Abstract
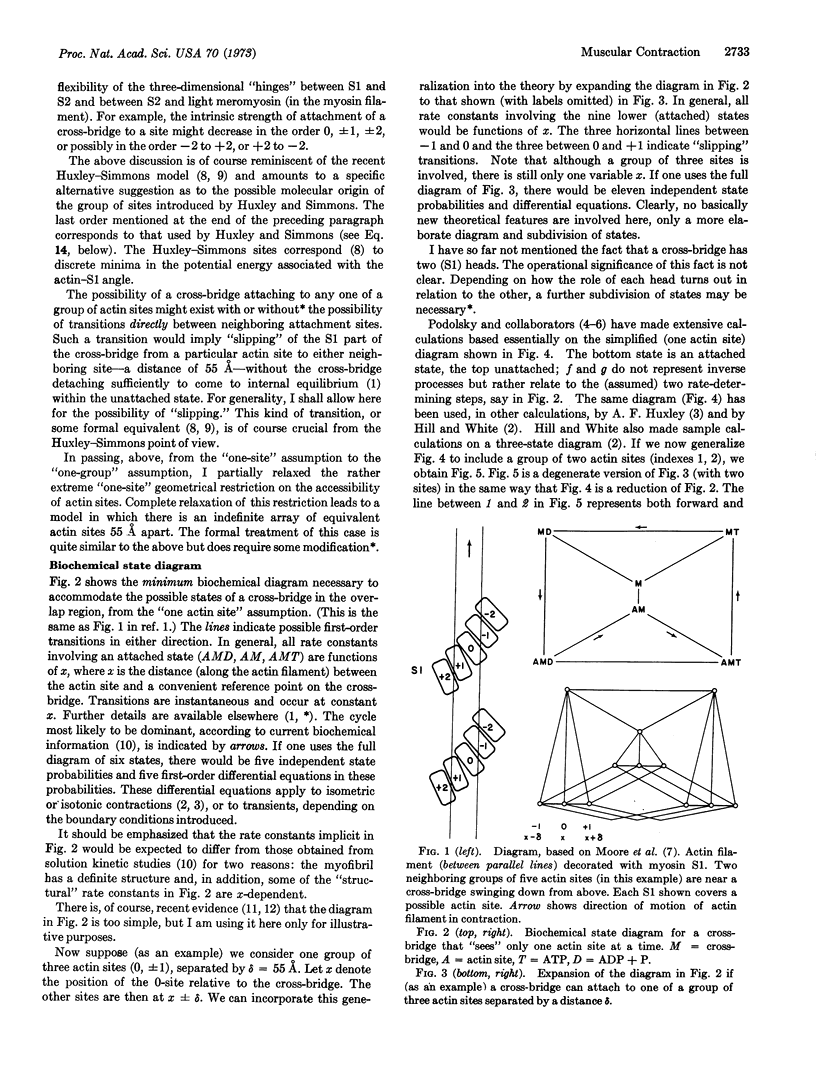
It was shown in an earlier paper how to connect, in principle, the biochemical states of a cross-bridge with the mechanics of muscular contraction, by the methods of statistical mechanics. The treatment applies to cross-bridges that are able to interact with only one actin site at a time. The present paper shows that it is a straightforward matter to extend the theory to groups of actin sites (three, five, etc.), say 55 Å apart, as suggested by the work of Moore, H. E. Huxley, and DeRosier. The possibility of the cross-bridge attachment slipping between sites is included. This provides an alternative molecular interpretation of the model introduced by A. F. Huxley and Simmons. A second possible interpretation is also suggested: their discrete stable angles correspond to different biochemical (attached) states. The Huxley-Simmons analysis of an example is rederived and extended somewhat (x averaging), from the point of view of the present theory. Their qualitative conclusions are left unchanged by the x averaging, but significant quantitative effects are possible. Possible consequences of fast slipping in isotonic contraction are discussed in a preliminary way.
Keywords: statistical mechanics, sliding filament model, biochemical state diagram, Huxley-Simmons analysis
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill T. L. On the sliding-filament model of muscular contraction, II. Proc Natl Acad Sci U S A. 1968 Sep;61(1):98–105. doi: 10.1073/pnas.61.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Sliding filament model of muscular contraction. V. Isometric force and interfilament spacing. J Theor Biol. 1970 Dec;29(3):395–410. doi: 10.1016/0022-5193(70)90105-0. [DOI] [PubMed] [Google Scholar]
- Hill T. L., White G. M. On the sliding-filament model of muscular contraction, IV. Calculation of force-velocity curves. Proc Natl Acad Sci U S A. 1968 Nov;61(3):889–896. doi: 10.1073/pnas.61.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Huxley H. E., DeRosier D. J. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970 Jun 14;50(2):279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Nolan A. C., Zaveler S. A. Cross-bridge properties derived from muscle isotonic velocity transients. Proc Natl Acad Sci U S A. 1969 Oct;64(2):504–511. doi: 10.1073/pnas.64.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]


