Abstract
AIM
To investigate whether the enhanced green fluorescent protein (EGFP) reporter gene could be transferred into human trabecular meshwork (HTM) cells by a HIV-based lentivirus both in vitro and ex vivo.
METHODS
The HIV-based lentivirus that contains an EF1-α promoter driving EGFP expression cassette was constructed following the standard molecular cloning methods. The cultured HTM cells were transduced at a range of multiplicity of infection (MOI) with HIV-based lentivirus. EGFP positive cell populations were detected by flow cytometry. Human anterior eye segments were cultured with perfusion culture system and transfected by HIV-based lentivirus with a 1×108 transducing unit (TU) virus in perfusion liquid. The intraocular pressure was recorded every 8h for 21d. The expression of EGFP in the anterior segment of the human eye was detected by fluorescence microscopy. Furthermore, the distribution of EGFP expression was confirmed by anti-EGFP immunohistochemical staining.
RESULTS
The HIV-based lentivirus which contains an EF1-α promoter driving EGFP expression cassette was constructed successfully. After HTM cells were transduced with HIV-based lentivirus containing EGFP in vitro, the ratio of EGFP positive cells to the total cell number reached 92.3%, with the MOI of 15. After the lentivirus containing EGFP were used to transduce human anterior eye segments, the EGFP could be directly detected by fluorescence microscopy in vivo. Immunohistochemistry staining revealed that 88.19% EGFP-positive trabecular meshwork (TM) cells were observed in the human anterior segment. Nevertheless, the intraocular pressure in the lentivirus-transduced group kept constant when compared with control group (P>0.05).
CONCLUSION
EGFP gene could be efficiently transferred into HTM cells both in vitro and ex vivo by using HIV-based lentivirus.
Keywords: gene transfer, trabecular meshwork, HIV-based lentivirus, glaucoma
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide[1] and elevated intraocular pressure (IOP) is its major risk factor which appears to result from increased trabecular meshwork (TM) outflow resistance. Clinically, surgical or pharmacological treatment targeting TM could lower elevated IOP[2]. Hence, TM has been considered as a promising therapeutic target for the treatment of glaucoma[3].
Several studies have identified candidate genes that might have a role in the pathogenesis of glaucoma[4]. These studies suggest the possibility of using genetically modified TM cells to treat glaucomatous IOP dysregulation. However, although TM cells are highly metabolically active, they appear to undergo a limited number of cellular division in vivo. Only less than 0.5% of total TM cells have been estimated to be mitotically active at any one time[5]. The challenge therefore, is how to efficiently transfer the therapeutic gene to TM cells. HIV-based lentivirus have the ability to transduce a broad range of cell types efficiently, including non-dividing, senescent and terminally differentiated cells[6]. In addition, HIV-based lentivirus can integrate into the host cell genome resulting in stable long-term transgene expression. In ocular tissues, HIV-based lentivirus has been shown to have a mediating effect and sustained transgene expression in uvea, corneal endothelial cells, retinal pigment cells and photoreceptors[7],[8]. Loewen et al[9] used the HIV-based lentivirus encoding β-galactosidase gene to transduce the human anterior segment of the eye. However, the β-galactosidase gene cannot be detected in living cells. Therefore, the capability of monitoring enhanced green fluorescent protein (EGFP) gene expression in vivo is an advantage for establishing gene delivery approaches. In the present study, we investigated whether HIV-based lentivirus can be used to quantify transgene expression in the human TM (HTM) cells in vitro and ex vivo. In addition, we also examined the IOP in cultured human anterior segments transfected with HIV-based lentivirus, since the effect of HIV-based lentivirus on IOP has never been fully assessed in previous studies.
MATERIALS AND METHODS
Plasmids and Human Eye Anterior Segment
Plasmids pWPLXd, pMD2G, psPAX2 were generously supplied by Tronolab lab. Human embryonic kidney 293T cells (HEK 293T cells) were provided by Prof. Jian-Feng Zhou who works in the Department of Hematology of Tongji Hospital. Plasmid pIRES2-EGFP and HeLa cells were kept in our lab. Human cadaveric eyes (ages, 16-80y) without glaucoma, uveitis, and other ophthalmic diseases were obtained less than 24h postmortem from the Red Cross Eye Bank of Wuhan (Wuhan, Hubei Province, China). Human donor tissue protocols were approved by the University Institutional Review Board of Huazhong University of Science and Technology. Eight pairs of donor eyes were obtained and used according to the provisions of the Declaration of Helsinki for research involving human tissue.
Reagent
Endonuclease BamHI, and EcoRI were purchased from TaKaRa (Japan). Polymerase chain reaction (PCR) reaction kit and T4 DNA ligase were provided by Fermantas (Lithuania). Lipofectamine™ 2000 was acquired from Invitrogen (American) and fetal calf serum and Dulbecco's modified eagle medium (DMEM) were bought from GIBICO (American). Penicillin, streptomycin, amphotericin B and gentamicin were obtained from Sigma-Aldrich (American). The DNA primer was synthesized by Invitrogen (Shanghai, China). The following primary antibodies were used in this study: anti-fibronetion (FN) and anti-laminin (LN) (Santa cruz, American), anti-neuron specific enolase (NSE) and anti-factor VIII-related antigen (Chemicon, American), anti-EGFP (Abcam, American). Human eye anterior segment perfusion culture system was provided by Pang, I.H. (Alcon Research, Ltd., Fort Worth, TX, USA). The perfusion pump was manufactured by B. Braun (German) and the sensitive pressure transducer was made by the Edwards Lifesciences, LLC (Irive, CA, USA).
Plasmid Construction
The EGFP reporter gene was amplified from plasmid pIRES2-EGFP by DNA primer which contains BamHI and EcoRI digestion site. The primer sequences are as following: 5′-CCGGATCCGGGACGTGGTTTTCCTTTG-3′, 5′-GCGAATTCCCTCTACAAATGTGGTATGGC-3′. The PCR product was digested by BamHI and EcoRI and harvested from agarose electrophoresis, then cloned to plasmid pWPXLd (which already contains EF1-α promoter) based on the standard protocol for Molecular Cloning (fifth edition, published by Cold Spring Harbor Laboratory Press). The recombinant plasmid containing EF1-α promoter driving EGFP expression cassette was confirmed by restrictive enzyme digestion and sequencing.
Preparation of HIV-based Lentivirus and Determination of Viral Titer
HIV-based lentiviral particles were generated according to the protocols previously described[10]. Briefly, HEK 293T cells were transfected with 6 µg of pMD2G envelope plasmid, 15 µg of psPAX2 packaging plasmid (pMD2G, psPAX2; Tronolab), and 20 µg of the HIV-based lentiviral transfer vector pWPXLd-EGFP. Vector particles were collected from the conditioned medium 48h post transfection. The virus-containing supernatants were collected 48h after transfection, filtered through 0.45 mm pore size Millipore filters, concentrated by passage through Centricon-plus columns (Millipore, Billerica, MA, USA), and kept at -80°C until use. Vector titers were determined by quantitative PCR on HeLa cells as described previously[10]. In brief, 5×104 HeLa cells were transduced with serial dilutions of HIV-based lentiviral preparations in the presence of polybrene (4-8 mg/ml; Sigma-Aldrich, St. Louis, MO, USA). Five days later, genomic DNA was extracted with a DNeasy kit according to the manufacturer's protocol (Qiagen, Valencia, CA, USA). Real-time PCR was performed with an ABI PRISM 7000 sequence detector (Applied Biosystems, Foster City, CA, USA) with primers and probe corresponding to the truncated gag gene present in all vectors. Serial dilutions of pWPXLd plasmid DNA were used to establish standard curves.
Culture of Human Trabecular Meshwork Cells
The culture of HTM cells was briefly described as follows: Trabecular specimens were obtained less than 24h postmortem. The TM tissue was microdissected under an operating microscope. Explants of dissected trabecular tissue were placed in DMEM/F12 with 10% fetal bovine serum (FBS). After migrating from the primary explants and seeding on plate, the TM tissue explants were removed. Passage of TM cells was performed at a ratio of 1:4 using 0.1% trypsin when they reached 90% confluence. Identification of HTM cell was confirmed by immunochemistry staining of FN, LN, NSE and Factor VIII relative antigen (FVIII Rag).
Infection of Human Trabecular Meshwork Cells with HIV-based Lentivirus and Assessment of Transduction
Third generation of cultured HTM cells, 1×105 cells per well, were seeded in 6-well plates and then randomly divided into 6 groups (n=5). The next day, cells were transduced with HIV-based lentivirus with a gradient dilution of multiplicity of infection (MOI)=0, 1, 5, 10, 15, 20 respectively. When EGFP could be detected by fluorescence microscopy, cells were harvested for flow cytometry analysis. The percentage of EGFP-positive cells was calculated by using Guava Express Plus analysis on a Guava Easy Cyte™ flow cytometer (Guava Technologies, Millipore, Billerica, MA, USA).
Culture of Anterior Segment of Human Eye and the Monitoring of the Intraocular Pressure
The procedures for the perfusion of the human eye anterior segment, according to the protocol previous described[11], are as follows: eyes were dissected along the equator. Iris, lens, ciliary body and vitreous were removed. The anterior segment of the eye was clamped in a custom-made Plexiglas culture dish and sealed in place with a Plexiglas O-ring. The anterior segments of the eye were perfused with Dulbecco's modified Eagle's medium containing antibiotics (penicillin: 10,000 U/100 mL solution, streptomycin: 10 mg, amphotericin B: 25 mg, and gentamicin: 17 mg, in 100 mL medium) at the constant flow rates (2.5 µL/min) using a perfusion pump at 37°C in 5% CO2 atmosphere. IOP of the anterior segments was continuously monitored by a second cannula attached to a sensitive pressure transducer. The IOP was recorded every 8h and data were averaged daily.
Infection of the Anterior Segment of the Human Eye with HIV-based Lentivirus Containing the Enhanced Green Fluorescent Protein
The anterior segments of eye were equilibrated for 3d before being infected with the HIV-based lentivirus containing EGFP. Those did not reach a stable baseline of IOP were discarded. For eachpair, one eye was treated with HIV-based lentiviral vector containing EGFP and the other one was perfused with a null-lentiviral virus medium. In total, 108 transducing units (TU) HIV-based lentiviral particles were applied as a bolus to the perfusion chambers (indicated by time point 0) and pressure was monitored for 21d. On day 21 (the end of the experiment), anterior segments were removed from the perfusion chambers for fluorescence microscopy analysis (data obtained from three samples collected for each group) and then fixed with 4% paraformaldehyde. Tissue sections were prepared for anti-EGFP staining by using streptavidin biotinperoxidase complex (SABC) methods. To measure transduction efficiency of lentivirus, each anterior segment was divided into four quadrants. A section from each quadrant was taken for anti-EGFP staining. Positive cell bodies and stained nuclei were manually counted and the percentage of EGFP expressing cells versus stained nuclei was calculated.
Statistical Analysis
Measurement data are presented as mean±S.E.M (standard error of mean). For in vitro EGFP positive ratio data analysis, one-way ANOVA was used. For ex vivo EGFP positive ratio data analysis, t-test was used. For IOP data, Repeated-measure ANOVA analysis was used for within group and t-test analysis for between groups. P<0.05 was considered statistically significant.
RESULTS
Successful Construction of HIV-based Lentivirus Containing the Enhanced Green Fluorescent Protein Gene
After vector pWPXLd/EGFP was constructed, its sequence was confirmed by restriction enzyme digestion and nucleotide sequencing analysis. After double endonuclease digestion of the vector pWPXLd/EGFP, the agarose electrophoresis results showed that it contains a fragment which is about 800 bp in length and similar to the size of its PCR product, whereas the null-vector control does not. Sequence testing confirmed that plasmid pWPXLd/EGFP contains the EGFP sequence without DNA mutation. When pWPXLd/EGFP was used for HIV-based lentivirus packing according to a standard protocol, all the vectors achieved a titration of 107 TU/mL before concentration and approximately 109 TU/mL after concentration.
Highly Purified Human Trabecular Meshwork Cells Were Cultured in Vitro
HTM cells of the third generation were identified by immunochemistry staining. These HTM cells showed morphological diversity: fusiform, triangle and irregular shape. There were many processes on body cells that were rich in cytoplasm and usually there were a few pigment granules found inside. The nucleus was circular or oval in shape. Further immunochemistry staining revealed that these cells were positive in anti-LN (Figure 1B), anti-NSE (Figure 1C), anti-FN (Figure 1D) staining, but negative in anti- FVIII Rag staining (Figure 1A).
Figure 1. Identification of HTM cells by immunochemistry staining.
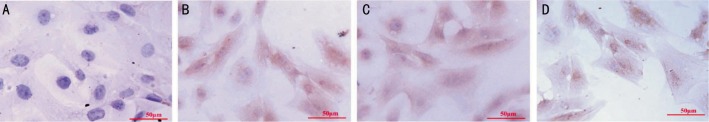
HTM cells of third generation were fixed and used for immunochemistry staining (SABC methods). Results showed that anti- FVIII Rag was negative in cultured HTM cells (1A), and anti-LN(1B), anti-NSE(1C), anti-FN (1D) were positive. Scale bar=50 µm.
HIV-based Lentivirus Efficiently Transduced Human Trabecular Meshwork Cell in Vitro
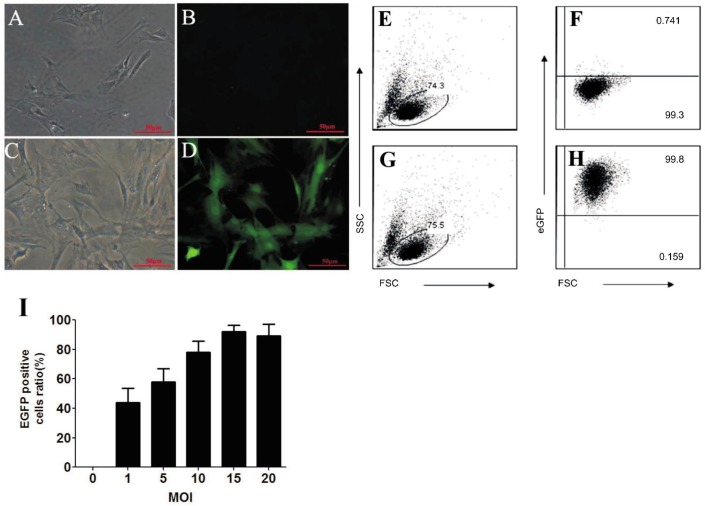
After HTM cells were transfected with HIV-based lentiviral vectors for five days, EGFP could be detected by fluorescence microscopy (Figure 2D). Flow cytometric analysis of the HTM cells showed that about 92.3% cells are EGFP positive when MOI is 15 (Figure 2G, 2H), which is the highest ratio of EGFP positive HTM cells obtained. The ratio of EGFP positive HTM cells between an MOI of 15 and 20 is not statistically significant, indicating that more viral particles will not increase the transfection ratio in a positive manner.
Figure 2. The efficiency of HIV-based lentivirus transducing Cultured HTM cells in vitro.
Cultured HTM cells were transduced with lentivirus containing EGFP gene. Five days later, EGFP could be detected in the transfected cells (A and B are null-vector controls for C and D, respectively; A and C are brightfield images, while B and D are fluorescent images; scale bar=50 µm). The ratios of EGFP positive cells were detected by flow cytometric analysis. Health HTM cells were gated based on forward scatter (FSC) and side scatter (SSC). Representative flow cytometry plots of E and F are from those cells transduced by null-vector controls, respectively the G and H are from those cells transduced by lentivirus containing EGFP with MOI of 15. E and G show cell population of interested, while F and H show EGFP positive cell ratio. After HTM cells were transfected with HIV-based lentiviral vector containing EGFP at a different range of MOI, the ratio of EGFP positive cells was calculated based on the following formula: (EGFP positive cells/HTM total cells)×100%, as illustrated in Figure 2I. Data are shown as mean ±SD (n= 3) of three independent experiments.
High Efficiency of HIV-based Lentivirus Transduced Human Trabecular Meshwork Cell ex Vivo
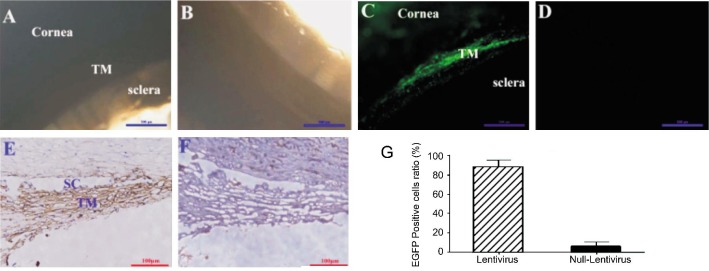
After cultured anterior segments of the human eye were perfused with HIV-based lentivirus for 21d, EGFP could be detected directly by fluoresce microscope (Figure 3A, 3C). Positive cells were mainly localized within TM and some in cornea tissue (Figure 3C). Further immunohistochemistry staining confirmed that EGFP positive staining mainly exist in TM, schlemm's canal (Figure 3E) with some in corneal endothelium (data is not shown). Cell count results showed that (88.19±7.13) % TM cells were EGFP positive.
Figure 3. Genetic modification of HTM cells in an ex vivo model of cultured anterior segment of human eye.
Cultured anterior segments of the human eye were transfected with HIV-based lentivirus which was delivered by perfusion system. Twenty-one days later, EGFP expression could be detected by fluorescence microscopy in transfected cells (A: Brightfield image, C: Fluorescent image, scale bar=500 µm), but not in control group (B: Brightfield image, D: Fluorescent image; scale bar=500 µm). Further immunochemistry study showed that EGFP was expressed both in TM and Schlemm's canal (E for EGFP contained HIV-based lentivirus, F for control virus, scale bar=100 µm). EGFP positive cell ratio were calculated by counting positive cells and the total cell nucleus (G for positive cell ratio, t-test, P<0.01).
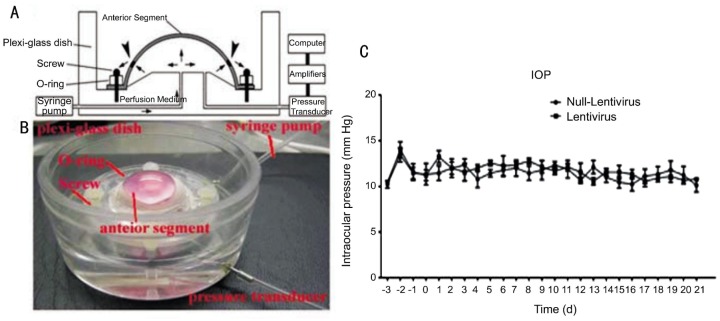
Intraocular Pressure Kept Constant When Cultured Anterior Segments of Human Eye Were Transfected With HIV-based Lentivirus
After equilibration for 3d, the IOP of human anterior segments reached a stable baseline. Right after lentivirus perfusion to anterior segment of the eye, the IOP increased slightly. However, on day 2 the IOP returned to baseline levels by day 3. Continual IOP monitoring showed that the IOP kept constant when compared with the baseline (P>0.05, repeated-measure ANOVA, Figure 4) and there was also no statistical difference when compared with control group (P>0.05, t-test, Figure 4).
Figure 4. Intraocular pressure of cultured anterior segment of the human eye receiving HIV-based lentivirus.
When cultured anterior segments of the human eye (Figure 4A, 4B) were transfected with HIV-based lentivirus, IOP was recorded every 8h. Figure 4C showed that IOP did not change when compared with the baseline (repeated-measure ANOVA, P>0.05) and control group (t-test, P>0.05).
DISCUSSION
Reducing IOP is the most important treatment for glaucoma and TM plays a pivotal role in the aqueous humor drainage from the anterior chamber. Gene therapy that targets the TM is a promising therapeutic strategy in lowering IOP. How to efficiently and safely transfer the therapeutic gene to TM cells is the major obstacle that needs to be overcome. Here we have successfully transferred an exogenous gene to HTM cells both in vitro and ex vivo using HIV-based lentivirus encoding EGFP gene. Furthermore, the expression of EGFP transferred by HIV-based lentivirus in HTM cells does not change IOP in an ex vivo model.
Several studies have tried to transfer an exogenous gene to HTM cells both in vivo and in vitro by various gene therapy vectors including adenovirus[12], adeno-associated virus (AAV)[13], [14], lentivirus[15], [16], and non-viral vectors[14]. Lentivirus are considered some of the most promising vehicles to effectively genetically modify cells for basic research and gene therapy approaches [17]. There are many types of engineered lentivirus such as, HIV, feline immunodeficiency virus (FIV), equine infectious anemia virus (EIAV), Simian immunodeficiency virus (SIV) and bovine immunodeficiency virus (BIV). EIAV vectors were shown to transduce mouse TM[18]. FIV vectors have already been tested in rodent, cat and no-human primates and the human model of the anterior chamber[15], [19]. Furthermore, HIV-based lentivirus has been the most extensively studied vector and it has been shown that it can efficiently transfer an exogenous gene into many eye tissues[7].
Our results demonstrated that an HIV-based lentivirus could efficiently transfer an exogenous gene to HTM cells both in vitro and ex vivo. For an in vitro study, it has shown very high transducing efficiency as 92.3% HTM cells were transfected by HIV-based lentivirus. In ex vivo studies, the EGFP gene is expressed in about 88.16% TM cells of human anterior segment after transduction by HIV-based lentivirus. Not only were we able to detect high levels of EGFP gene expression in HTM cells of the anterior segment, but we also found that the IOP kept constant when transfected with lentivirus. To some extent, this indicated that HIV-based lentivirus could safely be used for HTM cells gene transfer. Furthermore, in this study, we directly monitored green fluorescent of EGFP transgene expression in HTM in vivo rather than in a fixed dye condition. It is therefore, possible to observe gene transfer and expression in future gene therapy in vivo. In this study, the housekeeping EF1-α promoter robustly drove the expression of EGFP gene, but it did not direct strictly HTM cell-specific expression of foreign genes, as EFGP fluorescence could be detected in some corneal endothelium cells. For specific targeting, additional strategies such as addressing tissue specific promoter matrix Gla protein (MGP) promoter may be useful in this regard[20].
Since glaucoma is a chronic disease, IOP requires long-term control. Lentivirus may be a good choice because it could stably integrate as an obligate part of the transduction process and result in long-lasting expression. This study has shown that gene transfer to TM can be achieved by the administration of lentivirus circulating into the anterior chamber. Therefore, the delivery of appropriate therapeutic genes to the TM using HIV-based lentiviral systems could reduce the IOP, providing hope for a potential treatment of glaucoma - an irreversible blindness affecting millions of people worldwide.
Acknowledgments
The authors thank Dr. Jian-Feng Zhou for providing us with the HEK 293T cells. The authors are grateful to Tronolab for HIV-based lentiviral packaging plasmids and technique and Pang I.H. for the Perfusion culture system of human eye anterior segment.
Foundations: Supported by Natural Science Foundation of China (No. 30901395); the Doctoral Fund of Ministry of Education of China (No. 20090142120012, No. 20110142120021).
Conflicts of Interest: Xiang Y, None; Li B, None; Wang JM, None; Li GG, None; Zhang H, None; Manyande A, None; Tian XB, None.
REFERENCE
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razeghinejad MR, Fudemberg SJ, Spaeth GL. The changing conceptual basis of trabeculectomy: a review of past and current surgical techniques. Surv Ophthalmol. 2012;57(1):1–25. doi: 10.1016/j.survophthal.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer E. Trabecular meshwork as a new target for the treatment of glaucoma. Drug News Perspect. 2006;19(3):151–158. doi: 10.1358/dnp.2006.19.3.985929. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Allingham RR, Qin X, Layfield D, Dellinger AE, Gibson J, Wheeler J, Ashley-Koch AE, Stamer WD, Hauser MA. Gene expression profile in human trabecular meshwork from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54(9):6382–6389. doi: 10.1167/iovs.13-12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimpel MW, Johnson DH. Factors influencing in vivo trabecular cell replication as determined by 3H-thymidine labelling; an autoradiographic study in cats. Curr Eye Res. 1992;11(4):297–306. doi: 10.3109/02713689209001783. [DOI] [PubMed] [Google Scholar]
- 6.Yi Y, Noh MJ, Lee KH. Current advances in retroviral gene therapy. Curr Gene Ther. 2011;11(3):218–228. doi: 10.2174/156652311795684740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi G, Calistri A, Curtarello M, Giudice GL, Piermarocchi S, Prosdocimo G, Palù G, Parolin C. HIV-1-mediated delivery of a short hairpin RNA targeting vascular endothelial growth factor in human retinal pigment epithelium cells. Br J Ophthalmol. 2009;93(2):244–248. doi: 10.1136/bjo.2008.138388. [DOI] [PubMed] [Google Scholar]
- 8.Valtink M, Stanke N, Knels L, Engelmann K, Funk RH, Lindemann D. Pseudotyping and culture conditions affect efficiency and cytotoxicity of retroviral gene transfer to human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2011;52(9):6807–6813. doi: 10.1167/iovs.11-7710. [DOI] [PubMed] [Google Scholar]
- 9.Loewen N, Fautsch MP, Peretz M, Bahler CK, Cameron JD, Johnson DH, Poeschla EM. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum Gene Ther. 2001;12(17):2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- 10.Tian X, Wang G, Xu Y, Wang P, Chen S, Yang H, Gao F, Xu A, Cao F, Jin X, Manyande A, Tian Y. An improved tet-on system for gene expression in neurons delivered by a single lentiviral vector. Hum Gene Ther. 2009;20(2):113–123. doi: 10.1089/hum.2008.018. [DOI] [PubMed] [Google Scholar]
- 11.Pang IH, Fleenor DL, Hellberg PE, Stropki K, McCartney MD, Clark AF. Aqueous outflow-enhancing effect of tert-butylhydroquinone: involvement of AP-1 activation and MMP-3 expression. Invest Ophthalmol Vis Sci. 2003;44(8):3502–3510. doi: 10.1167/iovs.02-0758. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Gonzalez P, Camras LJ, Navarro I, Qiu J, Challa P, Stamer WD. Optimizing gene transfer to conventional outflow cells in living mouse eyes. Exp Eye Res. 2013;109:8–16. doi: 10.1016/j.exer.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borras T, Xue W, Choi VW, Bartlett JS, Li G, Samulski RJ, Chisolm SS. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J Gene Med. 2006;8(5):589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 14.Demetriades AM. Gene therapy for glaucoma. Curr Opin Ophthalmol. 2011;22(2):73–77. doi: 10.1097/ICU.0b013e32834371d2. [DOI] [PubMed] [Google Scholar]
- 15.Barraza RA, Rasmussen CA, Loewen N, Cameron JD, Gabelt BT, Teo WL, Kaufman PL, Poeschla EM. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum Gene Ther. 2009;20(3):191–200. doi: 10.1089/hum.2008.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borras T. Advances in glaucoma treatment and management: gene therapy. Invest Ophthalmol Vis Sci. 2012;53(5):2506–2510. doi: 10.1167/iovs.12-9483o. [DOI] [PubMed] [Google Scholar]
- 17.Benabdellah K, Cobo M, Munoz P, Toscano MG, Martin F. Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines. PLoS One. 2011;6(8):e23734. doi: 10.1371/journal.pone.0023734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JW, Naylor S, Ali RR. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8(3):275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 19.Khare PD, Loewen N, Teo W, Barraza RA, Saenz DT, Johnson DH, Poeschla EM. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol Ther. 2008;16(1):97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez P, Caballero M, Liton PB, Stamer WD, Epstein DL. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest Ophthalmol Vis Sci. 2004;45(5):1389–1395. doi: 10.1167/iovs.03-0537. [DOI] [PubMed] [Google Scholar]