Abstract
The goal of the present study was to investigate developmental differences in the effectiveness of parent support to alleviate hypothalamic-pituitary-adrenal (HPA) axis stress responses of children (ages 9-10, N = 40) and adolescents (ages 15-16, N = 41). We experimentally manipulated the provision of parent support during the speech preparation period before a modified Trier Social Stress Test (TSST) and examined its effect on levels of salivary cortisol secreted in response to this laboratory stressor. Analyses revealed a significant interaction of condition and age group such that social support from the parent (versus a stranger) significantly eliminated the cortisol stress response in children, but had no effect on the response among adolescents.
Keywords: stress, social buffering, adolescence, parent support, cortisol, HPA axis
Adolescence is a period of dramatic neurobehavioral reorganization that is marked by an increase in the activity of stress-response systems and in indices of emotional reactivity (Crone & Dahl, 2012; Dahl & Gunnar, 2009; Spear, 2000). It has been suggested that these changes may underlie the increased prevalence of psychopathology and emotion dysregulation observed during this developmental stage (Merikangas et al., 2010; Nelson, Leibenluft, McClure, & Pine, 2005; Spear, 2000). In addition to examining the underpinnings for this pattern of enhanced stress and emotion reactivity, it is equally important to understand whether there are developmental changes in the mechanisms that could reduce or buffer stress-responding.
Social support is one such mechanism that plays critical roles in stress-regulation throughout the lifespan (Hostinar, Gunnar, & Sullivan, 2014; Taylor, 2007; Uchino, Cacioppo, & Kiecolt-Glaser, 1996). There are a variety of pathways through which social support can protect against the negative effects of threatening or stressful experiences (Cohen & Wills, 1985; Thoits, 2011). The focus in the present report is on one particular phenomenon termed social buffering, which can be defined as a reduction in acute physiological stress responses with the presence or assistance of a conspecific during an otherwise stressful event. We chose to focus on the reactivity of the HPA stress system given the widely documented links between glucocorticoids and numerous aspects of physical or mental health (McEwen, 2008). The buffering of HPA axis stress responses by social partners or stimuli has been noted across development and in multiple species (Hennessy, Kaiser, & Sachser, 2009; Hostinar et al., 2014; Kikusui, Winslow, & Mori, 2008).
In humans, this phenomenon has been almost exclusively studied in adulthood or early childhood. In adults, one of the earliest investigations of social buffering used the Trier Social Stress Test (TSST) to elicit cortisol responses after participants spent the period preparing for the test with their close relationship partner or with the experimenter. The results showed that preparing with their significant other nearly blocked the cortisol response to the TSST for men, whereas the same manipulation had little effect for women (Kirschbaum et al., 1995; replicated by Heinrichs et al., 2003). In women, partner support coupled with a brief neck-and-shoulder massage has the same cortisol-dampening effect (Ditzen et al., 2007). Studies also show that women receiving support from a close female friend during a laboratory social stress test experience a significant reduction in their cardiovascular responses (Fontana, Diegnan, Villeneuve, & Lepore, 1998; Uno, Uchino, & Smith, 2002), suggesting that the same may be true for the HPA axis. Stranger support does not seem to be able to block stress responses in either males or females (Kirschbaum et al., 1995; but see Smith, Loving, Crockett, & Campbell, 2009 for an experimental manipulation enhancing closeness to strangers through self-disclosure), suggesting that a certain level of intimacy may be required to instantiate social stress-buffering effects. Consistent with this interpretation, one study found that despite friendly and positive nonverbal signals from the two judges observing the TSST, the task still increased cortisol stress responses compared to a no-audience condition (Taylor et al., 2010).
The social regulation of the HPA axis by parents during early human development is also well-documented (for a review, see Gunnar & Donzella, 2002). This has been studied by examining the effectiveness of the parent in secure attachment relationships to reduce or prevent cortisol increases to fear stimuli (Nachmias, Gunnar, Mangelsdorf, Parritz & Buss, 1996; Spangler & Schieche, 1998) and to physically painful events (Gunnar, Brodersen, Nachmias, Buss & Rigatuso, 1996) in samples approximately 12- to 18-months-old. It must be noted that parent presence can reduce HPA reactivity even when infants continue to exhibit fear or distress behaviorally, suggesting possible dissociations between different stress and emotion systems (Nachmias et al., 1995). Additionally, maternal touch has also been associated with reduced cortisol reactivity (Feldman, Singer, & Zagoory, 2010) or recovery (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008) in infants. It is apparent that in early childhood the parent's presence is needed for buffering to occur. Thus, although attachment security moderated the cortisol response of toddlers visiting and adapting to center-based child care when their mothers were with them, the effects of attachment security were not observed during the first weeks when the child was in the center without the mother present (Ahnert, Gunnar, Lamb, & Bartel, 2004).
Despite the importance of parental social buffering in infancy and early childhood, we know surprisingly little about whether parental presence and availability retains its potent effects on acute stress reactivity later in childhood. To our knowledge, the only previous study examining this question was conducted with 7- to 12-year-old girls and used contact with the mother immediately following the Trier Social Stress Test for Children (TSST-C) to test its effects on cortisol and oxytocin production (Seltzer, Ziegler, & Pollak, 2010). After completing the stress test, children were either reunited with their mothers, were allowed to talk to their mothers on the phone or had no contact with their mothers and spent time with the experimenters. Full contact with the mother nearly eliminated the cortisol response, reducing peak responses and rapidly returning cortisol levels to baseline, whereas phone contact provided some stress alleviation but was much less effective. Thus, based on this study it seems likely that parental social buffering remains potent throughout childhood, at least for girls. However, there is a major gap in the literature regarding the extent to which parental presence remains a potent stress buffer into the adolescent years. The current report aimed to address this gap.
Parent-Child Relationship History and Later Stress Reactivity
Even though there has been a dearth of research on parental buffering of acute stress responses in later childhood, there have been numerous studies examining the extent to which the quality of the relationship with the parent is correlated with children and adolescents’ responses to stressors when the parent is not present. This literature is relevant to the question of how parental support early in development may become internalized and may shape the individual to become less stress-responsive later, regardless of the parent's presence. For instance, studies have shown differential responses to the TSST-C in youth by levels of parental structure (i.e., organization and consistency) in middle childhood (Ellenbogen & Hodgins, 2009) or based on home measures of parental responsiveness when the participants were 4-years-old in a sample of low-income African American youth (Hackman et al., 2013). Bereaved youth also show altered cortisol responses assessed 5 years after the loss of their parent (Dietz et al., 2013). Similarly, lasting effects of early parent-child relationships have been noted when examining adult stress reactivity (Luecken, 1998; Pierrehumbert et al., 2012; but see also the null result in Ditzen et al., 2008). Finally, a number of studies have reported correlations between various aspects of the parent-child relationship and both HPA and autonomic activity assessed under baseline or only very mildly challenging conditions (Boyce et al., 2006; Chaplin et al., 2012; Doan & Evans, 2011; Dorn et al., 2009; Ellenbogen, Hodgins, Walker, Couture, & Adam, 2006; Marceau, Dorn & Susman, 2012). Together, these studies suggest that it is important to measure the supportive qualities of the relationship when examining the influence of parents on child and adolescent stress reactivity.
Reviews of the literature also show that risky families (i.e., replete with conflict, aggression, cold and harsh relationships) foster altered HPA or sympathetic nervous system reactivity and unhealthy biobehavioral profiles that engender higher levels of adult physical and mental illness (Repetti, Taylor, & Seeman, 2002; Seeman & McEwen, 1996). Indeed, naturalistic observations have shown that children who are routinely reprimanded by their parents show elevated levels of glucocorticoids (Flinn, 1999; Flinn & England, 1997). Laboratory studies also show alterations in cortisol reactivity for participants exposed to negative family interactions, for example during toddlerhood (Sturge-Apple, Davies, Cicchetti, & Manning, 2012) and adolescence (Spies, Margolin, Susman, & Gordis, 2011). Given these results, it is plausible that both positive and negative parent-child relationship aspects that change between childhood and adolescence might help explain emerging differences in the stress-alleviating role of parent support.
Puberty and Social-Developmental Changes During Adolescence
Previous research has also largely neglected to examine the ways in which normative developmental changes (e.g., puberty) may impact the concurrent effectiveness of parent support to dampen stress responses. We examine the theoretical rationale for focusing on these developmental changes next.
The brain and the HPA axis undergo important changes under the influence of gonadal and adrenal hormones during puberty, as shown by animal models and human studies (Andersen & Teicher, 2008; Klein & Romeo, 2013; McCormick, Mathews, Thomas, & Waters, 2010). Basal cortisol levels have been shown to increase from childhood to adolescence (for a review, see Gunnar & Vazquez, 2006) and there is also mounting evidence that cortisol reactivity to both performance and peer rejection stressors may increase during this period (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009; Sumter, Bokhorst, Miers, Van Pelt, & Westenberg, 2010; van den Bos, de Rooij, Miers, Bokhorst, & Westenberg, 2013). These changes are thought to be related to the pubertal stage (Adam, 2006; Oskis, Loveday, Hucklebridge, Thorn, & Clow, 2009). Other studies have also found sex differences in puberty-related changes in HPA activity, with females but not males showing higher basal cortisol post-puberty (Netherton, Goodyer, Tamplin & Herbert, 2004; Stroud, Papandonatos, Williamson, & Dahl, 2011) as well as increasing total cortisol output following CRH challenge with pubertal development in females (Stroud et al., 2004; 2011). Although a number of studies have not found sex differences in HPA reactivity with puberty (Gunnar et al., 2009; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Stroud et al., 2009; Sumter et al., 2010), others indicate that males tend to have higher HPA responses to performance stressors than females, especially later in puberty (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, 2009; Klimes-Dougan et al., 2001; Zijlmans, Beijers, Mack, Pruessner, & de Weerth, 2013). This is the same pattern that has been observed in adults with respect to performance stressors (Kudielka, & Kirschbaum, 2005). Some have argued that the heightened activity of the HPA axis may play a role in the elevated levels of various types of psychopathology observed in adolescence (Spear, 2000), supporting the importance of studying stress-alleviating factors in adolescence.
These pubertal changes are concomitant with massive transformations in adolescents’ social world. For instance, they report decreasing levels of emotional intimacy with parents and increasing closeness with peers across this period (Cauce, 1986; Harris, 1995; Hartup, 1996; Hunter & Youniss, 1982). Parents remain providers of nurturance throughout adolescence and young adulthood (Collins & Laursen, 2004; Furman & Buhrmester, 1992), however these relationships evolve and acquire different functions as peers gain salience (Laursen & Collins, 2009). For instance, parents provide more advice whereas peers are more likely to be relied on for intimate disclosure (Buhrmester & Furman, 1987; Hunter & Youniss, 1982).
Despite a general pattern of increases in negative interactions and negative affect between parents and their offspring during adolescence (Kim, Conger, Lorenz, & Elder, 2001; Laursen, DeLay, & Adams, 2010), it is not the case that all dyads experience negative interactions or that conflict increases linearly with age or pubertal status (Laursen & Collins, 1994). Even though increases in mild bickering and negativity seems to be quite prevalent, a smaller number of dyads experience more intense conflict (Shanahan et al., 2007; Steinberg & Morris, 2001). Importantly, studies suggest that it is primarily the dyads entering the adolescent transition with higher levels of negativity that experience the greatest subsequent deterioration in relationship quality (Laursen et al., 2010).
There is some evidence that the increase in the emotional distance between parents and adolescents and in the tendency to experience negativity or conflict coincides with the timing of puberty (Hill et al., 1985a, b; Steinberg, 1987, 1988). Rates of conflict that do increase in early adolescence decline towards mid- to late-adolescence (Laursen, Coy, & Collins, 1998); this is likely due to improvements in adolescent problem-solving and withdrawal from conflict as a coping strategy of mothers, fathers and adolescents themselves (van Doorn, Branje, & Meeus, 2011). When intense conflict does occur, whether with parents or friends, it is associated with maladaptive outcomes for adolescents (Adams & Laursen, 2007; Ehrlich, Dykas, & Cassidy, 2012), as well as with altered patterns of cortisol reactivity as described previously.
For these reasons, we aimed to examine the role of parent support in two samples that were developmentally situated either before or after the onset of puberty in order to best highlight developmental differences in social networks and in stress reactivity that have been noted to emerge with puberty. Furthermore, we included measures of relational quality (support and negative interactions) with the parent to examine whether any significant differences between children and adolescents on these relationship dimensions would explain changes in the effectiveness of parent support with development.
Study Hypotheses
The present study investigated the effect of experimentally-provided parent support on the cortisol response to a modified TSST in 9-10-year-olds and 15-16-year-olds. The quality of parent support was rated by experimenters in real-time and later assessed by two additional independent coders watching the videotaped interactions. Based on the prior evidence described above and the measures we collected, we derived the following four hypotheses: H1) parental support provision would dampen the cortisol response to the TSST in the experimental group compared to the control group in the entire sample; however, H2) the parental buffering effect would be diminished in adolescents compared to children; additionally, H3) adolescents would show a stronger response to the social-evaluative task than children and higher basal cortisol levels; and H4) we tested which dimensions of parent-child relationship quality (support and negative interactions, observed and self-rated) differed between children and adolescents and tested whether these differences explained the hypothesized reduction in the effectiveness of parental buffering. Lastly, the effect of sex and its interactions with age and condition were also explored, but no directional predictions were made given the mixed prior evidence.
Methods
Participants
A sample of 81 typically-developing participants was recruited, half of whom were children (N = 40, M age = 9.97 years, SD = .52, range 9.1 - 11.1; 50% females) and half who were adolescents (N = 41, M age = 16.05 years, SD = .39, range 15.2 -16.8; 48.8% females). The sample size was calculated a priori based on the large effect sizes observed in adult studies for the difference between peak cortisol responses in the Stranger Support versus Romantic Partner Support conditions (e.g., Kirschbaum et al., 1995: Cohen's d = .83). All participants were raised by their birth families in a large urban Midwestern area. Exclusion criteria were Autism Spectrum Disorder, Fetal Alcohol Syndrome, or any other major developmental disorder; and use of steroid medications (due to their interference with cortisol assay results). Parents reported a range of annual household income from under $35,000 for 3.7% of the sample to over $200,000 for 11.1%, with a median yearly family income of $100,000-$125,000. Only two parents did not report their household income. Parental education ranged from less than a high school degree to doctorate-level, with a median of 16 years of education for the parents attending the session as well as their spouses or partners.
Procedure
Stress paradigm
The following research protocol was approved by the Institutional Review Board at the University of Minnesota. Using a cross-sectional developmental design to compare children with adolescents, 9-10 and 15-16-year-olds were recruited. The study had a balanced 2 x 2 x 2 design (Age Group x Sex x Experimental Condition), with 10 subjects per cell in all but one cell with N = 11. Participants completed an adapted version of the TSST using the story stem from the Modified Trier Social Stress Test (TSST-M, Yim, Quas, Cahill, & Hayakawa, 2010; an adaptation of the TSST-C by Buske-Kirschbaum et al., 1997). This paradigm consisted of a public speaking task (introducing oneself to a hypothetical new classroom of students) and a mental arithmetic task (subtracting out loud by 7s from 758 for adolescents or by 3s from 307 for children). The participant was alone in the room when giving the speech and performing the mental arithmetic in front of a two-way mirror and a conspicuously placed video camera. The participant was told that the experimenter and two other teachers (one male, one female) would watch them from the other side of the mirror and rate their speech performance and their arithmetic accuracy. This was accomplished using an audio recording of two adults who sternly provided instructions for the speech. Replacing an audience of three judges with a two-way mirror has succeeded in elevating cortisol in 9-year-olds (Jansen et al., 2000). At the end of the session, all participants and parents were debriefed about the protocol and given positive feedback on their performance.
Session timeline
Participants were accompanied by one of their parents to two laboratory sessions spaced up to one week apart, with all start times scheduled between 3:30 -4:30 pm in order to control for the diurnal variation in cortisol. Session 1 included the following: consent process and reading leisurely (25 min.), participant moving to adjacent room with either the parent or a female experimenter (based on random assignment) and receiving TSST instructions (5 min.), speech preparation with parent or stranger (5 min.), completing the TSST (10 min.), relaxing with parent (10 min.), and completing questionnaires (approximately one hour). In the Parent Support condition, the parents (91% mothers) were instructed to help their child or adolescent in any way they found useful. In the Stranger Support condition, the female stranger stated that she was ready to help in any way participants found useful. In this latter condition the parent remained in the waiting room and did not accompany the participant into the testing area. Salivary cortisol was collected four times during Session 1 (45, 65, 85, and 105 minutes after arrival), corresponding to 20 minutes since the end of the relaxation period and 20, 40, and 60 minutes after the end of the stress task.
Session 2 was conducted to collect two resting cortisol samples (45 and 65 minutes from arrival) and additional questionnaire measures. Prior work suggests that baseline cortisol samples are ideally collected in this fashion: in the same laboratory conditions, at the same time of day but on a different day, and during rest (Lovallo, Farag, & Vincent, 2010). This also eliminated the novelty of arriving to the laboratory and any anticipatory stress responses, since participants were told in advance that this session would only include “filling out paperwork”, without any challenging tasks. These samples were important for examining whether adolescents differed from children in resting basal cortisol levels, in addition to differences in reactivity.
Measures
Salivary cortisol
Participants expelled saliva through a straw into pre-labeled vials. They were instructed to refrain from eating large, protein-filled meals and consuming caffeine or energy drinks two hours before arriving to the laboratory. The samples were stored in a laboratory freezer at –20°C until being shipped to the University of Trier, Germany to be assayed using a time-resolved fluorescence immunoassay (dissociation-enhanced lanthanide fluorescent immunoassay [DELFIA]; intra-assay CV < 7%, inter-assay CV < 10%). All of the samples from each participant were included in the same assay batch, and the assay batches were balanced by group, age, and condition. Samples were assayed in duplicate and averaged.
Daily Diaries
The parent and the child each completed a daily diary on session days, for which they reported information about the participant relevant to cortisol collection: time of wake-up, medication usage, caffeine consumption, distressing events experienced that day (e.g., arguments with siblings or parents), and number of hours of sleep during the previous night. Child reports were the primary source of information. However, for type of medication used by the offspring, the parents's report on this variable was used instead when the child's information was missing, too vague or incomplete. All other child-reported variables were either complete or missing less than 5% of data so imputation was not necessary.
Child Life Events Scale (Boyce et al., 1995)
This is a widely-used and ecologically valid measure of life stressors for children. The scale asked the parent to select any major life events that occurred in their child's life in the 3 months prior to testing (from a list of 40 possible events –e.g., serious illness of parent, death of a grandparent, change in schools, problems with teachers, moving to a new home, etc.). One of the items is an open category allowing the parent to add any life events not previously listed. A total score was created by adding 1 for each event experienced by children and adolescents during this period (M = 1.42, SD = 1.77, range = 0-10).
Self-Assessment Manikin (Lang, 1980)
Towards the end of the session, participants used 5-point Likert-type scales (from 1 = “calm” to 5 = “very high stress”) to rate how stressed they felt at five time points during the session: upon arrival to the laboratory, during speech preparation, while giving the speech, during the subtraction task, and when completing this questionnaire (at least 30 minutes post-stressor).
Male and Female Puberty Scales (Petersen, Crockett, Richards, & Boxer, 1988)
This questionnaire assessed participants’ physical changes indicative of puberty, which were used to verify that 9-10-year-olds were indeed pre-pubertal and 15-16-year-olds were pubertal. Both the parent and the child completed these questionnaires for increased accuracy. The scale yields a mean score from 1 to 4 and participants are considered pubertal if average scores are at least 2.5. All of the 9-10-year-olds were pre-pubertal and all but one adolescent were pubertal according to self-report (this female scored a 2.4, however the mother's report indicated a score of 3.5, thus she likely underestimated or was too embarrassed to endorse all the physical changes).
Observational ratings of parent support
Speech preparation with the parent (for participants randomized to this condition) was rated in real time by the experimenter using 5-point Likert-type items yielding two subscales: Parent Support (4 items: parental sensitivity, positive affect towards the child, effective communication, and validation of the child's perspective; Cronbach's α = .92) and Parent Negativity (3 items measuring intrusiveness, criticism, and authoritarianism; scale Cronbach's α = .74). For each item, the experimenter rated how often (1 = “never” to 5 = “the entire time”) the parent engaged in a type of behavior (e.g., being intrusive). Parent behavior from the same segment was also later double-coded from videotapes by two other independent coders (with good reliability between these two coders: intra-class correlation = .92). This scale had more items because coders could re-watch the videos. Coders completed 5-point Likert-type scales to yield a measure of Parent Support (6 items: encouragement, validation, coping assistance, positivity, sensitivity, and helpfulness; Cronbach's α = .71) and a measure of Parent Negativity (2 items: criticism and intrusiveness; Cronbach's α = .75). Experimenter ratings and the average of the two coders’ ratings were standardized and averaged to create one measure of observed Parent Support and one of Parent Negativity.
Network of Relationships Inventory: Social Provisions Version (NRI-SPV) (Furman & Burhmester, 1985, 2009). The NRI-SPV is a well-validated questionnaire that was designed to be used with children and adolescents. It assesses ten relationship qualities (with three questions for each) including seven aspects of support (Companionship, Instrumental Aid, Intimate Disclosure, Nurturance, Affection, Reassurance of Worth, and Reliable Alliance), two negative relationship features (Conflict, Antagonism) and a measure of Relative Power. For instance, participants completed Likert-type items rating “how much does this person like or love you” or “how much do you and this person get upset with or mad at each other” using scales from 1 (“little or none”) to 5 (“the most”). We used subjects’ ratings of these qualities for the parent attending the session (91% mothers) to extract a summary measure of Support by averaging the 7 support subscales (Cronbach's alpha = .89) and a measure of Negative Interactions based on averaging the Conflict and Antagonism subscales (Cronbach's alpha = .94).
Data Analysis Plan
Data preparation
Preliminary analyses were conducted to identify outliers in cortisol concentrations and values more than 3 SD from the mean were Winsorized (3 values for Day 1 and 3 values for Day 2) and replaced with the value at the 99.7th percentile. Because cortisol measures displayed high skewness and kurtosis, a log10 transformation was applied to these concentrations after Winsorizing to normalize their distributions and meet assumptions for statistical analyses.
Statistical Analyses
Hierarchical Linear Modeling (HLM, Raudenbush, 2001; Raudenbush & Bryk, 2002) was used to analyze the cortisol data because it is ideal for autocorrelated samples collected from the same individual and it allows for greater statistical power than traditional RM ANOVA models (Raudenbush & Bryk, 2002). Analyses were implemented using the PROC MIXED procedure for linear mixed modeling in the SAS 9.2 Software (SAS Institute, Inc., 2009). The Level 1 model represented individual change in levels of cortisol as a function of time. We anticipated based on previous research (Lam, Dickerson, Zoccola, & Zaldivar, 2009; Smith et al., 2009) and the sampling times in this design that both a linear and quadratic term for time would be necessary to model cortisol reactivity (i.e., sample time and time-squared were entered as predictors). Indeed, the linear and quadratic time terms included in the model were both significant: F(1, 217) = 8.09, p = .005 and F(1, 161) = 36.45, p < .001, suggesting the expected significant peaked cortisol response with the TSST. Time was coded as 0, 1, 2, and 3 given that the samples were equally spaced at 20-minute intervals. The linear time term represented the instantaneous rate of change in cortisol and the quadratic time term represented the curvature of the cortisol trajectory. We also added a peak term to the intercept (a dummy code indicating whether the value was sample 2 or not) to superimpose upon the quadratic model (similar to methodology for modeling the cortisol awakening response as a peak elevation above the diurnal rhythm curve, Adam, Hawkley, Kudielka, & Cacioppo, 2006). The Level 2 model explained between-subjects differences based on multiple independent variables: three dummy variables coding for Age group (child versus adolescent), Sex, and Condition (Stranger versus Parent support), as well as their interactions. Type 3 F tests of fixed effects were reported for each main or interaction effect and specific β parameters were used to follow up on significant interactions and interpret directionality of effects through simple slope analysis (Aiken & West, 1991). Continuous variables were mean-centered. Kenward-Roger adjusted degrees of freedom were used (Kenward & Roger, 1997).
Cortisol covariates
Cortisol is sensitive to numerous sleep, diet, medication, experiential, and demographic factors (Cohen, Doyle, & Baum, 2006; Kudielka, Hellhammer, & Wüst, 2009). Of the Daily Diary and demographic factors relevant for cortisol measures (time since wake-up, number of hours slept the previous night, number of distressing events experienced the day of testing, number of major life events in the previous 3 months, caffeine consumption, and medication usage coded according to Granger, Hibel, Fortunato, & Kapelewski, 2009), the only variables that were significantly or marginally associated with any cortisol measures on the experimental day were time since wake-up and family income (see Table 1 for bivariate correlations). Thus these two measures were included as covariates for intercepts, linear and quadratic terms in every model. Results did not change when models were run without these covariates. Participants did not differ by condition on any of these demographic and Daily Diary measures (p > .32), suggesting that randomization was successful.
Table 1.
Descriptive statistics and bivariate correlations between main study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Log Cortisol 1 (nmol/L) | - | .74** | .66** | .56** | −0.21 | 0.10 | 0.09 | −0.19 | 0.20 | 0.08 |
| 2. Log Cortisol 2 (nmol/L) | - | .90** | .82** | −0.21 | 0.21 | 0.08 | −0.08 | 0.16 | 0.05 | |
| 3. Log Cortisol 3 (nmol/L) | - | .92** | −0.18 | .32** | 0.16 | −0.17 | 0.16 | 0.03 | ||
| 4. Log Cortisol 4 (nmol/L) | - | −0.22 | .32** | 0.12 | −0.22 | 0.12 | 0.02 | |||
| 5. Time since wake-up | - | −0.10 | −0.27 | 0.08 | −0.01 | 0.06 | ||||
| 6. Household income ± | - | −0.29 | 0.25 | −0.02 | 0.01 | |||||
| 7. Observed Negative Interactions | - | −.45** | 0.12 | −0.25 | ||||||
| 8. Observed Parent Support | - | −.32* | 0.19 | |||||||
| 9. Self-reported Negative InteractionsΔ | - | −.34** | ||||||||
| 10. Self-reported Parent SupportΔ | - | |||||||||
| Mean | 0.52 | 0.57 | 0.47 | 0.37 | 8.46 | 6.77 | −0.02 | 0.00 | 2.15 | 3.68 |
| SD | 0.27 | 0.33 | 0.29 | 0.25 | 1.99 | 2.09 | 0.77 | 0.54 | 1.08 | 0.72 |
Household income was measured on a 1-11 scale, where 1 = “less than $15,000 per year” and 11 = “more than $200,000 per year”. A mean of 6.77 fell in the "$75,000-$100,000/year" range.
Possible scores on these NRI scales ranged from 1 to 5, where 1 = “None or little” and 5 = “The most”.
p < .05
p < .01
Models tested
We began by testing the first three hypotheses simultaneously in a model including age, condition, sex, and their interactions as predictors of cortisol, with the previously mentioned covariates at every level of the model. Non-significant two-way and three-way interactions were dropped from this and subsequent models. We then tested the same model, with the same covariates, but predicting perceived, self-reported stress levels across the session. Self-reported stress levels were modeled using HLM in the same fashion as cortisol levels, given that the trajectories showed similar curvilinearity. Lastly, to test whether parent-child relationship qualities explained developmental differences between children and adolescents, we retested the first model but controlling for the effect of any relationship dimension that differed significantly between the two age groups. Four dimensions were considered (observed and self-reported Parent Support and Negative Interactions). Those differing between children and adolescents were entered at every level of the HLM.
Results
Descriptive statistics and bivariate correlations involving the main study variables are presented in Table 1.
Cortisol Reactivity Differences by Condition, Age, and Age x Condition: Hypotheses 1, 2, and 3
To test the first three hypotheses, one model was used to simultaneously test the fixed effects of age, condition, sex, and their two-way and three-way interactions on cortisol trajectories, controlling for time since wake-up and family income at each level of the model. Given that the interactions of sex x condition and age x sex x condition did not have significant effects on intercepts, the linear or quadratic terms (p values >.18), and that significant results did not change with or without including these interactions, these higher order terms were dropped (see Table 2 for results of F tests for the fixed effects in this trimmed model).
Table 2.
Type 3 F tests for the model used to test the first three hypotheses.
| Fixed Effect | Num. DF | Den. DF | F | P |
|---|---|---|---|---|
| Linear | 1 | 202 | 1.18 | 0.28 |
| Quadratic | 1 | 158 | 11.88 | <0.001** |
| Peak (effect of being sample 2) | 1 | 158 | 7.83 | <0.01** |
| Time since wake-up | 1 | 86.3 | 0.81 | 0.37 |
| Time since wake-up×Linear | 1 | 216 | 0 | 0.98 |
| Time since wake-up×Quadratic | 1 | 158 | 0 | 0.96 |
| Income | 1 | 86.3 | 0.12 | 0.73 |
| Income×Linear | 1 | 216 | 3.61 | 0.06 |
| Income×Quadratic | 1 | 158 | 1.05 | 0.31 |
| Age Group | 1 | 86.3 | 11.83 | <0.001** |
| Age Group×Linear | 1 | 216 | 0.01 | 0.93 |
| Age Group×Quadratic | 1 | 158 | 0.42 | 0.52 |
| Sex | 1 | 86.3 | 0.05 | 0.82 |
| Sex×Linear | 1 | 216 | 3.51 | 0.06 |
| Sex×Quadratic | 1 | 158 | 3.95 | 0.049* |
| Condition | 1 | 86.3 | 0.05 | 0.83 |
| Condition×Linear | 1 | 216 | 2.94 | 0.09 |
| Condition×Quadratic | 1 | 158 | 2.63 | 0.11 |
| Age Group×Sex | 1 | 86.3 | 1.75 | 0.19 |
| Age Group×Sex×Linear | 1 | 216 | 4.15 | 0.04* |
| Age Group×Sex×Quadratic | 1 | 158 | 5.73 | 0.02* |
| Condition×Age Group | 1 | 86.3 | 0.18 | 0.67 |
| Condition×Age Group×Linear | 1 | 216 | 4.88 | 0.03* |
| Condition×Age Group×Quadratic | 1 | 158 | 4.2 | 0.04* |
p < .05
p < .01.
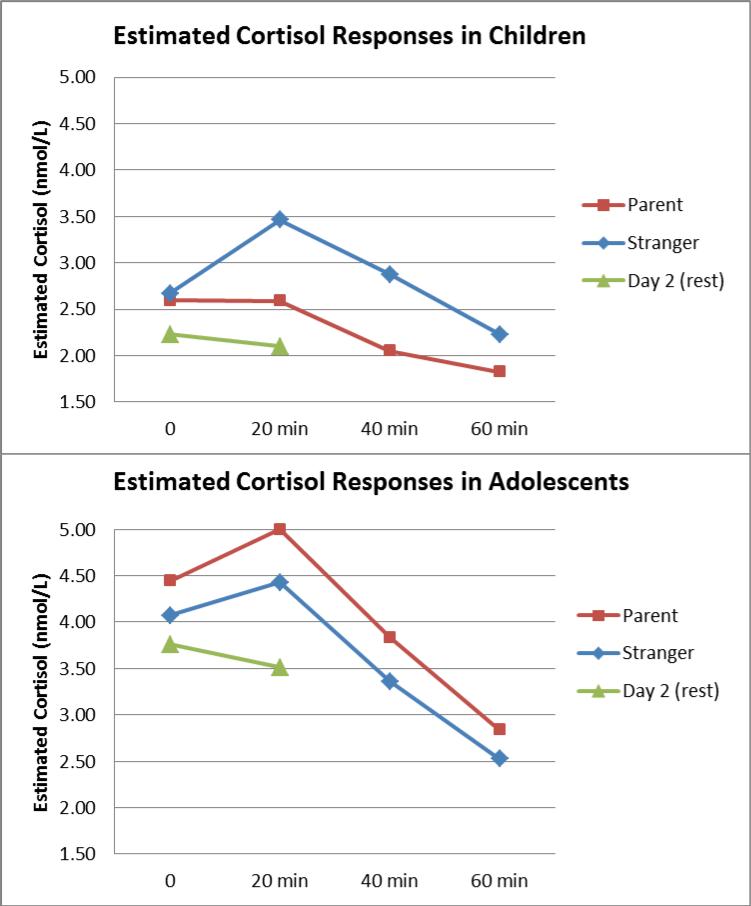
The first hypothesis posited dampened cortisol reactivity with parent support in the entire sample. The main effect of condition (Parent vs. Stranger Support) was not statistically significant (effect on linear term: F(1, 216) = 2.94, p = .09; on quadratic term: F(1,158) = 2.63, p = .11). The second hypothesis predicted that parent support would be less effective in adolescents compared to children. In support of this notion, there was a significant interaction of condition x age group in predicting cortisol trajectories (linear term, F(1,216) = 4.88, p = .03; quadratic term: F(1,158) = 4.2, p = .04). Simple slope tests for condition when each of the age groups was coded to be the comparison group revealed a significant effect of condition for 9-10-year-olds, such that they had greater cortisol reactivity in the Stranger condition (effect on linear term: β = .16, SE = .06, t(216) = 2.75, p = .007; on quadratic term: β = -.04, SE = .02, t(158) = - 2.57, p = .01) and a dampened cortisol response for 9-10-year-olds in the Parent condition (see Figure 1 for estimated cortisol values, Table 3 for raw observed means). As expected given random assignment, children’s cortisol intercepts (i.e., baseline levels) did not differ by Parent vs. Stranger condition, β = .01, SE = .08, t(86.3) = .15, p = .88. Among adolescents, there was no effect of condition on the intercept, linear or quadratic terms (p's > .65), thus parents did not affect stress reactivity compared to strangers.
Figure 1.
Estimated cortisol samples based on HLM results by age group and condition (Stranger Support or Parent Support). The TSST lasted 10 minutes and ended at the 0 minute mark on this graph. Analyses were conducted with log-transformed cortisol and estimated values were exponentiated to present nmol/L concentrations. Day 2 samples were estimated in a separate linear model controlling for the same covariates.
Table 3.
Observed Cortisol level means (standard errors of the mean in parenthesis) by age group and condition. Values reported in nmol/L.
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
|---|---|---|---|---|
| Children -Parent Condition | 3.20 (.53) | 3.08 (.49) | 2.53 (.45) | 2.01 (.25) |
| Children -Stranger Condition | 3.31 (.57) | 4.48 (.80) | 3.79 (.77) | 2.69 (.46) |
| Adolescents -Parent Condition | 4.61 (.39) | 6.00 (.77) | 4.17 (.48) | 3.11 (.29) |
| Adolescents -Stranger Condition | 4.80 (.65) | 5.85 (.84) | 4.29 (.75) | 3.11 (.38) |
The third hypothesis concerned developmental differences in cortisol reactivity and basal cortisol between children and adolescents. As expected, there was a significant effect of age group on the cortisol intercept, F(1, 86.3) = 11.83, p < .001, with adolescents having higher initial values than children. This was evident for both the experimental day and the rest day (see Figure 1). There were no other age differences in cortisol curve parameters indexing reactivity (Table 2).
Sex differences were also considered in this first model. Main effects were not interpreted given that there was a significant interaction of age x sex on the linear term, F(1,216) = 4.15, p =.04, and quadratic term, F(1,158) = 5.73, p =.02. Follow-up simple slope analyses indicated that 9-10-year-old girls had greater cortisol reactivity than 9-10-year-old boys (linear term: β = .16, SE = .06, t(216) = 2.79, p = .006; quadratic term: β = -.05, SE = .02, t(158) = -3.13, p = .002). Among adolescents there were no sex differences in cortisol intercepts or slopes (p's >.27).
It must be noted that the age x condition interaction was specific to HPA axis reactivity and was not observed for self-reported stress ratings. Using the Self-Assessment Manikin, growth-curve modeling captured significant increases in ratings of subjective stress from the beginning of the session through the math segment (linear term, F(1, 316) = 274.4, p < .001; quadratic term, F(1, 316) = 345.8, p < .001) with the parent's presence having no effect on self-reported stress, and likewise no effects of age group, sex or any of their two-way or three-way interactions on intercepts, linear or quadratic terms (p's >.16).
Relationship Quality as an Explanation for Reduced Buffering: Hypothesis 4
Lastly, we aimed to examine whether changes in parent-child relationship quality between childhood and adolescence may explain the pattern of reduced parental buffering in adolescents. We first tested which relationship qualities differed between these two age groups (observed or self-reported parent support, and negative interactions), then tested whether our main finding would become non-significant when controlling for variance due to relationship qualities that differed significantly between children and adolescents. Observational measures of laboratory behavior and self-reported measures of overall relational quality were analyzed separately because they were not significantly correlated with each other (Table 1) and likely captured slightly different aspects of the relationships.
Observational measures of support could not explain the developmental differences noted with cortisol reactivity, given that ANOVAs revealed no significant differences in age, sex, or age x sex in terms of either observed support, F(1, 37) = 3.13, p = .09, partial η2 = .08; F(1, 37) = .004, p = .95, partial η2 <.001; and F(1, 37) = .98, p = .33, partial η2 = .03, or observed negativity, F(1, 37) = .93, p = .34, partial η2 = .02; F(1, 37) = .09, p = .77, partial η2 = .002; and F(1, 37) = .10, p = .75, partial η2 = .003.
Developmental differences in perceived Support and Negative Interactions derived from the NRI scales were examined next. ANOVAs with age, sex, and age x sex as predictors revealed that adolescents reported significantly more Negative Interactions in their relationship with the parent than children did, F(1, 77) = 10.88, p < .001, partial η2 = .12; estimated M Adolescents = 2.51, SE = .16, estimated M Children = 1.78, SE = .16; there was also a significant age x sex interaction, F(1, 77) = 5.76, p = .02, partial η2 = .07, such that adolescent females reported higher Negative Interactions than males (p = .03, estimated M females = 2.86, SE = .22, M males = 2.17, SE = .22), but children did not differ by sex (p = .24). The main effect of sex on Negative Interactions was not significant, F(1, 77) = .51, p = .48, partial η2 = .01, and there were also no significant age, sex, or age x sex differences in self-reported support from the parent, F(1, 77) = .23, p = .63, partial η2 = .003; F(1, 77) = .41, p = .52, partial η2 = .005; and F(1, 77) = .19, p = .66, partial η2 = .002, respectively.
Given that self-reported Negative Interactions was the only dimension that differed significantly between children and adolescents and it was also significantly correlated with age (r(79) = .35, p < .001), we examined the possibility that age group might simply act as a proxy measure of negativity, confounding the results. We re-tested our first model, including age and age by condition effects, while controlling for variance due to self-reported Negative Interactions by adding its effects on intercepts, linear and quadratic terms. None of these three effects were significant (p > .32), and the age x condition interaction previously noted remained significant (linear term: F(1, 216) = 4.68, p = .03; quadratic term: F(1, 158) = 3.95, p = .048), as did the main effect of age on basal cortisol (F(1, 86.4) = 8.46, p = .005). Follow-up simple slope comparisons controlling for Negativity also yielded identical results, showing significantly reduced reactivity in children receiving parent support and no significant differences by condition in adolescents. Results from the first model also remained identical when controlling for each of the relational qualities that did not reach statistical significance in terms of age group differences (observed support, observed negativity, or self-reported support).
Discussion
To our knowledge, this is the first experimental study showing that parental social buffering has diminished effectiveness in reducing cortisol stress responses in adolescence compared to childhood. Parent support appeared to nearly completely block mean increases in cortisol among the child participants, but had no buffering effects among adolescents. There are several possible underlying mechanisms for this finding. One possibility is that we are observing a puberty-related phenomenon whereby interactions with the parent cease to have the same potent buffering effect on the HPA axis that they did in infancy and childhood, even when adolescents continue to report high levels of perceived support from their parents. It is well-recognized that adolescents from many species and human cultures leave their native family group (Schlegel & Barry, 1991; Steinberg, 1988). It may be that there are puberty-related maturational changes in neurobiology that switch off the anti-stress protective features of the caregiver, which might then ease the adolescent's leaving of the natal group. There is some evidence that psychological distancing from parents increases with pubertal maturation specifically and not just with chronological age (Hill et al., 1985a, b; Steinberg, 1987, 1988). Future studies should examine whether it is puberty onset or simply increasing age that is associated with the waning effectiveness of the parent's presence as a social buffer.
The second possibility is that as children start spending more time with peers, reliance on parents for support and help diminishes. Certainly, adolescence brings about increased affiliative tendencies towards peers and potential romantic partners (Collins, Welsh, & Furman, 2009; Forbes & Dahl, 2010), thus it is possible that the rewarding nature of these social interactions and the sheer increase in the amount of time spent with peers (Larson & Richards, 1991) leads to a shift in the relative saliency of peers versus parents as sources of social buffering. These socioemotional changes have long been observed in questionnaire data collected from adolescents (Buhrmester & Furman, 1987; Furman & Buhrmester, 1992; Hunter & Youniss, 1982). If these shifts in social network configuration are the driving mechanism, then we might expect a more gradual change in the effectiveness of parental social buffering, as youth turn increasingly to peers for support across the entire span of adolescence. A previous correlational study would support the role of peers as stress buffers, showing that 10-11-year-olds had lower levels of cortisol after negative events recorded in their daily diaries if they reported being with their best friend at the time (Adams, Santos, & Bukowski, 2011). However, experimental paradigms which invite a best friend to the laboratory to provide assistance before the stressor are needed before drawing any definitive conclusions. Additionally, even though our paradigm is ecologically valid and resembles school contexts, where children and adolescents often have to introduce themselves or speak in front of others to be evaluated, it is possible that a different pattern of results would be obtained in other social contexts. For instance, parents might remain the primary social buffer when adolescents are confronted with scary medical procedures or physical illness, and future studies should examine when and if parental protection in these other contexts ever wears off during later development.
Thirdly, the fact that adolescence is a period of enhanced self-conscious emotions and neural activation to social evaluation (Somerville et al., 2013) may render social-evaluative stressors particularly difficult to buffer by any support figure during this period. In addition, other studies have also reported an increase in HPA axis and autonomic activity to this type of performance stressor across adolescence (Gunnar et al., 2009; Stroud et al., 2009; van den Bos et al., 2013), supporting the hypothesis that this stressor may be more potent at this developmental stage. Even though we did not find evidence of greater cortisol reactivity in adolescents compared to children in the Stranger condition, after controlling for adolescents’ higher baseline cortisol, it is still possible that HPA responses to social-evaluative threat might be more difficult to contain or regulate in adolescence, regardless of whether they differ in absolute magnitude. Future studies should examine the effect of various stress task components on HPA responses across development, with or without support from a wide variety of social partners, to examine the extent to which social evaluation may be Achilles’ heel for adolescents, that perhaps no one can buffer, or whether for instance close friends can serve this role.
Lastly, it could be argued that the underlying mechanism is increased negativity in the parent-child relationship, occurring as adolescents negotiate their increasing needs for autonomy. This was, indeed, the only parent-child relationship dimension that adolescents and children differed on in this sample. However, the present study did not support this interpretation as this dimension was not a significant predictor of cortisol reactivity in our sample and the developmental differences in parental buffering remained significant when parsing out cortisol variance due to self-reported negative interactions. Conflict and disagreement with the parent are known to have important repercussions for adolescent adjustment and development (Adams & Laursen, 2007), but our findings suggest that they may not be the explanation for the reduced efficiency of parental social buffering of the HPA axis in adolescence. Indeed, it is notable that we obtained evidence of a loss of parental social buffering effectiveness in adolescence even in this low-risk sample, where scores for negative interactions with the parents were generally low. To improve generalizability, future studies should examine families expressing a broader range of parent-youth relationship qualities and use conflict discussion tasks in the laboratory to elicit and assess these behaviors. In addition, future research should recruit participants from broader socioeconomic strata, given the known effects of severe poverty and family stress on parent-child relationships and on stress biomarkers (Evans & English, 2002).
It must be noted that developmental differences in social buffering were only observed with respect to HPA reactivity, and were not evident when using participants’ subjective ratings of experienced stress. This is consistent with the decoupling between self-report measures and hormonal output observed in the majority of adult studies using the TSST (reviewed by Campbell & Ehlert, 2012) –e.g., women reported feeling more supported than men but men were the only ones exhibiting diminished cortisol responses with support from romantic partners in one study (Kirschbaum et al., 1995). Consistent with studies of early development showing dissociations between HPA reactivity and behavioral signs of fear or distress (Nachmias et al., 1996), there are likely true dissociations among different emotional, cognitive, and physiological stress response systems in many situations (Campbell & Ehlert, 2012). The effects of social desirability biases and motivational engagement on self-reports cannot be ruled out either. For these reasons, HPA measures continue to be critical in investigations of stress-alleviating factors, as they provide unique and sometimes divergent information compared to self-reports.
The neurobiological mechanisms through which parents buffer the HPA axis for children are insufficiently understood, but based on animal models and adult neuroimaging studies it has been suggested that warmth and safety cues from the parent (visual stimuli, vocalizations, touch) may work through oxytocinergic activity and/or prefrontal cortex-mediated inhibition of fear and stress neurocircuitry to lower cortisol responses (Hostinar et al., 2014). As discussed above, there are several scenarios that may explain the reduced efficiency of parental buffering for adolescents, but the neurobiology underlying this developmental difference is not yet known.
With respect to developmental differences in HPA axis activity, adolescents exhibited higher cortisol levels overall, a pattern that is consistent with prior reports of increasing average basal cortisol levels with puberty (for a review, see Gunnar & Vazquez, 2006). When examining the combined effects of sex and age, 9-10-year-old females had higher cortisol output than 9-10-year-old males. Previous studies have not found sex differences in responses to the TSST for children in the 9-12 age range (e.g., Gunnar et al., 2009; Yim et al., 2009), thus this pattern would need to be replicated before any clear conclusions can be drawn. Among adolescents, there were no sex differences in cortisol reactivity. Prior findings on sex differences in TSST responses in similarly-aged samples are mixed, with some reporting no sex differences after puberty (Gunnar et al., 2009; Kudielka et al., 2004; Stroud et al., 2009; Sumter et al., 2010), whereas others indicate that males tend to have higher HPA responses to performance stressors, especially in the later stages of puberty (Klimes-Dougan et al., 2001; Zijlmans et al., 2013). It is possible that studies finding sex by age interactions post-puberty differ in the task demands on the participants. For instance, studies emphasizing competition (e.g., Zijlmans et al., 2013) tend to find higher levels of reactivity in males compared to females. The paradigm used here (hypothetically introducing oneself to a new classroom of students and believing that there is one male and one female judge) may have equalized the demands of the task for boys and girls.
The present study had a number of strengths, including the experimental control of the stressor and the manipulation of support provision in the laboratory. Correlational studies reporting lower cortisol reactivity in children with supportive parents cannot rule out the possibility of gene-environment correlations, such that low-reactive parents would perhaps be both more responsive and transmitting genetic factors for lower HPA reactivity to their children. Our results cannot be explained by gene-environment correlations, given that the cortisol response was only blocked in children randomly assigned to the parent support condition, and that the experimental and control groups were carefully matched on age and sex. Additionally, the results provide novel and important results regarding the social buffering of stress by parent support in two previously neglected developmental periods: late childhood and adolescence.
The study also had a number of limitations. The cross-sectional design makes it difficult to draw conclusions about developmental change, urging future replication of the same process with a longitudinal study. However, there is some evidence that repeating the Trier Social Stress Test causes some habituation of HPA axis reactions (Schommer, Hellhammer, & Kirschbaum, 2003), making the current design preferable in this regard. Information from both types of designs should be combined to inform and strengthen conclusions. The second limitation was the omission of adolescents’ ratings of the perceived helpfulness of the parent during speech preparation. However, these ratings likely would have been highly correlated with participants’ overall ratings of support given that relationship histories provide context for and can bias perceptions of parent-child interactions compared to observer ratings (Dykas, Woodhouse, Ehrlich, & Cassidy, 2012). Lastly, the study had a relatively modest sample size for detecting two-way and three-way interactions at Level 2 of the hierarchical linear model, thus future studies should increase enrollment and use the estimates obtained here to adequately power examinations of these higher-order interactions. Subsequent studies should also collect more cortisol data points to allow for a more precise estimation of the shape of the growth curve.
Despite these limitations, the present study opens an important avenue of research on the developmental changes in the social regulation of HPA axis stress reactivity. Future studies should examine whether adolescents who normatively shift from seeking parental support to peer support but fail to establish close friendships are at increased risk of experiencing stress and emotional dysregulation during this developmental period.
Research Highlights.
Parents are powerful stress buffers for children during early development, and our study suggests they can still serve this role for 9-10-year-old children
Even though parent support nearly eliminated the cortisol stress response to a laboratory public speaking challenge for 9-10-year-old children, it had no effect on the HPA stress responses of adolescents aged 15-16
One implication of these findings may be that adolescents who normatively shift from seeking parental support to peer support but fail to establish close friendships may be left without social buffers and experience increased stress
Acknowledgements
We would like to thank the families from the Institute of Child Development Participant Pool for their participation. This work was supported by the Eva O. Miller Fellowship to Camelia E. Hostinar, a predoctoral Interdisciplinary Training Program in Cognitive Science Fellowship (NIH T32HD007151) to Anna E. Johnson, a seed grant from the NIMH Early Experience, Stress and Neurobehavioral Development Center (Grant P50 MH078105 to Megan R. Gunnar), a small grant award from the Institute of Child Development, and by travel awards from the Center of Neurobehavioral Development at the University of Minnesota.
References
- Adam E. Transactions among trait and state emotion and adolescent diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. doi:10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):17058–63. doi: 10.1073/pnas.0605053103. doi:10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RE, Santos JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2011;47(6):1786–1791. doi: 10.1037/a0025401. doi:10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Development. 2004;75(3):639–650. doi: 10.1111/j.1467-8624.2004.00698.x. doi:10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; Newbury Park: 1991. [Google Scholar]
- Albers EM, Riksen-Walraven JM, Sweep FCGJ, De Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology and Psychiatry. 2008;49:97–103. doi: 10.1111/j.1469-7610.2007.01818.x. doi:10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. doi:10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress: Effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34(6):884–93. doi: 10.1016/j.psyneuen.2009.01.003. doi:10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(12):1510–20. doi: 10.1097/01.chi.0000237706.50884.8b. doi:10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Buhrmester D, Furman W. The development of companionship and intimacy. Child Development. 1987;58(4):1101–1113. doi: 10.1111/j.1467-8624.1987.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–26. doi: 10.1097/00006842-199707000-00012. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9251162. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–34. doi: 10.1016/j.psyneuen.2011.12.010. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Cauce AM. Social networks and social competence: Exploring the effects of early adolescent friendships. American Journal of Community Psychology. 1986;14:607–628. doi: 10.1007/BF00931339. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Sinha R, Simmons JA, Healy SM, Mayes LC, Hommer RE, Crowley MJ. Parent-adolescent conflict interactions and adolescent alcohol use. Addictive Behaviors. 2012;37(5):605–12. doi: 10.1016/j.addbeh.2012.01.004. doi:10.1016/j.addbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. doi:10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. doi:10.1037/0033-2909.98.2.310. [PubMed] [Google Scholar]
- Collins WA, Laursen B. Changing relationships, changing youth: Interpersonal contexts of adolescent development. Journal of Early Adolescence. 2004;24(1):55–62. doi:10.1177/0272431603260882. [Google Scholar]
- Collins WA, Welsh DP, Furman W. Adolescent romantic relationships. Annual Review of Psychology. 2009;60:631–52. doi: 10.1146/annurev.psych.60.110707.163459. doi:10.1146/annurev.psych.60.110707.163459. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. doi:10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dietz LJ, Stoyak S, Melhem N, Porta G, Matthews KA, Walker Payne M, Brent DA. Cortisol response to social stress in parentally bereaved youth. Biological Psychiatry. 2013;1573(4):379–87. doi: 10.1016/j.biopsych.2012.08.016. doi:10.1016/j.biopsych.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. doi:10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schmidt S, Strauss B, Nater UM, Ehlert U, Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. Journal of Psychosomatic Research. 2008;64:479–486. doi: 10.1016/j.jpsychores.2007.11.011. doi:10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Doan SN, Evans GW. Maternal responsivity buffers the relationship between allostatic load and working memory. Development and Psychopathology. 2011;23:873–88. doi: 10.1017/S0954579411000368. doi:10.1017/S0954579411000368. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Kolko DJ, Susman EJ, Huang B, Stein H, Music E, Bukstein OG. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biological Psychology. 2009;81(1):31–9. doi: 10.1016/j.biopsycho.2009.01.004. doi:10.1016/j.biopsycho.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykas MJ, Woodhouse SS, Ehrlich KB, Cassidy J. Do adolescents and parents reconstruct memories about their conflict as a function of adolescent attachment? Child Development. 2010;81(5):1445–59. doi: 10.1111/j.1467-8624.2010.01484.x. doi: 10.1111/j.1467-8624.2010.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Dykas MJ, Cassidy J. Tipping points in adolescent adjustment: Predicting social functioning from adolescents’ conflict with parents and friends. Journal of Family Psychology. 2012;10:776–783. doi: 10.1037/a0029868. doi:10.1037/a0029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S. Structure provided by parents in middle childhood predicts cortisol reactivity in adolescence among the offspring of parents with bipolar disorder and controls. Psychoneuroendocrinology. 2009;34(5):773–85. doi: 10.1016/j.psyneuen.2008.12.011. doi:10.1016/j.psyneuen.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31(10):1164–80. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Developmental Science. 2010;13(2):271–278. doi: 10.1111/j.1467-7687.2009.00890.x. doi:10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Flinn MV. Family environment, stress, and health during childhood. In: Panter-Brick C, Worthman C, editors. Hormones, health, and behavior. Cambridge University press; Cambridge: 1999. pp. 105–138. [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. American Journal of Physical Anthropology. 1997;102(1):33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. doi:10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W, Buhrmester D. Children's perceptions of the personal relationships in their social networks. Developmental Psychology. 1985;21(6):1016–1024. doi:10.1037//0012-1649.21.6.1016. [Google Scholar]
- Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Development. 1992;63(1):103–115. doi: 10.1111/j.1467-8624.1992.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Furman W, Buhrmester D. The Network of Relationships Inventory: Behavioral Systems Version. International Journal of Behavioral Development. 2009;33(5):470–478. doi: 10.1177/0165025409342634. doi:10.1177/0165025409342634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman W, Simon VA, Shaffer L, Bouchey HA. Adolescents’ working models and styles for relationships with parents, friends, and romantic partners. Child Development. 2002;73(1):241–255. doi: 10.1111/1467-8624.00403. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14717255. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. doi:10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso R. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. doi:10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Developmental Neuroscience. 2nd ed. Vol. 2. Wiley; New York: 2006. pp. 533–577. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. doi:10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Kobrin L, Hurt H, Farah MJ. Selective impact of early parental responsivity on adolescent stress reactivity. PLoS One. 2013;8(3):e58250. doi: 10.1371/journal.pone.0058250. doi:10.1371/journal.pone.0058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR. Where is the child's environment? A group socialization theory of development. Psychological Review. 1995;102:458–489. [Google Scholar]
- Hartup WW. The company they keep: Friendships and their developmental significance. Child Development. 1996;67:1–13. [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. doi:10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. doi:10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hill J, Holmbeck G, Marlow L, Green T, Lynch M. Pubertal status and parent-child relations in families of seventh grade boys. Journal of Early Adolescence. 1985a;5:31–44. doi: 10.1007/BF02089236. [DOI] [PubMed] [Google Scholar]
- Hill J, Holmbeck G, Marlow L, Green T, Lynch M. Menarcheal status and parent-child relations in families of seventh grade girls. Journal of Youth and Adolescence. 1985b;14:301–316. doi: 10.1007/BF02089236. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. doi:10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–82. doi: 10.1037/a0032671. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter FT, Youniss J. Changes in functions of three relations during adolescence. Developmental Psychology. 1982;18:806–811. [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Van der Gaag RJ, ten Hove F, Willemsen-Swinkels SWM, Harteveld E, van Engeland H. Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, Multiple Complex Developmental Disorder. Psychoneuroendocrinology. 2000;25:753–764. doi: 10.1016/s0306-4530(00)00020-2. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society. Biological Sciences. 2008;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Conger RD, Lorenz FO, Elder GH. Parent-adolescent reciprocity in negative affect and its relation to early adult social development. Developmental Psychology. 2001;37(6):775–790. doi:10.1037//0012-1649.37.6.775. [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. Retrieved from http://www.psychosomaticmedicine.org/content/57/1/23.long. [DOI] [PubMed] [Google Scholar]
- Klein ZA, Romeo RD. Changes in hypothalamic-pituitary-adrenal stress responsiveness before and after puberty in rats. Hormones and Behavior. 2013:1–7. doi: 10.1016/j.yhbeh.2013.01.012. doi:10.1016/j.yhbeh.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11523855. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. doi:10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis response to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. doi:10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. Ablex; Norwood, NJ: 1980. pp. 119–l37. [Google Scholar]
- Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Development. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Laursen B, Collins WA. Interpersonal conflict during adolescence. Psychological Bulletin. 1994;115:197–209. doi: 10.1037/0033-2909.115.2.197. doi:10.1037//0033-2909.115.2.197. [DOI] [PubMed] [Google Scholar]
- Laursen B, Collins WA. Parent-child relationships during adolescence. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology (3rd ed.). Vol. 2: Contextual influences on adolescent development. Wiley; New York: 2009. pp. 3–42. [Google Scholar]
- Laursen B, Coy KC, Collins WA. Reconsidering changes in parent-child conflict across adolescence: A meta-analysis. Child Development. 1998;69(3):817–32. [PMC free article] [PubMed] [Google Scholar]
- Laursen B, DeLay D, Adams RE. Trajectories of perceived support in mother-adolescent relationships: The poor (quality) get poorer. Developmental Psychology. 2010;46(6):1792–8. doi: 10.1037/a0020679. doi:10.1037/a0020679. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Vincent AS. Use of a resting control day in measuring the cortisol response to mental stress: Diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology. 2010;35(8):1253–1258. doi: 10.1016/j.psyneuen.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine. 1998;60(6):765–72. doi: 10.1097/00006842-199811000-00021. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9847038. [DOI] [PubMed] [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent--adolescent relationships. Psychoneuroendocrinology. 2012;37(8):1286–98. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition. 2010;72(1):73–85. doi: 10.1016/j.bandc.2009.06.003. doi:10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: U Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. doi:10.1016/j.ejphar.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication –Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. doi:10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Development. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. doi:10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. doi:10.1016/S0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34(3):307–16. doi: 10.1016/j.psyneuen.2008.09.009. doi:10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: Reliability and validity of a self-report measure. Journal of Youth and Adolescence. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Ansermet F, Borghini A, Halfon O. Adult attachment representations predict cortisol and oxytocin responses to stress. Attachment & Human Development. 2012;14(5):453–76. doi: 10.1080/14616734.2012.706394. doi:10.1080/14616734.2012.706394. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Schlegel A, Barry III H. Adolescence: An anthropological inquiry. The Free Press, Maxwell MacMillan International; New York, NY: 1991. [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the Hypothalamus-Pituitary-Adrenal Axis and the Sympathetic-Adrenal-Medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. doi:10.1097/01.PSY.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS. Impact of social environment characteristics on neuroendocrine regulation. Psychosomatic Medicine. 1996;58(5):459–71. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings. Biological sciences / The Royal Society. 2010;277(1694):2661–6. doi: 10.1098/rspb.2010.0567. doi:10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan L, McHale SM, Osgood DW, Crouter AC. Conflict frequency with mothers and fathers from middle childhood to late adolescence: Within- and between-families comparisons. Developmental Psychology. 2007;43:539–550. doi: 10.1037/0012-1649.43.3.539. [DOI] [PubMed] [Google Scholar]
- Slatcher RB, Robles TF. Preschoolers’ everyday conflict at home and diurnal cortisol patterns. Health Psychology. 2012;31(6):834–8. doi: 10.1037/a0026774. doi:10.1037/a0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Loving TJ, Crockett EE, Campbell L. What's closeness got to do with it? Men's and women's cortisol responses when providing and receiving support. Psychosomatic Medicine. 2009;71(8):843–851. doi: 10.1097/PSY.0b013e3181b492e6. doi:10.1097/PSY.0b013e3181b492e6. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science. 2013;24(8):1554–62. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the Strange Situation: The differential function of emotional expression. International Journal of Behavioral Development. 1998;22(4):681–706. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spies LA, Margolin G, Susman EJ, Gordis EB. Adolescents’ cortisol reactivity and subjective distress in response to family conflict: the moderating role of internalizing symptoms. The Journal of Adolescent Health. 2011;49(4):386–92. doi: 10.1016/j.jadohealth.2011.01.014. doi:10.1016/j.jadohealth.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Impact of puberty on family relations: Effects of pubertal status and pubertal timing. Developmental Psychology. 1987;23:451–460. [Google Scholar]
- Steinberg L. Reciprocal relation between parent-child distance and pubertal maturation. Developmental Psychology. 1988;24(1):122–128. doi:10.1037//0012-1649.24.1.122. [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. doi:10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to corticotropin-releasing hormone challenge. Annals of the New York Academy of Sciences. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology. 2011;36:1226–1238. doi: 10.1016/j.psyneuen.2011.02.017. doi:10.1016/j.psyneuen.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Interparental violence, maternal emotional unavailability and children's cortisol functioning in family contexts. Developmental Psychology. 2012;48(1):237–49. doi: 10.1037/a0025419. doi:10.1037/a0025419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers a C., Van Pelt J, Westenberg PM. Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35(10):1510–6. doi: 10.1016/j.psyneuen.2010.05.004. doi:10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Social support. In: Friedman HS, Silver R. Cohen, editors. Foundations of health psychology. Oxford University Press; New York: 2007. pp. 145–171. [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. doi:10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98(1):47–56. doi: 10.1037/a0016563. doi:10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior. 2011;52:145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. doi:10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Uno D, Uchino BN, Smith TW. Relationship quality moderates the effect of social support given by close friends on cardiovascular reactivity in women. International Journal of Behavioral Medicine. 2002;9(3):243–262. doi: 10.1207/s15327558ijbm0903_06. [DOI] [PubMed] [Google Scholar]
- van den Bos E, de Rooij M, Miers AC, Bokhorst CL, Westenberg PM. Adolescents’ increasing stress response to social evaluation: Pubertal effects on cortisol and alpha-amylase during public speaking. Child Development. 2013 doi: 10.1111/cdev.12118. [Epub]. doi:10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- van Doorn MD, Branje SJT, Meeus WHJ. Developmental changes in conflict resolution styles in parent-adolescent relationships: A four-wave longitudinal study. Journal of Youth and Adolescence. 2011;40:97–107. doi: 10.1007/s10964-010-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayakawa CM. Children's and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 2010;35(2):241–8. doi: 10.1016/j.psyneuen.2009.06.014. doi:10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Beijers R, Mack S, Pruessner JC, De Weerth C. Cortisol responses to social evaluation in 10- to 15-year-old boys and girls. Stress. 2013;16(4):393–401. doi: 10.3109/10253890.2013.764494. doi:10.3109/10253890.2013.764494. [DOI] [PubMed] [Google Scholar]