Dear Sir,
Eye drops (single use) commonly used in clinics do have varying pH values. The use of such drops in the initial management of chemical eye injury may influence the accuracy of pH measurement of the eye, and subsequently influence its management.
Chemical eye injury is an ophthalmic emergency, which may be caused by exposure to an acidic (pH<4) or an alkali (pH>10) solution to the eye. Rinsing the eye decreases the concentration of these solutions on ocular surface. The initial management would be copious irrigation with clean or sterile water or Ringer's solution (pH 7.3-7.4) or its equivalent, of a near neutral pH. Irrigating volumes of up to 20 litres or more are sometimes required to achieve physiological pH level[1]–[4]. The eye is then assessed for any damage. This would include serial pH measurements, with the indication that should the pH not be neutralised, more irrigation is required[5]. On many occasions local anaesthetic eye drops are used to reduce eye pain, which can help in better eye opening for detailed examination; and fluorescein is often used to assess corneal damage, prior to pH measurement. The instillation of such eye drops may have an implication on the subsequent measurement of the ocular surface pH. This is because the various eye drops have varying pH values, which is shown clinically using universal pH indicator paper (Figure 1).
Figure 1. The pH values of the commonly available eye drops in eye clinics and emergency departments are shown here with the pH meter.
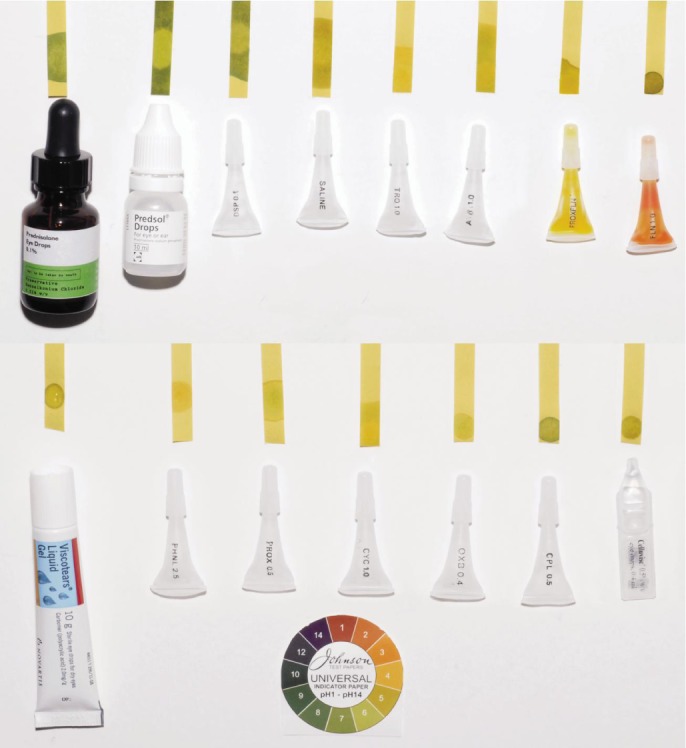
Saline shown in the figure is used as a control. The eye drops from right to left starting from the top row are as follows: Prednisolone eye drops 0.1%, Prednisolone 0.5% eye drops, Dexamethasone sodium phosphate 0.1%, Saline (control), Tropicamide 1%, Atropine 1%, combination drops of proxymethocaine and fluoresceine, fluorescein 1% eye drops, carbomer artificial tears, phenylephrine 2.5%, proxymethocaine 2.5%, cyclopentolate 1%, oxybuprocaine 0.4%, chloramphenicol 0.5% eye drops and carmellose sodium 0.5% eye lubricant. All these drops apart from saline have non-neutral pH.
It is vital to reassess the ocular surface pH after irrigation, in the event of a chemical eye injury. This constitutes an important step in the clinical assessment by confirming neutralisation before stopping irrigation[6]. However, patients may feel intense pain after initial irrigation caused by both epithelial defect and ciliary body irritation. Therefore, under more practical circumstances, the attending clinician in the eye casualty or emergency department may choose to instil cycloplegics and topical local anaesthesic drops (acidic) before reassessing the eye after the initial irrigation. For instance, cyclopentolate hydrochloride eye drops 1.0% (pH 4.5) and tropicamide ophthalmic solution (pH 4.0 to 5.8) are often used for cycloplegia. Proparacaine hydrochloride ophthalmic solution (pH 3.5 to 6.0) is often used as topical anaesthetic drops. Moreover, formulations of antibiotic eye drops also contain HCl to adjust pH. For example, ofloxacin ophthalmic solution is unbuffered and formulated with a pH of 6.4 (range: 6.0 to 6.8). Occasionally fluorescein drops (alkali) are used to assess corneal damage after the initial irrigation. Due to the non-neutral pH of those eye drops, the reassessed pH value of the eye may not reflect the actual pH and thus compromise optimum treatment.
In the context of an alkali burn, the use of ophthalmic drops may cause decrease in pH to normal levels, prompting medical staff to discontinue instillation due to this temporary decrease. On the other hand, medical staff may rinse the eye more than enough in an acidic trauma. One should be aware of the effects of eye drops on pH particularly just after the instillation. Thus, during the management of chemical ocular injury, physicians should measure the pH without use of any ophthalmic drops.
Our previous experience demonstrates that when the ocular surface pH is made acidic (by using eye drops), the return to pre-existing pH level can take between 20-40min. This is consistent with previous findings from Coles and Jaros[7], who investigated the dynamics of ocular surface pH. They found the normal physiological pH of ocular surface in humans to be 7.11±1.5. They also showed an increase in ocular surface pH early in the morning and a gradual increase in pH to more alkali levels during the day. Additionally one hour of lid closure causes decrease in pH. Data from the study suggests that following instillation of 1 drop of tropicamide (pH 6.0) and 1 drop of phenylephrine (pH 4.8), pH of the ocular surface shows a sudden decrease up to 4.6 and returns to the normal levels with flushing effect of normal tearing within 40min.
Kompa et al[8] explored the effects of different irrigating solutions on intracameral pH, intraocular pressure and histological findings after induced alkali burns in rabbit eyes. With regards to pH measurements, their findings suggest that the diluting effect produced by hypo-osmolar irrigants was effective in inhibiting elevated pH levels. However, to this date, there is no similar animal study, which also explores the effects of eye drops on ocular surface pH in the context of chemical eye injury. Further studies are required to explore the impact of commonly used eye drops on ocular surface pH following an acid or alkali burn. In conclusion, we hope this communication will alert colleagues to the importance of making sure that the measurement of eye pH is not influenced by the usage of various commonly used eye drops—a point that can be easily overlooked in the management of acute chemical eye injury.
Acknowledgments
Conflicts of Interest: Lim LT, None; Ah-Kee EY, None; Collins CE, None.
REFERENCES
- 1.Gicquel JJ. Management of ocular surface chemical burns. Br J Ophthalmol. 2011;95(2):159–161. doi: 10.1136/bjo.2010.187104. [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Tyagi M, Kumar Y, Gupta KK, Sharma PD. Ocular chemical injuries and their management. Oman J Ophthalmol. 2013;6(2):83–86. doi: 10.4103/0974-620X.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor AJ, Severn P. Use of a control test to aid pH assessment of chemical eye injuries. Emerg Med J. 2009;26(11):811–812. doi: 10.1136/emj.2008.067439. [DOI] [PubMed] [Google Scholar]
- 4.Chau JP, Lee DT, Lo SH. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9(3):129–138. doi: 10.1111/j.1741-6787.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 5.Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Curr Opin Ophthalmol. 2010;21(4):317–321. doi: 10.1097/ICU.0b013e32833a8da2. [DOI] [PubMed] [Google Scholar]
- 6.Subash M, Sheth HG. Rapid, accurate and easy pH assessment in ocular chemical injury. J Emerg Med. 2011;41(3):301. doi: 10.1016/j.jemermed.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Coles WH, Jaros PA. Dynamics of ocular surface pH. Br J Ophthalmol. 1984;68(8):549–552. doi: 10.1136/bjo.68.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kompa S, Redbrake C, Hilgers C, Wüstemeyer H, Schrage N, Remky A. Effect of different irrigating solutions on aqueous humour pH changes, intraocular pressure and histological findings after induced alkali burns. Acta Ophthalmol Scand. 2005;83(4):467–470. doi: 10.1111/j.1600-0420.2005.00458.x. [DOI] [PubMed] [Google Scholar]


