Abstract
AIM
To assess choroidal thickness in patients with severe obstructive sleep apnea syndrome (OSAS) and compare them with healthy controls, using spectral domain optical coherence tomography (OCT).
METHODS
In this observational, cross-sectional study, choroidal thicknesses of 23 newly severe OSAS patients and 23 body mass index- age- and sex-matched healthy subjects were measured using a high-speed, high-resolution frequency domain-OCT device (λ=840 nm, 26000 A-scans/s, 5 µm axial resolution). All patients underwent a complete ophthalmic examination before the measurements. OCT measurements were taken at the same time of day (9:00 a.m.), in order to minimize the effects of diurnal variation.
RESULTS
There was a statistically significant difference in median choroidal thickness between the OSAS patients (201 µm; range 145-237 µm) and the controls (324 µm; range 296-383 µm; P<0.001). There were significant differences at all measurement points (P<0.001 for all). The apnea-hypopnea index (AHI) values were more than 30 in all OSAS patients and the mean AHI was 48.57±6.54. The interexaminer intraclass correlation coefficient (ICC) for the mean choroidal thickness was 0.938 (95%CI, 0.908-0.985) and ICC was greater than 0.90 for all measurement points.
CONCLUSION
The decreased choroidal thickness of patients with severe OSAS might be related to the the autonomic disregulation associated with this disease. Further studies are needed to evaluate the etiopathologic relationship between choroidal thickness and OSAS.
Keywords: choroidal thickness, optical coherence tomography, obstructive sleep apnea syndrome
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is defined as repeated episodes of upper airway obstruction during sleep, leading to periods of hypoxemia and hypercapnia[1]. It is characterized by snoring, excessive daytime sleepness, chronic fatigue, and decreased cognitive abilities. The prevelance of OSAS is 2%-4% in the middle age population. Risk factors include obesity, male gender, thick neck, consumption of alcohol, and abnormal upper respiratory tract. Diagnosis of OSAS is made by overnight polisomnography (PSG)[2],[3].
OSAS-related hypoxia, hypercapnia, and decreased oxygen saturation have been shown to generate systemic and pulmonary hypertension, atherosclerosis, coronary artery disease, and increased intracranial pressure[4],[5]. Recent evidence suggests that inflammatory processes, oxidative stress, endothelial dysfunction and vascular remodelling may play roles in the pathogenesis of vascular complications in OSAS[6],[7]. OSAS can also be responsible for autonomic dysfunction with high sympathetic tone and elevated muscle sympathetic nerve activity[7].
There have been several reports that OSAS is also associated with ophthalmic disordes, including glaucoma, floppy eyelid syndrome, nonarteritic anterior ischemic optic neuropathy (NAION), optic disc edema, retinal vein occlusions, and central serous chorioretinopathy (CSCR) [8]–[14].
In several recent studies, optical coherence tomography (OCT) was introduced as an effective tool for evaluating choroidal thickness[15]. New-generation spectral domain OCT devices with enhanced scanning speed provide the opportunity to achieve high-resolution images and more accurate measurements. Thus, it has become possible to evaluate ocular tissues located at deeper levels than that of the retina, which was not possible with time domain OCT[16]–[18].
In recent studies, a decreased retinal nerve fiber layer thickness and increased optic nerve head area-volume parameters measured with OCT have been reported in patients with OSAS[19],[20]. But, there have not been any reports to investigate choroidal thickness in patients with OSAS. Therefore, we intended to evaluate the choroidal thickness of patients with severe OSAS and to compare them with that of healthy controls.
SUBJECTS AND METHODS
Twenty-three newly diagnosed OSAS patients who have apnea-hypopnea index (AHI) >30 (regarded as severe OSAS) (20 males and 3 females) and 23 control subjects (20 males and 3 females) were consecutively enrolled in this case-control prospective study. Patients suspected of clinically of having OSAS examinated and underwent standart overnight PSG in the “Sleep Unit” of the Pulmonary Medicine department of our university. OSAS was diagnosed and graded according to the AHI values. Diagnosis of OSAS was made when the AHI was over 5. When the AHI was between 5/h and 15/h, OSAS was regarded as “mild”, between 16/h and 30/h as “moderate”, and when it was over than 30/h, it was regarded as “severe”. After polysomnographic study, patients with an AHI ≥30 were considered as severe OSAS and AHI <5 were included as control subjects that were matched 1:1 with OSAS patients for body mass index (BMI), sex, and age.
The study protocol was approved by the local ethics committee of Baskent University School of Medicine. The research adhered to the tenets of the Declaration of Helsinki, and a detailed written informed consent form was obtained prior to each individual's participation in the study. Patients with a history of ocular surgery, ocular trauma, anterior or posterior segment disease, glaucoma, heavy smoking, alcohol abuse, bronchial asthma, interstitial lung diseases, cerebrovascular disease, systemic hypertension, diabetes mellitus were excluded from the study. Patients who were using any systemic or ocular drug were also excluded. Blood pressure values were between 110/80 mm Hg and 130/90 mm Hg in all patients. Moreover, in order to obtain clear images and to minimize the effect of axial length on choroidal thickness, patients with best corrected visual acuity worse than 20/20 and a refractive error higher than 1 D were excluded from the study[21].
All patients underwent a detailed ophthalmic examination, including visual acuity testing, refraction assessment, biomicroscopy, intraocular pressure measurement with non-contact tonometry, fundus examination, and choroidal thickness measurements by OCT. One eye per patient was randomly selected for OCT measurement. Randomization was done using a random number generator.
Choroidal thickness measurements were performed by the same experienced technician using a high-speed and high-resolution spectral-domain OCT device (λ=840 nm, 26000 A-scans/s, and 5 µm axial resolution), and results were analyzed using Optovue RTVue software version 3.5 (Optovue Inc., Fremont, CA, USA). The scan pattern was the retina cross line, consisting of 2 orthogonally oriented 6-mm lines that contained 1024 A-scans. By automatically inverting the image, the chorioretinal interface became adjacent to the zero delay. The retina cross-line scan had 32 frames in average, 16 per direction, without tracking[22].
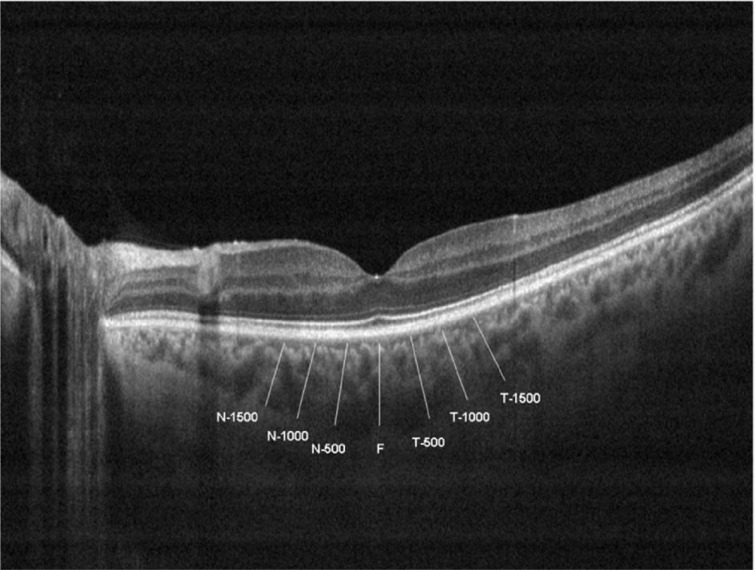
Choroidal thickness was measured perpendicularly from the outer edge of the retinal pigment epithelium to the choroid-sclera boundary at the fovea and at 7 more points located at fovea, at 500 µm nasal to the fovea, 1000 µm nasal to the fovea, 1500 µm nasal to the fovea; 500 µm temporal to the fovea, 1000 µm temporal to the fovea, and 1500µm temporal to the fovea (Figure 1). Choroidal thickness measurements were performed by two masked physicians (Karalezli A and Eroglu FC). All basal OCT scans were performed at the same time of the day (in the morning) to avoid diurnal fluctuations[23]. The average of the two measurements was taken; the differences between readings of the masked physicians were found to be within 10 µm of the mean. The inter examiner reproducibility of the choroidal thickness measurements was assessed by measuring the intraclass correlation coefficient (ICC).
Figure 1. Choroidal thickness measurements of a patient.
T-1500: Choroidal thickness at 1500 µm temporal to the fovea; T-1000: Choroidal thickness at 1000 µm temporal to the fovea; T-500: Choroidal thickness at 500 µm temporal to the fovea; F: Choroidal thickness at fovea; N-500: Choroidal thickness at 500 µm nasal to the fovea; N-1000: Choroidal thickness at 1000 µm nasal to the fovea, N-1500: Choroidal thickness at 1500 µm nasal to the fovea.
Statistical Analysis
Statistical analysis was performed using the statistical package SPSS v15.0 (SPSS Inc., Chicago, USA, 2006). Data were analyzed by Mann-Whitney U test. The categorical variables between the groups were analyzed using the χ2 test and to quantify the reproducibility of manual re-measurements of the choroidal thickness in cases of alignment errors, the ICC was calculated. The P value <0.05 was considered statistically significant
RESULTS
The mean age of the OSAS patients was 58.6±9.7y (range 42-84y) and that of the controls was 58.2±7.1y (range 43-82y). There was no statistically significant difference in age between the groups (P=0.765). The AHI values were more than 30 in all OSAS patients and the mean AHI was 48.57±6.54 (regarding as several OSAS).
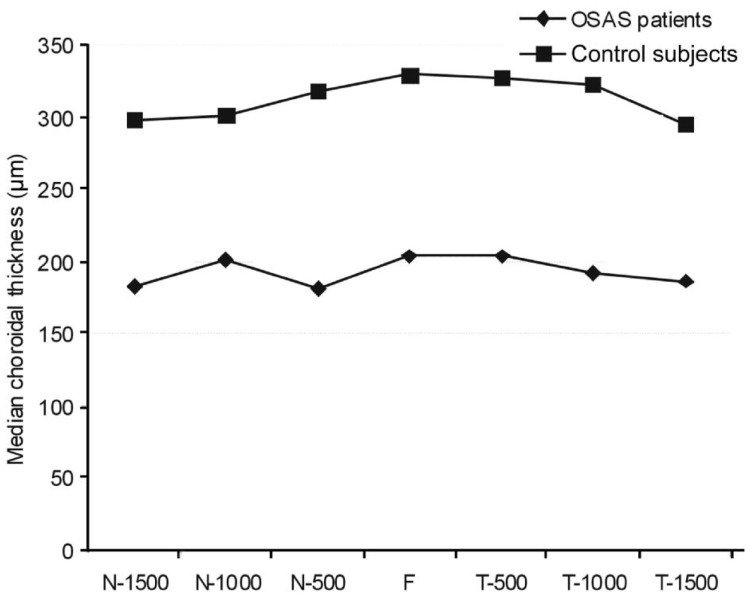
The median choroidal thickness was measured as 201 µm (range 145-237 µm) in OSAS patients and 324 µm (range 296-383 µm) in the control subjects. The median choroidal thickness was statistically significant between groups (P<0.001). Moreover, as shown in Figure 2, the choroidal thicknesses of the patients with OSAS were all thinner than those of the controls in all eyes and at all seven measurement points.
Figure 2. Choroidal thickness measurements in OSAS patients and control subjects.
N-1500: Choroidal thickness at 1500 µm nasal to the fovea; N-1000: Choroidal thickness at 1000 µm nasal to the fovea; N-500: Choroidal thickness at 500 µm nasal to the fovea; F: Choroidal thickness at fovea; T-500: Choroidal thickness at 500 µm temporal to the fovea; T-1000: Choroidal thickness at 1000 µm temporal to the fovea; T-1500: Choroidal thickness at 1500 µm temporal to the fovea.
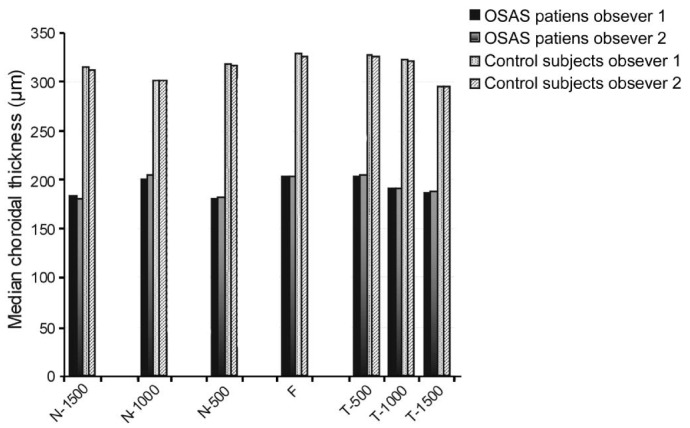
The interexaminer ICC for the choroidal thickness was 0.938 (95%CI, 0.908-0.985) and ICC was greater than 0.90 for all measurement points. Figure 3 shows the comparison of the median chroidal thichness measurements by two different observers in OSAS and control groups.
Figure 3. Comparison of the choroidal thickness measurements of the OSAS patients and control subjects between two different observers.
N-1500: Choroidal thickness at 1500 µm nasal to the fovea; N-1000: Choroidal thickness at 1000 µm nasal to the fovea; N-500: Choroidal thickness at 500 µm nasal to the fovea; F: Choroidal thickness at fovea; T-500: Choroidal thickness at 500 µm temporal to the fovea; T-1000: Choroidal thickness at 1000 µm temporal to the fovea; T-1500: Choroidal thickness at 1500 µm temporal to the fovea.
DISCUSSION
There are many reports in the literature that show the association between OSAS and many ocular disorders[8]–[14]. Intermittent airway obstruction in OSAS causes hypoxia and decrease main saturation of oxygen. Recurrent hypoxia and reperfusion episodes can lead to oxidative stress and inflammation, and also damage vascular endothelium and decreased responsiveness to vasodilatator agents, such as nitric oxide (NO). Moreover, drop in oxygen saturation leads to activation of the adrenergic system, high sympathetic tone and elevated muscle sympathetic nerve activity[7]. All of these conditions lead to vascular dysregulation, autonomic dysfunction and altered blood flow, because of imbalanced of vasodilatation and vasoconstriction[7],[24]. This vascular phenomenon might compromise optic nerve perfusion and oxygenation, and several authors have mentioned a high prevalence of NAION and glaucoma in OSAS patients[11],[12]. Retinal vein occlusions have been associated with OSAS as they may be result of a slowdown of blood circulation secondary to hypoxemia[13]. OSAS also has been recognized to be a risk factor for CSCR, because of elevated levels of circulating catecholamines in patients with OSAS[5].
The ocular blood flow can be assessed using color duplex imaging, laser speckle method, or Doppler flowmetry[25]–[27]. However, because the choroid receives aproximately 95% of all ocular blood flow, changes in its structure would help to evaluate choroidal, thus ocular blood flow[25]–[27].
Therefore, a precise clinical understanding of choroidal morphology should be important for understanding of the pathogenesis of many retinal and choroidal disease[28]. Certain characteristics of the choroid have been challenging to study histologically. To date, spectral domain OCT featuring high resolution and scanning speeds has allowed the choriod to be accesed in vivo[22]. Spaide et al[29] described a “enhanced deep imaging” (EDI) technique to optimize the parameters of OCT and this tecnique allows to visualise the full thickness of the choroid.
In the recent clinical studies, choroidal thickness was reported significantly decreased in patients with pathologic myopia, age-related macular degeneration, glaucoma and diabetic retinopathy or increased in patients with Voght-Koyanagi-Harada disease and CSCR[30]–[34]. In these studies, the authors speculated that abnormal choroidal thickness may be associated with choroidal circulation. Sogawa et al[35] reported that there were no correlation between the choroidal thickness and choroidal blood flow in the healthy young subjects. But, in two different studies, the authors mentioned that sildenafil citrate increases choroidal thickness because of a vasodilatory effect of sildenafil citrate on the choroidal circulation[36],[37].
There are several studies related to choroidal blood flow in the patients with OSAS[38],[39]. Tonini et al[38] reported that choroidal vascular response to hypoxia or hyperoxia does not alter in the patients with OSAS. Khayi et al[39] also reported that choroidal vascular changes in blood pressure were similar to healthy subjects. This result was explained with an autoregulation mechanism of the choroid. Contrary to retinal and optic nerve head vasculature, choroid vessels are subjected to autonomic regulation. But, ability to authoregulate of the choroid may be altered in smokers, age-related maculopathy, CSCR and glaucoma[40]–[43].
In our study, we showed that the median choroidal thickness was statistically thinner in the eye of patients with severe OSAS than that of the controls. We think that hypoxia and reperfusion episodes are responsible for pathophysiology of the OSAS, and these episodes cause oxidative stress, inflammation, damage to vascular endothelium and decreased responsiveness of the vessels to nitric oxide. Insufficient vasodilatation of vessels may lead to decrease in choroidal blood flow. We speculated that impaired autoregulation of the choroid in severe OSAS may be responsible for thinner choroidal thickness than that of controls.
There is a shortcoming regarding our study. A larger cohort would have allowed more meaningful analysis on the relation between the severity of OSAS and choroidal thickness; however, this can be achieved in a further study based on the preliminary findings of this study. Moreover, the design of the study with age, BMI and sex-matched control group and high ICCs between the two operators (ICC>0.90, for all measurement points) make the results more valuable.
In conclusion, patients with severe OSAS tend to have thinner choroids than healthy individuals, which in turn, might be a result of the autonomic disregulation associated with this disease. Further studies with larger subgroups of this disease, including mild-modarete OSAS, are necessary to come to a more definite conclusion.
Acknowledgments
Conflicts of Interest: Karalezli A, None; Eroglu FC, None; Kivanc T, None; Dogan R, None.
REFERENCES
- 1.Kiekens S, De Groot Veva, Coeckelbergh T, Tassignon MJ, van de Heyning P, De Backer Wilfried, Verbraecken J. Continuous positive airway pressure therapy in associated with an increase in intraocular pressure in obstructive sleep apnea syndrome. Invest Ophtalmol Vis Sci. 2008;49(3):934–940. doi: 10.1167/iovs.06-1418. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilleminault C. Clinical features and evaluation of obstructive sleep apnea. In: Karger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. London: Sauders; 1994. pp. 165–170. [Google Scholar]
- 4.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30(3):291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 5.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103(3):722–727. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 6.Arnaud C, Dematteis M, Pepin JL, Baguet JP, Lévy P. Obstructive sleep apnea, immuno-inflamation, and atherosclerosis. Semin Immunopathol. 2009;31(1):13–125. doi: 10.1007/s00281-009-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mojon DS, Hess CW, Goldblum D, Boehnke M, Koerner F, Gugger M, Bassetti C, Mathis J. Normal tension glaucoma is associated with sleep apnea syndrome. Ophthalmologia. 2002;216(3):180–184. doi: 10.1159/000059625. [DOI] [PubMed] [Google Scholar]
- 9.Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC. Prevelance of glaucoma in patients with obstructive sleep apnoea- a cross-sectional case-series. Eye (Lond) 2008;22(9):1105–1109. doi: 10.1038/sj.eye.6702846. [DOI] [PubMed] [Google Scholar]
- 10.Karger RA, White WA, Park WC, Rosales AG, McLaren JW, Olson EJ, Woog JJ. Prevalence of floppy eyelid syndrome in obstructive sleep apnea syndrome. Ophthalmology. 2006;113(9):1669–1674. doi: 10.1016/j.ophtha.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Mojon DS, Mathis J, Zulauf M, Koerner F, Hess CW. Optic neuropathy associated with sleep apnea syndrome. Ophthalmology. 1998;105(5):874–877. doi: 10.1016/S0161-6420(98)95030-8. [DOI] [PubMed] [Google Scholar]
- 12.Bucci FA, Jr, Krohel GB. Optic nerve swelling secondary to the obstructive sleep apnea syndrome. Am J Ophthalmol. 1986;105(4):428–430. doi: 10.1016/0002-9394(88)90318-2. [DOI] [PubMed] [Google Scholar]
- 13.Glacet-Bernard A, Leroux les Jardins G, Lasry S, Coscas G, Soubrane G, Souied E, Housset B. Obstructive sleep apnea among patients with retinal vein occlusion. Arch Ophthalmol. 2010;128(12):1533–1538. doi: 10.1001/archophthalmol.2010.272. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Kaines A, Schwartz S. Bilateral central serous chorioretinopathy resolving rapidly with treatment for obstructive sleep apnea. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1037–1039. doi: 10.1007/s00417-009-1257-5. [DOI] [PubMed] [Google Scholar]
- 15.Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, Quigley HA. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118(8):1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus-HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–329. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27(1):45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Sander B, Larsen M, Thrane L, Hougaard JL, Jongersen TM. Enhanced optical coherence tomography imaging by multiple scan averaging. Br J Ophthalmol. 2005;89(2):207–212. doi: 10.1136/bjo.2004.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PW, Friedman M, Lin HC, Chang HW, Pulver TM, Chin CH. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):585–593. doi: 10.1007/s00417-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 20.Casas P, Ascaso FJ, Vicente E, Tejero-Garcés G, Adiego MI, Cristóbal JA. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea hypopnea syndome (OSAHS) Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1625–1634. doi: 10.1007/s00417-013-2268-9. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across the three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119(1):119–123. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 24.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and respond to treatment. Am J Respir Cirt Care Med. 2004;169(3):348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010;88(7):723–729. doi: 10.1111/j.1755-3768.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 26.Portman N, Gugleta K, Kochkorov A, Polunina A, Flammer J, Orgul S. Choroidal blood flow response to isometric exercise in glaucoma patients and patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2011;52(10):7068–7073. doi: 10.1167/iovs.11-7758. [DOI] [PubMed] [Google Scholar]
- 27.Bill A. Circulation in the eye. In: Renkin EM, Michel CC, editors. Handbook in physiology. Microcirculation, Part 2. The American Physiological Society, Bethesda, MD; 1984. pp. 1001–1035. [Google Scholar]
- 28.Polska E, Polak K, Luksch A, Fuchsjager-Mayrl G, Petternel V, Findl O, Schmetterer L. Twelve hour reproducibility of choroidal blood flow parameters in healthy subjects. Br J Ophthalmol. 2004;88(4):533–537. doi: 10.1136/bjo.2003.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polipoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118(5):840–845. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Kubota T, Jonas JB, Naumann GO. Decreased choroidal thickness in eyes with secondary age ange glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol. 1993;77(7):430–432. doi: 10.1136/bjo.77.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmaeelpour M, Povazay B, Hermann B, Hofer B, Kajic V, Hale SL, North RV, Drexler W, Sheen NJ. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 33.Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):502–509. doi: 10.1097/IAE.0b013e3182083beb. [DOI] [PubMed] [Google Scholar]
- 34.Immura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 35.Sogawa K, Nagaoka T, Takahashi A, Tanano I, Tani T, Ishibazawa A, Yoshida A. Relationship between choroidal thickness and choroidal circulation in healthy young subjects. Am J Ophthalmol. 2011;153(6):1129–1132.e1. doi: 10.1016/j.ajo.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Vance SK, Imamura Y, Freund KB. The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina. 2011;31(2):332–335. doi: 10.1097/IAE.0b013e3181eef0ae. [DOI] [PubMed] [Google Scholar]
- 37.Kim DY, Silverman RH, Chan RV, Khanifar AA, Rondeau M, Lloyd H, Schlegel P, Coleman DJ. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra®) Acta Ophthalmol. 2013;91(2):183–188. doi: 10.1111/j.1755-3768.2011.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonini M, Khayi H, Pepin JL, Renard E, Baguet JP, Lévy P, Romanet JP, Geiser MH, Chiquet C. Choroidal blood-flow responses to hyperoxia and hypercapnia in men with obstructive sleep apnea. Sleep. 2010;33(6):811–818. doi: 10.1093/sleep/33.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khayi H, Pepin JL, Geiser MH, Tonini M, Tamisier R, Renard E, Baguet JP, Levy P, Romanet JP, Chiquet C. Choroidal blood flow regulation after posture change or isometric exercise in man with obstructive sleep apnea syndrome. Invest Ophthalmol Vis Sci. 2011;52(13):9489–9496. doi: 10.1167/iovs.11-7936. [DOI] [PubMed] [Google Scholar]
- 40.Wimpissinger B, Resch H, Berisha F, Weigert G, Schmetterer L, Polak K. Response of choroidal blood flow to carbogen breathing in smokers and non-smokers. Br J Ophthalmol. 2004;88(6):776–781. doi: 10.1136/bjo.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pournaras CJ, Logean E, Riva CE, Petrig BL, Chamot SR, Coscas G, Soubrane G. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(4):1581–1586. doi: 10.1167/iovs.05-0434. [DOI] [PubMed] [Google Scholar]
- 42.Tittl M, Maar N, Polska E, Weigert G, Stur M, Schmetterer L. Choroidal hemodynamic changes during isometrix exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2005;46(12):4717–4721. doi: 10.1167/iovs.05-0268. [DOI] [PubMed] [Google Scholar]
- 43.Emre M, Orgul S, Gugleta K, Flammer J. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol. 2004;88(5):662–666. doi: 10.1136/bjo.2003.032110. [DOI] [PMC free article] [PubMed] [Google Scholar]