Abstract
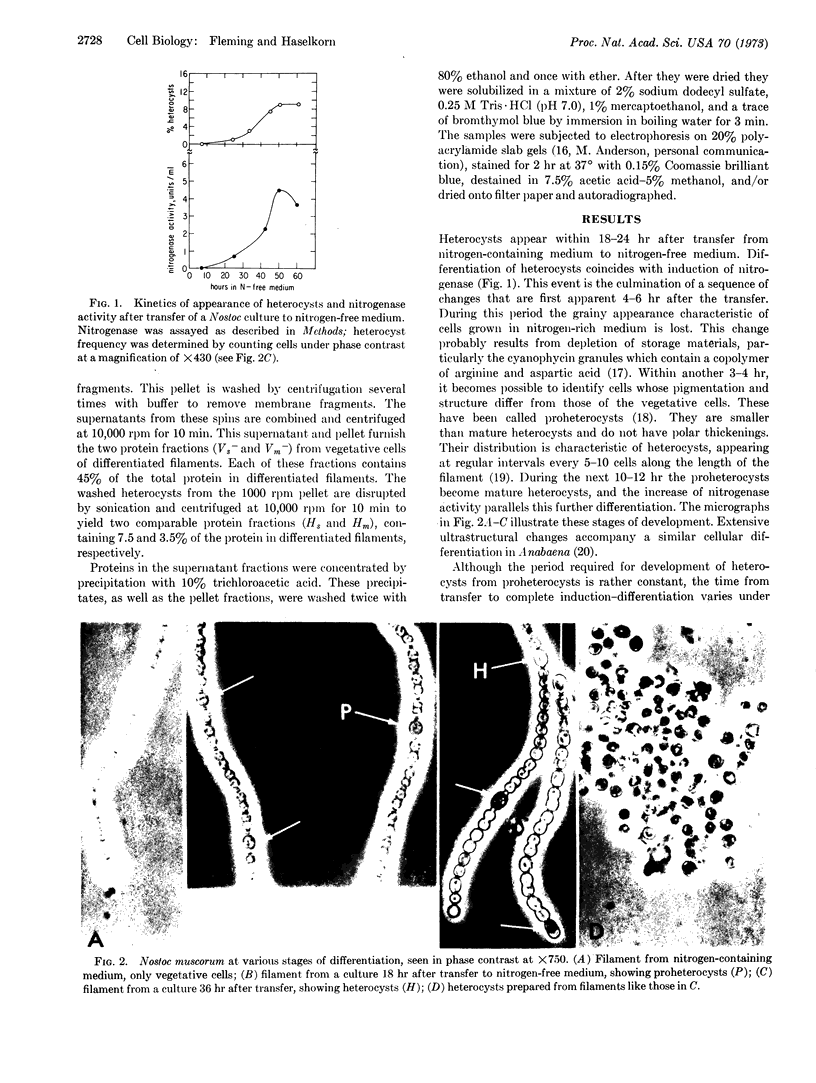
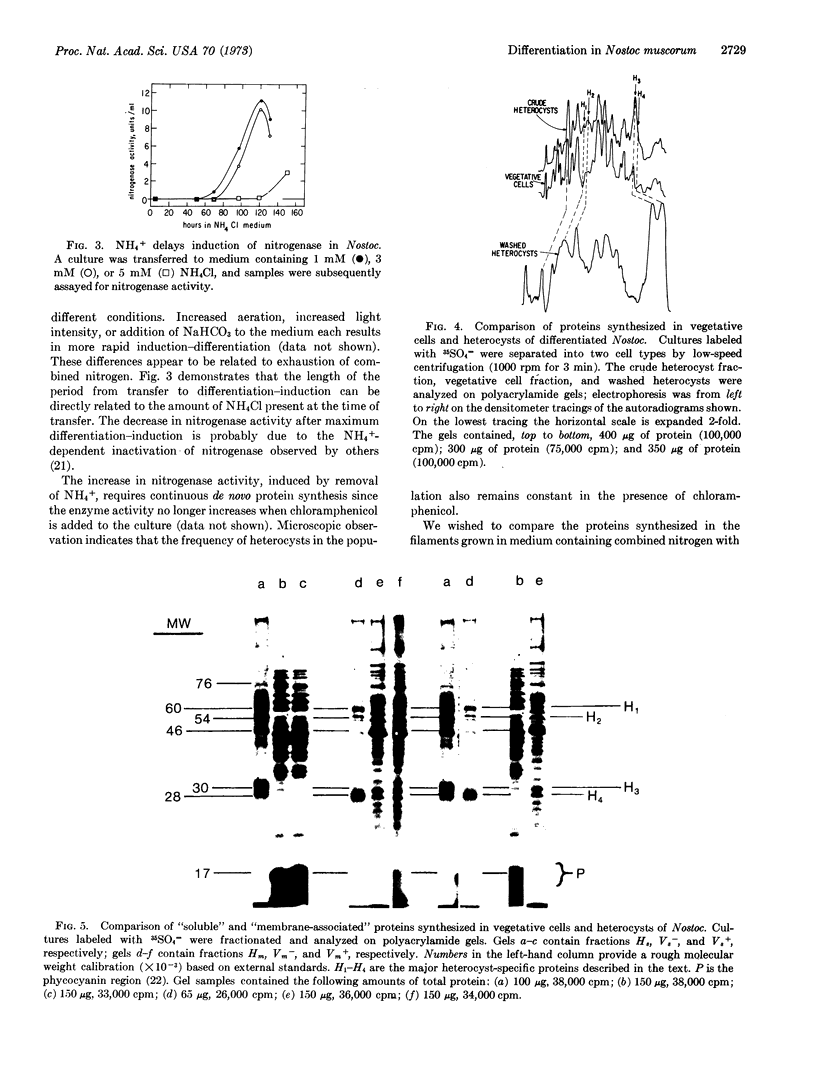
Cellular differentiation can be observed in certain filamentous blue-green algae after transfer of the cells from medium containing NH4+ or NO3- to nitrogen-free medium. The appearance of differentiated cells (heterocysts) is accompanied by an increase in the activity of nitrogenase, an enzyme complex that reduces N2 to NH3. We have separated vegetative cells from heterocysts in differentiated filaments of Nostoc muscorum, and analyzed their proteins by polyacrylamide-gel electrophoresis. The pattern of incorporation of 35SO4- into proteins of the two cell types during differentiation indicates that the increase in nitrogenase activity is due to the de novo synthesis of nitrogenase proteins predominantly, if not exclusively, in heterocysts.
Keywords: nitrogenase induction, cellular differentiation, polyacrylamide-gel electrophoresis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. C., Holsten R. D., Hardy R. W. Isolation by crystallization of the Mo-Fe protein of Azotobacter nitrogenase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):90–99. doi: 10.1016/0006-291x(70)90762-x. [DOI] [PubMed] [Google Scholar]
- COX R. M., FAY P., FOGG G. E. NITROGEN FIXATION AND PHOTOSYNTHESIS IN A SUBCELLULAR FRACTION OF THE BLUE-GREEN ALGA ANABAENA CYLINDRICA. Biochim Biophys Acta. 1964 Jul 29;88:208–210. doi: 10.1016/0926-6577(64)90168-8. [DOI] [PubMed] [Google Scholar]
- Dalton H., Morris J. A., Ward M. A., Mortenson L. E. Purification and some properties of molybdoferredoxin, a component of nitrogenase from Clostridium pasteurianum. Biochemistry. 1971 May 25;10(11):2066–2072. doi: 10.1021/bi00787a016. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Dunn J. H., Wolk C. P. Composition of the cellular envelopes of Anabaena cylindrica. J Bacteriol. 1970 Jul;103(1):153–158. doi: 10.1128/jb.103.1.153-158.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Cell differentiation and pigment composition in Anabaena cylindrica. Arch Mikrobiol. 1969;67(1):62–70. doi: 10.1007/BF00413682. [DOI] [PubMed] [Google Scholar]
- Fay P., Stewart W. D., Walsby A. E., Fogg G. E. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968 Nov 23;220(5169):810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G. Subunit structure of the phycobiliproteins of blue-green algae. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1398–1401. doi: 10.1073/pnas.68.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasooriya S. A., Lang N. J., Fay P. The heterocysts of blue-green algae. 3. Differentiation and nitrogenase activity. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):199–209. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Morphology of a virus of blue-green algae and properties of its deoxyribonucleic acid. J Virol. 1967 Apr;1(2):344–361. doi: 10.1128/jvi.1.2.344-361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. Components of cell-free extracts of Clostridium pasteurianum required for ATP-dependent H2 evolution from dithionite and for N2 fixation. Biochim Biophys Acta. 1966 Sep 26;127(1):18–25. doi: 10.1016/0304-4165(66)90470-3. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. Subunit structure of azoferredoxin from Clostridium pasteurianum W5. Biochemistry. 1971 Feb 2;10(3):455–458. doi: 10.1021/bi00779a016. [DOI] [PubMed] [Google Scholar]
- Neilson A., Rippka R., Kunisawa R. Heterocyst formation and nitrogenase synthesis in Anabaena sp. A kinetic study. Arch Mikrobiol. 1971;76(2):139–150. doi: 10.1007/BF00411788. [DOI] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Reduction of azide by the N2-fixing enzyme system. Proc Natl Acad Sci U S A. 1967 May;57(5):1317–1323. doi: 10.1073/pnas.57.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. D. Cyanophycin Granules from the Blue-Green Alga Anabaena cylindrica: A Reserve Material Consisting of Copolymers of Aspartic Acid and Arginine. Proc Natl Acad Sci U S A. 1971 Feb;68(2):265–267. doi: 10.1073/pnas.68.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Evans M. C. Soluble nitrogenase from vegetative cells of the blue-green alga Anabaena cylindrica. Nature. 1970 Mar 28;225(5239):1253–1254. doi: 10.1038/2251253a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Haystead A., Pearson H. W. Nitrogenase activity in heterocysts of blue-green algae. Nature. 1969 Oct 18;224(5216):226–228. doi: 10.1038/224226a0. [DOI] [PubMed] [Google Scholar]
- Walsby A. E., Nichols B. W. Lipid composition of heterocysts. Nature. 1969 Feb 15;221(5181):673–674. doi: 10.1038/221673a0. [DOI] [PubMed] [Google Scholar]
- Wilcox M. One-dimensional pattern found in blue-green algae. Nature. 1970 Nov 14;228(5272):686–687. doi: 10.1038/228686a0. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J Bacteriol. 1968 Dec;96(6):2138–2143. doi: 10.1128/jb.96.6.2138-2143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Physiological basis of the pattern of vegetative growth of a blue-green alga. Proc Natl Acad Sci U S A. 1967 May;57(5):1246–1251. doi: 10.1073/pnas.57.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]