotype.
METHODS
Institutional review board approval was obtained for this retrospective study. Charts of patients with genetically-confirmed CFEOM2(2, 3) examined by the authors from 2002–2012 were reviewed for best-corrected visual acuities, cycloplegic refractions, and results of ancillary testing such as visual fields and standard electroretinography (ERG; ISCEVR standard [http://www.iscev.org/]), as well as other potentially relevant clinical details. All patients had multiple examinations performed over a period of years in multiple clinics. Patients without clearly documented visual acuities or cycloplegic refractions were excluded. Genetic sequencing of PHOX2A was by previously-described methods.(2)
RESULTS
Clinical data are summarized in Table 1. Eighteen patients (four families; 13 males, five females; age range of 5–62 years [median 10 years]) were identified, all with the classic CFEOM2 phenotype (Figure 1). Genetic results and neurological observations for some family members were previously reported(2, 3) except for the family of patient 18. Recessive homozygous PHOX2A mutations were confirmed in all patients except one who did not undergo genetic testing because several affected relatives had already been genetically confirmed (patient 9). Three families harbored the homozygous c.215C>T (p.A72V) mutation (families BD, BB, AL) while the fourth harbored a homozygous splice (IVS2, G>A, −1) mutation (Family Z).
SUMMARY OF PATIENTS
| ID | FAMILY | AGE | SEX | BCVA | REFRACTION | GOLDMANN VISUAL FIELD | ERG BOTH EYES | LID/EOM SURGERY | COMMENTS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BD (2) | 5 | M | 20/40 20/40 |
+3.50+0.50×090 +3.50+0.50×090 |
--- --- |
--- | no | --- |
| 2 | BD | 8 | F | 20/60 20/60 |
−4.50+2.00×060 +3.00+1.50×180 |
--- --- |
--- | no | --- |
| 3 | BD | 5 | F | HM 20/70 |
+1.00+1.50×020 −1.50+0.75×120 |
--- --- |
--- | no | --- |
| 4 | BD | 8 | M | 20/125 LP |
−1.25+0.50×100 +0.50+1.00×150 |
--- --- |
--- | no | --- |
| 5 | BB (4) | 20 | M | 20/50 HM |
−3.00 −8.00 |
nasal step --- |
delayed & depressed rod & cone |
yes | developing keratoconus |
| 6 | BB (5) | 13 | M | 20/30 20/50 |
+1.00+1.00×090 plano+2.00×090 |
--- --- |
delayed & depressed rod & cone |
yes | --- |
| 7 | BB (6) | 7 | M | 20/50 20/70 |
plano+1.50×180 +0.75+1.50×180 |
--- --- |
delayed & depressed rod & cone |
yes | --- |
| 8 | BB (7) | 5 | M | HM 20/60 |
+2.25 +1.50 |
--- --- |
delayed & depressed rod & cone |
yes | resolved childhood seizures; nystagmus right eye |
| 9 | BB (9) | 14 | M | 20/50 HM |
plano plano |
slight constriction inferotemporal island | delayed & depressed rod |
yes | --- |
| 10 | BB (10) | 6 | M | 20/200 20/30 |
+2.50+2.00×080 +1.50+1.00×040 |
--- | --- | yes | --- |
| 11 | BB (11) | 33 | M | HM 20/50 |
plano plano |
--- nasal step (HVF) |
delayed & depressed rod & cone |
yes | MRI suggests small optic nerves |
| 12 | BB (12) | 11 | F | LP 20/40 |
−7.00 −0.50+1.50×105 |
--- --- |
--- | no | --- |
| 13 | BB | 26 | M | CF CF |
−17.00 −17.00 |
--- --- |
delayed & depressed rod & cone |
no | --- |
| 14 | BB | 62 | M | 20/160 20/80 |
−4.50+5.00×095 −17.50+3.00×180 |
--- --- |
delayed & depressed rod & cone |
yes | high myope (30mm axial length); intraocular lens right eye |
| 15 | BB | 11 | M | 20/60 20/100 |
−5.50+1.00×175 −4.50+4.50×170 |
--- --- |
delayed & depressed rod |
no | query retinal arteriolar attenuation and abnormal macular reflex |
| 16 | Z (13) | 21 | F | CF 20/50 |
−8.50+1.50×105 −2.00−1.50×170 |
superior arcuate island slight constriction |
--- | no | hypoplastic right optic nerve; question of frontal atrophy by CT |
| 17 | Z (14) | 7 | M | 20/100 20/80 |
+4.00+2.50×090 +1.50+3.00×125 |
slight constriction superonasal arcuate defect |
delayed & depressed rod & cone |
yes | idiopathic scleritis left eye |
| 18 | AL | 9 | F | LP 20/80 |
+6.50 +4.50 |
--- --- |
--- | yes | --- |
BCVA: best-corrected visual acuity; ERG: electroretinography; EOM: extraocular muscle; M: male; F: female; HM: hand motion; CF: count fingers; LP: light perception; HVF: Humphrey visual field rather than Goldmann; MRI: magnetic resonance imaging scan; CT: computed tomography scan; “---”: not available
“rod” refers to testing under scotopic conditions; “cone” refers to testing under photopic conditions
Where relevant, for a given patient top line refers to right eye and bottom line refers to left eye
For FAMILY column, number in parenthesis refers to patient identification in reference 3
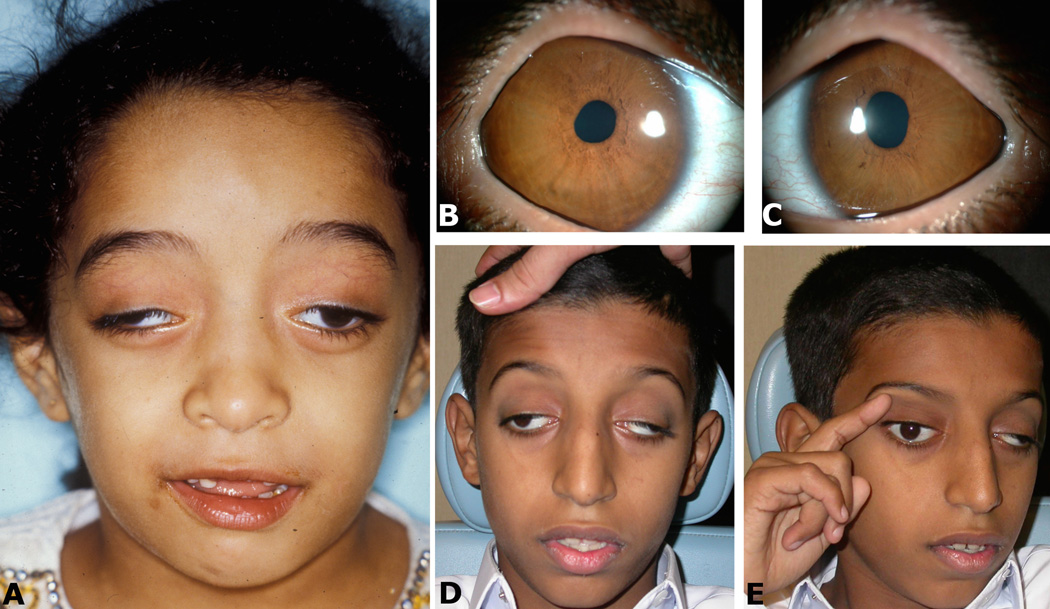
Figure 1. Clinical examples.
A (patient 2): The typical ptosis and very large angle exotropia can be appreciated in primary position; (B,C: patient 9): Irregular pupils (not pharmacologically dilated) can be appreciated in both the right (B) and left (C) eyes; (D,E: patient 1): (D) The typical ptosis and very large angle exotropia can be appreciated in primary position; (E) The patient uses a finger to lift a ptotic lid and better visualize an object of regard, which is not an uncommon maneuver.
No patient had 20/20 vision in either eye. Three patients had equally depressed best-corrected visual acuity in both eyes (20/40, 20/60, and count fingers). For the other 15 patients, two patients had 20/30 best-corrected vision in the preferred eye with better visual acuity but most were worse than 20/50. Subnormal vision was typically profound in the non-preferred eye of these 16 patients, often at the level of hand motion (five patients) or light perception (three patients). No patient had increased measured intraocular pressure. Crystalline lenses and ocular media were clear in all patients except for one older adult who had had unilateral cataract surgery with intraocular lens placement (Table 1, patient 14). The fundus examination was grossly normal in all patients with the exception of one patient who seemed to have narrowing of arterioles with questionable foveal discoloration (Table 1, patient 15) and another whose optic nerve heads appeared small (Table 1, patient 16). No patient complained of night vision loss, progressive visual loss, or color discrimination difficulties.
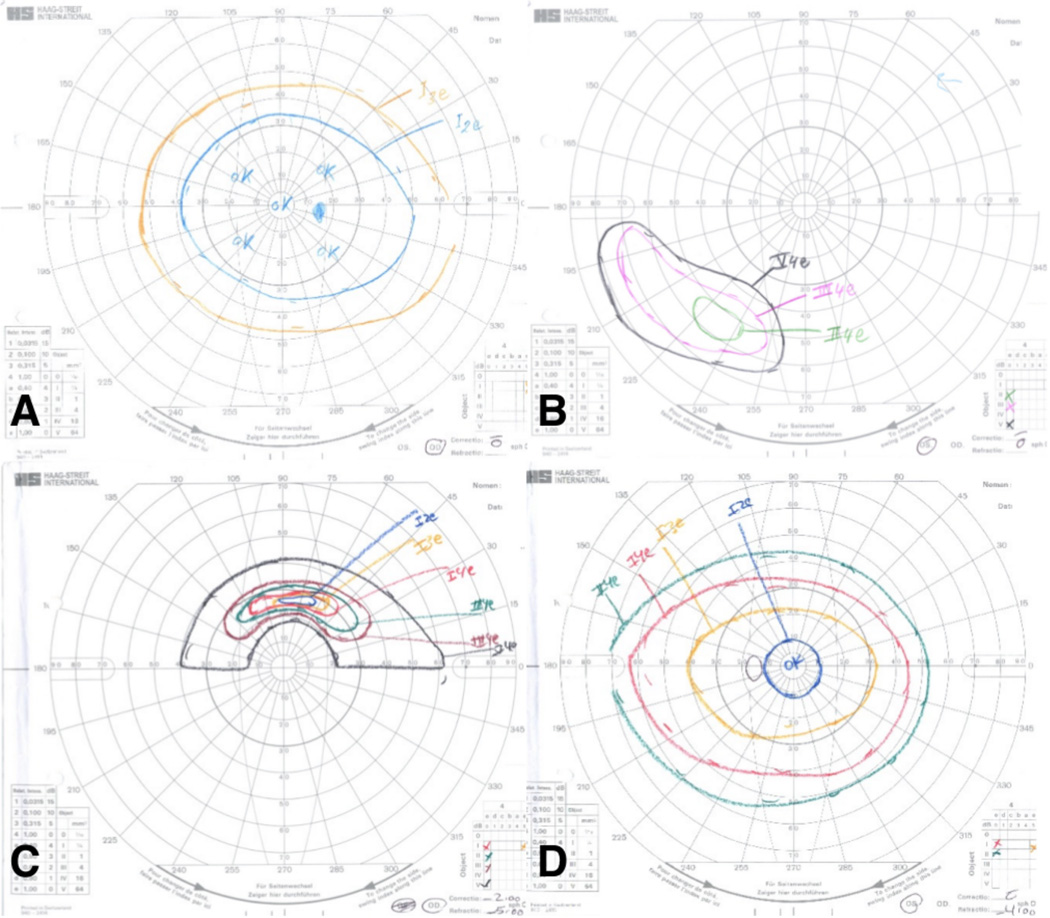
Visual fields were available for five patients, all of whom had abnormalities including mild constriction, arcuate defect, and residual island of vision. (Figure 2). ERG was performed for 10 patients, all of whom had delayed and depressed responses (both rod and cone for eight patients, only rod for two patients). An example of an abnormal tracing is provided in Figure 3.
Figure 2. Goldmann visual field examples): (A,B: patient 9.
A: The preferred right eye has a slightly constricted field that is otherwise normal; B: The non-preferred left eye has only an inferotemporal island of vision. (C,D; patient 16): C: The non-preferred right eye has only a superior arcuate island of vision; D: The preferred left eye has a slightly constricted field that is otherwise normal.
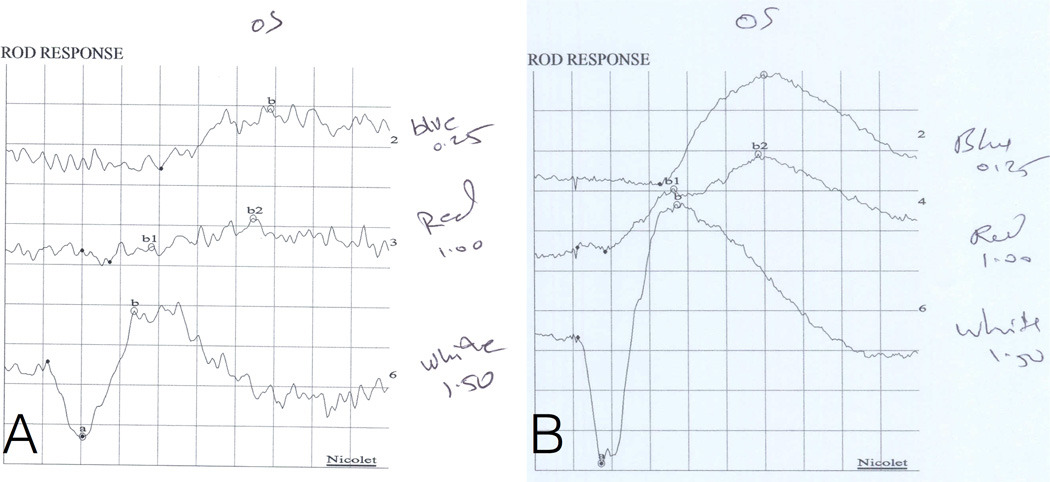
Figure 3. Scotopic electroretinography [ERG] example, patient 9.
(A,B) The abnormal left eye (OS) of patient 9 and a normal control for comparison. (A) For the left eye of patient 9, the white flash a-wave (combined rod-cone response) implicit time was delayed (21 ms vs normal mean[range] 14 [11–17]) and its amplitude is at the low range of normal (169 millivolts [mv] vs 271 [164–378]). Responses to blue (rod isolated) and red (cone isolated) stimuli under scotopic conditions are also depicted, and both also show delayed implicit times (blue 118 ms vs 92 [69–115]; red 56 ms vs 47 [42–53]). In addition, the amplitude of the response to red is depressed (red 36mv vs 124 [51–197]). (B) A normal control is shown for comparison. The white flash a-wave implicit time was 14 ms. Each box corresponds to 100 mv and 20 ms and normal means (ranges) are as follows: a-wave implicit time 14 (11–17) ms; a-wave amplitude 271 (164–378) mv; b-wave implicit time 51 (42–60) ms; b-wave amplitude 242 (103–380) mv.
DISCUSSION
In this cohort of 18 CFEOM2 patients, all had subnormal best-corrected visual acuity varying from 20/30 to light perception. This subnormal vision was not consistent with solely amblyogenic visual loss, as substantiated by visual field defects not consistent with amblyopia and abnormal ERGs in all patients who underwent such testing. These results suggest retinal dysfunction is a recurrent feature of the CFEOM2 phenotype.
Amblyopia is subnormal vision from abnormal visual experience during childhood. It can be strabismic, refractive, or occlusive in origin, and is not associated with significant structural or functional changes in the retina.(9) Previous reports of CFEOM2 did not emphasize visual acuities. Instances of subnormal visual acuity in CFEOM2 patients were typically considered amblyopia-related(3, 7, 8) although Wang and colleagues(7) did suggest subnormal retinal function as a possibility that deserved further investigation.
Strabismic amblyopia does not appear to explain visual loss in our cohort. All CFEOM2 patients were exotropic, even after strabismus surgeries. However, isolated exotropia typically does not cause strabismic amblyopia as the central visual fields of the two eyes do not overlap and strabismic amblyopia is unlikely to develop without competition between the two eyes when focusing on an object of regard.(10, 11) This is particularly true for large angle exotropia, which all of these patients had during early childhood.
Refractive amblyopia also does not adequately explain visual loss in our cohort. Many refractive errors in our cohort neither fit the commonly-observed refractive clinical profile of amblyopia(12) nor were in the range considered amblyogenic.(13) For those who did have larger anisometropias or absolute refractive errors, subnormal vision was lower than what would be expected from anisometropia alone. Light perception or hand motion vision was frequent in the non-preferred eye in this cohort, but uncorrected anisometropia by itself is not associated with such severe visual impairment.(14) In addition, visual acuity was also typically subnormal in the less ametropic preferred eye of these CFEOM2 patients.
Ptosis-induced occlusive amblyopia is also not an adequate explanation for subnormal vision in this cohort, particularly in the preferred eye. Ptosis-related amblyopia is typically not occlusive(15, 16) but rather is usually associated with anisometropia and/or esotropia.(17) Moreover, amblyopia related to ptosis usually occurs in the setting of monocular ptosis rather than binocular ptosis(15) and is not typically to the degree of light perception or hand motion vision.
The five patients who underwent visual field testing had visual field loss not consistent with amblyopia. In amblyopia, standard monocular visual field testing generally shows depressed central sensitivity and occasionally milder depressed sensitivity into the near periphery.(18) However, in our CFEOM2 patients other visual field abnormalities and sometimes only residual islands of visual field were documented (Table 1, Figure 2). These visual field abnormalities may have been influenced by patient positioning given the typical large-angle exotropia and ophthalmoplegia of CFEOM2; however, they were still not the type of field defects expected in amblyopia-related visual loss.
ERG abnormalities are not typical for amblyopia,(9) but all 10 patients who underwent ERG testing in our cohort had abnormalities, i.e., delayed and depressed rod and cone function in eight and delayed and depressed rod function in two (Figure 3). No pigmentary changes were noted in the posterior pole of any patient but the peripheral retina could not always be completely assessed because of globe positioning. Repeat ERG testing was not performed, so it is unclear whether retinal dysfunction was stationary or progressive; however, no patient complained of progressive visual loss or had an unexplained change in visual acuity documented on repeat examinations. Additional ancillary testing to further characterize these findings such as color vision assessment, fundus autofluorescence, and ocular coherence tomography would have been ideal but was not performed.
PHOX2A is a homeodomain transcription factor gene expressed in certain differentiating neurons of the central and peripheral nervous system.(4) In the knockout mouse model, both oculomotor and trochlear nerve nuclei are absent,(5) consistent with the human CFEOM2 phenotype of ptosis, exotropia, ophthalmoplegia, and absent oculomotor nerves by neuroimaging.(3) PHOX2A does not appear to play a role in human strabismus phenotypes other than CFEOM2.(19) Unlike the human CFEOM2 phenotype, the knockout mouse dies soon after birth and has additional neurologic abnormalties such as absence of the locus coeruleus (the main noradrenergic center of the brain), atrophy of cranial sensory gangilia, and absence of parasympathetic ganglia of the head. Transient expression of dopamine beta-hydroxylase in neuroblasts is also abolished in the knockout mouse model. Thus phox2a in the mouse appears necessary for catecholamine function. This may be relevant to retinal dysfunction in patients with PHOX2A mutations, as dopamine has complex roles in retinal development, growth, and maintenance.(20–22) Another potential mechanism for abnormal retinal development in CFEOM2 patients is the orbital dysinnervation itself, as has been described for optic nerve development in the context of orbital dysinnervation.(23, 24)
There are several qualifications to this study. The severe exotropia of CFEOM2 meant that neither eye could be brought to primary position, which can sometimes complicate the examination of both visual acuity and the fundus. The coincidence of strabismus, refractive errors, and ptosis may have led to a unique, additive amblyogenic effect. These amblyogenic factors were difficult to treat in CFEOM2 patients because strabismus surgery was ineffective in bringing eyes into primary position, the extent of lid surgery was limited by the lack of a Bell phenomenon, and spectacles provided only partially effective correction of refractive errors because of strabismus with ophthalmoplegia. It is also possible that restricted positioning for Goldmann perimetry and ERG contributed to abnormal results on these tests. Nevertheless, no other amblyogenic circumstance is known to commonly result in light perception or hand motion visual acuity, and positioning is not expected to affect ERG implicit times. At the very least, CFEOM2 as a CCDD is distinctly unusual in that reduced afferent visual functioning is common. A retinal developmental abnormality due to PHOX2A mutations currently seems to be the most likely causative mechanism.
Acknowledgements
This work was supported in part by National Institutes of Health, Bethesda, Maryland: R01-EY012498.
Footnotes
Financial disclosures: none
REFERENCES
- 1.Gutowski NJ, Bosley TM, Engle EC. 110th ENMC international workshop: The congenital cranial dysinnervation disorders (CCDDs). Naarden, the Netherlands, 25–27 october, 2002. Neuromuscul Disord. 2003 Sep;13(7–8):573–578. doi: 10.1016/s0960-8966(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 2.Nakano M, Yamada K, Fain J, Sener EC, Selleck CJ, Awad AH, et al. Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet. 2001 Nov;29(3):315–320. doi: 10.1038/ng744. [DOI] [PubMed] [Google Scholar]
- 3.Bosley TM, Oystreck DT, Robertson RL, al Awad A, Abu-Amero K, Engle EC. Neurological features of congenital fibrosis of the extraocular muscles type 2 with mutations in PHOX2A. Brain. 2006 Sep;129(Pt 9):2363–2374. doi: 10.1093/brain/awl161. [DOI] [PubMed] [Google Scholar]
- 4.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997 Oct;124(20):4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 5.Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997 Mar;18(3):411–423. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- 6.Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, et al. Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999 Nov;24(3):555–566. doi: 10.1016/s0896-6273(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang SM, Zwaan J, Mullaney PB, Jabak MH, Al-Awad A, Beggs AH, et al. Congenital fibrosis of the extraocular muscles type 2, an inherited exotropic strabismus fixus, maps to distal 11q13. Am J Hum Genet. 1998 Aug;63(2):517–525. doi: 10.1086/301980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazdani A, Chung DC, Abbaszadegan MR, Al-Khayer K, Chan WM, Yazdani M, et al. A novel PHOX2A/ARIX mutation in an iranian family with congenital fibrosis of extraocular muscles type 2 (CFEOM2) Am J Ophthalmol. 2003 Nov;136(5):861–865. doi: 10.1016/s0002-9394(03)00891-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong AM. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012 Oct;47(5):399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Haldi BA. Ninth annual Richard G. Scobee memorial lecture. sensory response in exotropia. Ophthalmology. 1979 Dec;86(12):2090–2100. doi: 10.1016/s0161-6420(79)35307-6. [DOI] [PubMed] [Google Scholar]
- 11.Romanchuk KG, Dotchin SA, Zurevinsky J. The natural history of surgically untreated intermittent exotropia-looking into the distant future. J AAPOS. 2006 Jun;10(3):225–231. doi: 10.1016/j.jaapos.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013 Mar;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JM, Harvey EM. Spectacle prescribing recommendations of AAPOS members. J Pediatr Ophthalmol Strabismus. 1998 Jan-Feb;35(1):51–52. doi: 10.3928/0191-3913-19980101-17. [DOI] [PubMed] [Google Scholar]
- 14.Repka MX, Kraker RT, Beck RW, Birch E, Cotter SA, Holmes JM, et al. Treatment of severe amblyopia with weekend atropine: Results from 2 randomized clinical trials. J AAPOS. 2009 Jun;13(3):258–263. doi: 10.1016/j.jaapos.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griepentrog GJ, Diehl N, Mohney BG. Amblyopia in childhood eyelid ptosis. Am J Ophthalmol. 2013 Jun;155(6):1125–1128. doi: 10.1016/j.ajo.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gusek-Schneider GC, Martus P. Stimulus deprivation amblyopia in human congenital ptosis: A study of 100 patients. Strabismus. 2000 Dec;8(4):261–270. doi: 10.1076/stra.8.4.261.687. [DOI] [PubMed] [Google Scholar]
- 17.Srinagesh V, Simon JW, Meyer DR, Zobal-Ratner J. The association of refractive error, strabismus, and amblyopia with congenital ptosis. J AAPOS. 2011 Dec;15(6):541–544. doi: 10.1016/j.jaapos.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Sireteanu R, Fronius M. Human amblyopia: Structure of the visual field. Exp Brain Res. 1990;79(3):603–614. doi: 10.1007/BF00229328. [DOI] [PubMed] [Google Scholar]
- 19.Khan AO, Khalil DS, Al-Sharif LJ, Al-Tassan NA. Mutations in KIF21A and PHOX2A are absent in 16 patients with congenital vertical incomitant strabismus. Ophthalmic Genet. 2009 Dec;30(4):206–207. doi: 10.3109/13816810903183613. [DOI] [PubMed] [Google Scholar]
- 20.Varella MH, de Mello FG, Linden R. Evidence for an antiapoptotic role of dopamine in developing retinal tissue. J Neurochem. 1999 Aug;73(2):485–492. doi: 10.1046/j.1471-4159.1999.0730485.x. [DOI] [PubMed] [Google Scholar]
- 21.Reis RA, Ventura AL, Kubrusly RC, de Mello MC, de Mello FG. Dopaminergic signaling in the developing retina. Brain Res Rev. 2007 Apr;54(1):181–188. doi: 10.1016/j.brainresrev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004 Jan;108(1):17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 23.Traboulsi EI. Congenital abnormalities of cranial nerve development: Overview, molecular mechanisms, and further evidence of heterogeneity and complexity of syndromes with congenital limitation of eye movements. Trans Am Ophthalmol Soc. 2004;102:373–389. [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AO, Shinwari J, Omar A, Khalil D, Al-Anazi M, Al-Amri A, et al. The optic nerve head in congenital fibrosis of the extraocular muscles. Ophthalmic Genet. 2011 Sep;32(3):175–180. doi: 10.3109/13816810.2011.567318. [DOI] [PubMed] [Google Scholar]