Abstract
The development of new and specific treatment options for kidney disease in general and glomerular diseases in specific has lagged behind other fields like heart disease and cancer. As a result, nephrologists have had to test and adapt therapeutics developed for other indications to treat glomerular diseases. One of the major factors contributing to this inertia has been the poor understanding of disease mechanisms. One way to elucidate these disease mechanisms is to study the association between the cardinal manifestations of glomerular diseases. Since many of these patients develop nephrotic syndrome, understanding the relationship of proteinuria, the primary driver in this syndrome, with hypoalbuminemia, hypercholesterolemia, hypertriglyceridemia, edema and lipiduria could provide valuable insight. The recent unravelling of the relationship between proteinuria and hypertriglyceridemia mediated by free fatty acids, albumin and the secreted glycoprotein Angiopoietin-like 4 (Angptl4) offers a unique opportunity to develop novel therapeutics for glomerular diseases. In this review, the therapeutic potential of mutant forms of Angptl4 in reducing proteinuria, and, as a consequence, alleviating the other manifestations of nephrotic syndrome is discussed.
Nephrotic syndrome is characterized by the presence of proteinuria in excess of 3.5 grams per 24 hours, hypoalbuminemia, and variable amounts of hyperlipidemia (hypertriglyceridemia and hypercholesterolemia), lipiduria and edema (1). Patients with primary glomerular diseases (e. g. minimal change disease (MCD), focal and segmental glomerulosclerosis (FSGS), membranous nephropathy (MN)) and systemic disorders (e. g. diabetes mellitus, systemic lupus erythematosus, amyloidosis) can present with nephrotic syndrome. Substantial research effort has been committed towards understanding the pathogenesis of each of the individual components. For example, several new proteins expressed in podocytes have been implicated in the pathogenesis of proteinuria (2–6), and at least one novel therapeutic approach is being developed based on this knowledge (1, 6). We now understand how salt retention through different tubular segments contributes towards edema (7), and this knowledge forms the basis of diuretic therapy, which is the mainstay of the treatment of edema. Changes in cholesterol uptake, and the cholesterol biosynthetic pathway in hepatocytes have shed some light towards the development of hypercholesterolemia (8). However, even though nephrotic syndrome (including the precursor term “nephrosis”) was recognized over a century ago, a molecular relationship between some of these has only very recently started becoming clear.
The molecular link between proteinuria and hypertriglyceridemia
A study published recently (9) established the first molecular link between proteinuria and the hypertriglyceridemia component of hyperlipidemia. While investigating elevated circulating levels of the secreted glycoprotein Angiopoietin-like-4 (Angptl4) in human MCD, FSGS, MN, non-HIV collapsing glomerulopathy, and in corresponding animal models, it was determined that levels rise after, but not before, the development of moderate to severe proteinuria. Elevated plasma levels of Angptl4, a previously known inhibitor of lipoprotein lipase (an enzyme that catalyzes conversion of triglycerides to monoglycerides and free fatty acids (FFA)), coincide with the development of hypertriglyceridemia and reduced lipoprotein lipase activity. In addition, Angptl4 is essential for the development of hypertriglyceridemia in nephrotic syndrome, since plasma triglyceride levels do not increase in nephrotic mice that lack Angptl4. The bulk of this circulating neutral or near neutral isoelectric point form of Angptl4 originates from skeletal muscle, heart, adipose tissue, and additionally from podocytes in MCD. Circulating Angptl4 differs significantly from a podocyte secreted hyposialylated, high isoelectric point form that causes the cardinal manifestations of human MCD, and does not appear in the circulation.
Using transgenic rats, knockout mice and recombinant rat and human Angptl4, it was determined that circulating Angptl4 reduces proteinuria in nephrotic rodents by binding to a protein present in glomerular endothelial cells at their interface with the glomerular basement membrane, the αvβ5 integrin. Using knowledge from prior human genetic studies (10), distinct mutant forms of human Angptl4 were developed. These Angptl4 mutants reduce proteinuria in nephrotic rodents without significantly elevating plasma triglyceride levels. By contrast, recombinant wild type human Angptl4 reduces proteinuria and elevates plasma triglyceride levels. These studies firmly establish that circulating Angptl4 reduces proteinuria, while also inducing hypertriglyceridemia in nephrotic syndrome (Figure 1), and that these effects involve different sites in this protein.
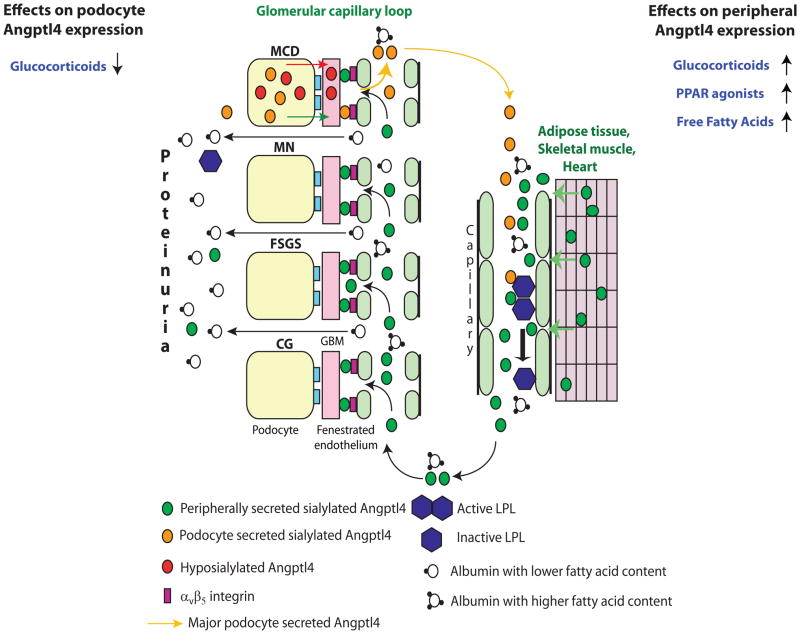
Figure 1.
Diagrammatic representation of the production of increased circulating Angptl4 protein and its biological effects. The circulating, sialylated form of Angptl4 is secreted from peripheral organs (mostly skeletal muscle, heart and adipose tissue) in minimal change disease (MCD), membranous nephropathy (MN), focal and segmental glomerulosclerosis (FSGS) and non-HIV collapsing glomerulopathy (CG). In addition, podocytes in MCD secrete two forms of Angptl4: a hypo-sialylated form that remains restricted to the kidney and induces proteinuria7; and a sialylated form that enters the circulation. Podocytes are not a significant source of circulating Angptl4 in MN, FSGS and CG. Circulating Angptl4 has two major effects in nephrotic syndrome: It binds to glomerular endothelial αvβ5 integrin and reduces proteinuria. It also inactivates endothelium bound LPL in skeletal muscle, heart and adipose tissue, some of which is lost in the urine, thereby reducing hydrolysis of triglycerides to FFA, and resulting in hypertriglyceridemia. The effects of existing drugs on podocyte and peripheral Angptl4 expression are also illustrated. (reproduced from reference 20)
Negative feedback loops in nephrotic syndrome
The development of novel therapeutics using recombinant Angptl4 requires a broad understanding of the pathways involved. Angptl4 is a known PPARα (11) and PPARγ (12) target gene, and free fatty acids (FFA) induce upregulation of Angptl4 expression in skeletal muscle and heart via a PPAR dependent mechanism (13–15). Circulating FFA are non-covalently bound to plasma albumin. Patients with nephrotic syndrome lose albumin with low FFA content in the urine, and retain albumin with higher FFA content in the circulation (16). Our studies in patients with nephrotic syndrome due to MCD and FSGS also noted the FFA to albumin ratio to be significantly lower in the urine compared to plasma. Longitudinal studies in Buffalo Mna rats, a model of FSGS, revealed the presence of relatively low FFA containing urine albumin even during mild proteinuria, which eventually leads to a significant increase in the plasma FFA to albumin ratio when proteinuria becomes moderate to severe (i. e. “nephrotic range”). Higher albumin bound FFA and the development of hypoalbuminemia both contribute towards the elevated FFA to albumin ratio, that promotes FFA uptake and induces Angptl4 upregulation, most likely via a PPAR dependent mechanism. Increased PPAR expression is noted in peripheral organs (heart, skeletal muscle, adipose tissue, liver) of nephrotic rats coinciding with Angptl4 upregulation. Finally, raising plasma FFA to albumin ratio in nephrotic Buffalo Mna rats by administering oleic acid, a monounsaturated ω9 fatty acid present naturally in animal and vegetable fats, or Intralipid, a commercially available lipid supplement, increases plasma Angptl4 levels and significantly reduces proteinuria.
These studies demonstrate the presence of two negative feedback loops in nephrotic syndrome, illustrated in Figure 2. The first loop is a systemic loop, that starts with proteinuria and ends with reduction of proteinuria, and includes urinary loss of albumin with low FFA content, elevated plasma FFA/albumin ratio, upregulation of Angptl4 expression and secretion from peripheral organs, and binding of circulating Angptl4 to the glomerular endothelium. The second loop is local and restricted to skeletal muscle, heart and adipose tissue, that express both Angptl4 and LPL. This loop starts with the entry of FFA into these organs, and ends with reduced entry of FFA, and includes the high plasma FFA/albumin ratio induced entry of FFA, increased production and secretion of Angptl4, inactivation of LPL activity by plasma Angptl4, reduced LPL mediated hydrolysis of triglycerides to monoglycerides and FFA, and reduced entry of FFA into these organs. The development of hypertriglyceridemia in nephrotic syndrome is a consequence of the local loop. The local loop limits the rise of plasma Angptl4 levels, and hence the ability of Angptl4 to reduce proteinuria via the systemic loop.
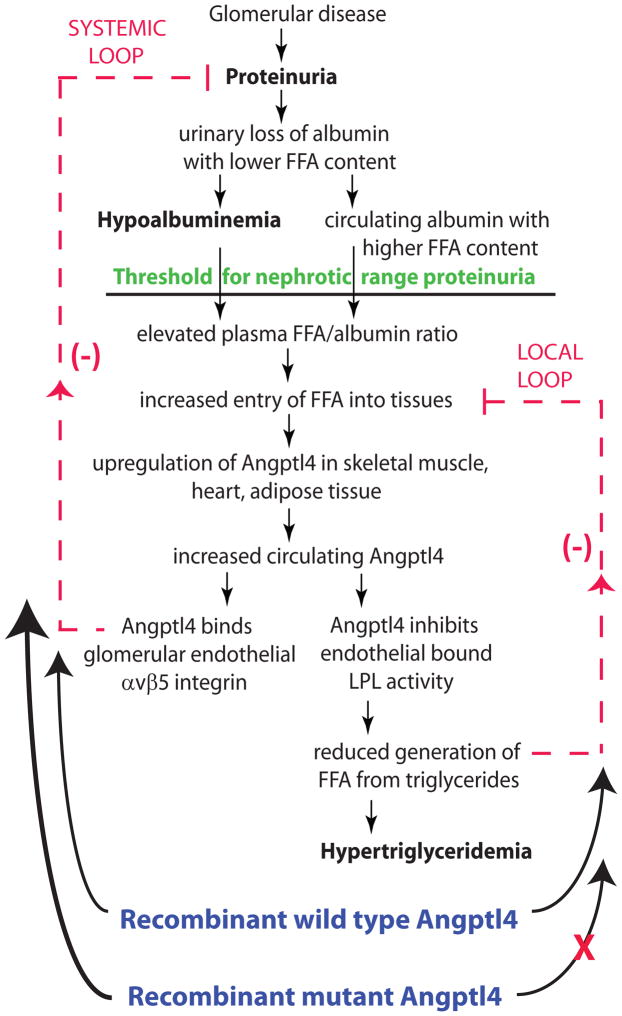
Figure 2.
Schematic illustration of negative feedback loops in the link between proteinuria, hypoalbuminemia and hypertriglyceridemia mediated by Angptl4 and free fatty acids (FFA). Plasma FFA are non-covalently bound to albumin. Because of the preferential loss of albumin with low FFA content in nephrotic syndrome, there is a relative increase in circulating albumin with higher FFA content. As glomerular disease progresses towards severe proteinuria, hypoalbuminemia develops, and the combination of high albumin FFA content and lower plasma albumin levels increases the FFA to albumin ratio. This promotes entry of FFA into skeletal muscle, heart and adipose tissue, which causes upregulation of Angptl4, at least partially mediated by PPARs. Angptl4 secreted from these organs participates in two feedback loops: In the systemic loop, it binds to glomerular endothelial αvβ5 integrin and reduces proteinuria, the principal driver of nephrotic syndrome. In a local loop, it inactivates LPL in the same organs from which it is secreted to reduce the uptake of FFA (see above), thereby curtailing the stimulus for its own upregulation. The participation of exogenously administered recombinant wild type and mutant Angptl4 in the feedback loops is also illustrated. (reproduced from reference 20)
Manipulating Angptl4 feedback loops using available drugs or therapeutic agents
Glucocorticoids reduce Angptl4 expression in podocytes (6) and increase Angptl4 expression in adipose tissue in experimental models (17) (Figure 1). Their efficacy in human MCD is likely related to their combined ability to reduce podocyte upregulation of hyposialylated Angptl4, the major molecular mediator of this disease (1), and increase circulating Angptl4, a potent anti-proteinuric protein, from peripheral tissue. On the same count, the partial efficacy of glucocorticoids in other forms of glomerular disease may be related to their ability to increase plasma Angptl4 levels from peripheral organs. PPAR agonist drugs, that include the thiazolidinediones, could potentially increase peripheral Angptl4 expression, since Angptl4 is a PPAR target gene. However, most of these drugs have been withdrawn from the market due to their side effect profile, which may be partly related to the relatively large number of PPAR target genes, some of which cause adverse effects. Oral FFA supplements, available over the counter, and Intralipid, used clinically as a nutritional supplement or a vehicle to administer lipid bound drugs, offer another option to increase peripheral Angptl4 secretion for an anti-proteinuric effect.
However, there are significant limitations of any approach that relies upon increasing the secretion of Angptl4 from peripheral tissues, best understood by examining the relationship between the local and systemic feedback loops. The local feedback loop limits the extent to which heart, skeletal muscle and adipose tissue can secrete Angptl4 into the circulation by reducing the entry of FFA into these tissues. This imposes a limit on the anti-proteinuric effect of Angptl4 mediated via the systemic loop. In other words, the local feedback loop is acting as a brake on the systemic feedback loop. Any therapy that aims at increasing the secretion of Angptl4 from these tissues is therefore likely to have limited efficacy.
Recombinant mutant forms of Angptl4 can bypass the local feedback loop
To circumvent these limitations, recombinant mutant forms of human Angptl4 with reduced ability to inhibit LPL were designed and tested. The LPL inhibiting sites of Angptl4 are confined to the N-terminal part, whereas the sites responsible for the anti-proteinuric effect are under investigation. Genetics studies show that people with the E40K variant of Angptl4 have very low plasma triglyceride levels (10) due to its reduced ability to inhibit LPL (18). Also, biochemical studies on wild type Angptl4 reveal a tendency to cleave between amino acids 161 and 164 following exposure to plasma (18). To reduce its ability to inhibit LPL and increase its half-life in circulation as an intact protein, mutations at two distinct sites were generated: (a) at or around amino acid 40 (b) between amino acids 161 and 164. A single intravenous injection of 55 μg of mutant protein significantly reduces proteinuria in nephrotic Buffalo Mna rats for two 2 weeks (maximum reduction nearly 65% of baseline) without affecting plasma triglyceride levels. Significant reduction of proteinuria by nearly 50% was also noted in Zucker Diabetic Fatty rats, a model of Type 2 diabetes and diabetic nephropathy, using a much smaller dose (15 μg). Therefore, exogenously administered recombinant mutant forms of Angptl4 are a superior strategy to reduce proteinuria than increasing production of native protein from peripheral tissues using existing drugs. Exogenously administered wild type human Angptl4 would be of limited use, since it will reduce proteinuria but participate in the local feedback loop and exacerbate hypertriglyceridemia in nephrotic syndrome (Figure 2). Moreover, long term administration and inhibition of LPL activity may have deleterious effects on skeletal muscle and heart function, since they rely heavily on FFA for energy requirements.
It is expected that long term reduction of proteinuria using mutant Angptl4 will also reduce the other cardinal manifestations of nephrotic syndrome. In the context of mechanism based therapeutics, there is unlikely to be major overlap between the Angptl4 related pathways and the renin angiotensin system (RAS), since RAS blockage has relatively mild effect on proteinuria in primary glomerular disorders, in which Angptl4 mutants demonstrate considerable efficacy. Indeed, the Angptl4 related pathway is likely to be one of several non-RAS related pathways that will be targeted by novel compounds or small molecules in the future.
Advancing the search for additional molecular mediators of nephrotic syndrome
There is consensus in the scientific community to go beyond pathology, and explore early molecular changes in glomerular diseases (19). Perhaps studying the symmetry of these early molecular changes will better explain concepts like selective proteinuria (20), and help replace traditional hypotheses that slow progress in an otherwise promising area of investigation. The discovery of Angptl4 as a key molecular mediator in nephrotic syndrome has helped clarify concepts beyond its role in proteinuria and hyperlipidemia. We now have for the first time a molecular basis for the nephrotic threshold i. e. why it nephrotic range proteinuria (> 3.5 grams / 24 hours) to develop hypertriglyceridemia. Whereas hyperlipidemia was mostly attributed to hypoalbuminemia in the past, the studies discussed above reveal FFA, Angptl4 and LPL as the true mediators of hypertriglyceridemia, with albumin serving as the carrier of FFA. Angptl4 is likely to be one of several members of a circulating glomerulophilic proteome that directly affects glomerular function in a favorable (e. g. Angptl4) or adverse manner, and develops as a systemic response to glomerular disease. It is also possible that these peripheral changes transition from being reversible to becoming irreversible, persist through end stage renal disease, and cause complications in renal allografts after transplantation. Identification of other components of this systemic response is likely to yield additional therapeutic strategies in the future.
Acknowledgments
Supported by the U.S. National Institutes of Health grants R01DK077073, R01DK090035 and R01DK101637 to Sumant S. Chugh, K01DK096127 to Lionel Clement, VA CDA-2 - 1 IK2 BX001942 to Caroline Marshall and T32DK007545 to Camille Macé. No editorial support was utilized in the preparation of this review. All authors have read the journal’s authorship agreement and conflict of interest policy. The manuscript was reviewed and approved by all named authors.
Footnotes
Conflict of interest statement
Sumant S. Chugh is Founder, President and Chief Executive Officer of GDTHERAPY LLC, and filed patents related to the use of Angpt4 mutants (PCT/US2011/039255) and precursors of sialic acid, including ManNAc (PCT/US2011/039058) for the treatment of nephrotic syndrome. He may benefit financially from these patents in the future. None of the other authors declared competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chugh SS, Clement LC, Macé C. New insights into human minimal change disease: Lessons from animal models. Am J Kid Dis. 2012;59:284–292. doi: 10.1053/j.ajkd.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000 Apr;24(4):349–54. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu G, Clement L, Kanwar YS, Avila-Casado C, Chugh SS. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem. 2006;281:39681–39692. doi: 10.1074/jbc.M606664200. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Clement LC. Telomerase at the center of collapsing glomerulopathy. Nat Med. 2012;18:26–27. doi: 10.1038/nm.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, et al. Podocyte – secreted Angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamm LL, Batuman V. Edema in the nephrotic syndrome: new aspect of an old enigma. J Am Soc Nephrol. 2003 Dec;14(12):3288–3289. doi: 10.1097/01.asn.0000102671.77794.33. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63:1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 9.Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating Angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HF, Hesselink MK, et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 14.Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, et al. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 16.Ghiggeri GM, Ginevri F, Candiano G, Oleggini R, Perfumo F, Queirolo C, et al. Characterization of cationic albumin in minimal change nephropathy. Kidney Int. 1987;32:547–553. doi: 10.1038/ki.1987.243. [DOI] [PubMed] [Google Scholar]
- 17.Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggins RC, Alpers CE, Holzman LB, He JC, Salant DJ, Chugh SS, et al. Glomerular disease: seeing beyond pathology. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.01450214. published online April 3 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chugh SS, Macé C, Clement LC, Del Nogal Avila M, Marshall CM. Angiopoietin-like 4 based therapeutics for proteinuria and kidney disease. Front Pharmacol. 2014;5:23. doi: 10.3389/fphar.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]