Abstract
AIM
To study the contribution of tonicity response element binding protein (TonEBP) in retinal ganglion cell (RGC) death of diabetic retinopathy (DR).
METHODS
Diabetes was induced in C57BL/6 mice by five consecutive intraperitoneal injections of 55 mg/kg streptozotocin (STZ). Control mice received vehicle (phosphate-buffered saline). All mice were killed 2mo after injections, and the extent of cell death and the protein expression levels of TonEBP and aldose reductase (AR) were examined.
RESULTS
The TonEBP and AR protein levels and the death of RGC were significantly increased in the retinas of diabetic mice compared with controls 2mo after the induction of diabetes. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL)-positive signals co-localized with TonEBP immunoreactive RGC. These changes were increased in the diabetic retinas compared with controls.
CONCLUSION
The present data show that AR and TonEBP are upregulated in the DR and TonEBP may contribute to apoptosis of RGC in the DR.
Keywords: aldose reductase, diabetes, tonicity response element binding protein, retinopathy
INTRODUCTION
Diabetic retinopathy (DR), a vascular disorder affecting microvasculature of the retina, is a leading cause of adult blindness and is the most common complication of diabetes[1]. But, retinal neurodegeneration, such as retinal ganglion cell (RGC) death and glial activation, is already present before any microvascular abnormalities are detected in ophthalmoscopic examinations[2]. Neural apoptosis is a feature of DR, and all neuronal cell types in the retina seem to be susceptible to hyperglycemia-induced apoptosis[3]. But the mechanism of the regulators in the apoptotic pathway in DR is still uncertain. Many biochemical pathways associated with hyperglycemia have been implicated in the development of diabetic complications including DR. These include glucose autoxidation, polyol pathway, prostanoid synthesis, protein glycation, protein kinase C activation, and hexosamine pathway[4]. And, increased polyol pathway activity has been linked to abnormalities such as increased osmotic and oxidative stress factors that have been cited as promoters of diabetic microvascular diseases[4]. Aldose reductase (AR), a rate-limiting enzyme of the polyol pathway, plays important roles in the pathogenesis of diabetic complications[5]. AR is tightly regulated by intracellular osmolality at the transcription level[6]. This is mediated through the osmotic response elements (ORE) located in the 5′ flanking sequences of the AR gene[7].
Tonicity response element binding protein (TonEBP) is a widely expressed transcription factor whose activity is regulated by extracellular tonicity and plays a key role in osmoprotection. Under hypertonic conditions, there is an increase of TonEBP levels in the nucleus[8]. Studies have shown that the expression of AR is upregulated by the TonEBP under high-glucose conditions in diabetic microvascular complication, especially, diabetic nephropathy[9]. But, until recently, few reports have evaluated the increase of TonEBP expression and the relationship between AR and TonEBP in DR.
In this study, we investigated retinal expression of TonEBP and whether TonEBP contributes to the ganglion cell death in the diabetic retinas.
MATERIALS AND METHODS
Materials
Male C57BL/6 mice (KOATEC, Pyeongtaek, Korea) were used in this study. All mice were maintained on a standard rodent diet and water ad libitum. All animal procedures for this study were in adherence to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and were kept in strict accordance with the Institutional Animal Care and Use Committee of Gyeongsang National University.
For induction of diabetes, mice were injected intraperitoneally with 55 mg/kg streptozotocin (STZ; Sigma, St. Louis, MO, USA) dissolved in 50 mmol/L sodium citrate (pH 4.5), once a day for 5 consecutive days, and control mice received buffer. All mice were killed 2mo after injections. Blood samples were obtained by tail puncture after 2h fasting, and the blood glucose levels were measured using a glucometer (Precision, UK). Diabetes was confirmed by blood glucose levels >13.9 mmol/L, 1wk after the fifth injection of STZ. Body weights and blood glucose levels in mice were recorded every week. This study was conducted in accordance with the Declaration of Helsinki, and was consistent with good clinical practice guidelines, and was approved by all institutional review boards.
Methods
Western blot analysis
Protein extraction and Western blot analysis were performed as described previously[10]. To determine the levels of TonEBP and AR protein expressed, total proteins were extracted from four different retinas per each group and we individually performed Western blot analyses on four independent retinal extracts for each group. Total proteins (30 µg) from retinas of each group of mice was subjected to 10% SDS-PAGE and then transferred to a nitrocellulose membrane. The blots were incubated in primary antibody against AR (Abcam 62795, rabbit polyclonal antibody) and TonEBP (from Dr. Kwon HM at UNIST, rabbit polyclonal antibody, UNIST, Korea) and in secondary antibody, HRP-conjugated anti-rabbit IgG (Rockford, IL, USA). The target proteins were visualized using an enhanced chemiluminescent kit (Pierce). Each blot was reprobed with alpha-tubulin or beta-actin to control for differences in loading. Data are representative of four independent experiments.
Measurement of changes in inner retinal thickness
To assess inner retinal thickness, the sections were stained with hematoxylin and eosin (H & E). Then, inner retinal thickness was measured as the length from the ganglion cell layer (GCL) to the tip of the inner nuclear layer (INL). Analysis of the inner retinal thickness in diabetic and control retinas was performed in four random fields of different retinas from each group.
Immunohistochemical and TUNEL staining
Two months after injection of streptozotocin or buffer, 12 µm frozen retinal sections were prepared as described previously[11]. The isolated retinas were immersed in 4% PFA for 6h, washed in phosphate-buffered saline (PBS), cryoprotected in 30% sucrose overnight at 4°C, frozen with the optimal cutting temperature (O.C.T.) compound (Sakura, Tokyo, Japan) in liquid nitrogen and then cryosectioned at a thickness of 12 µm using a cryostat (Leica 8400E; Leica, Tokyo, Japan). After blocking with serum, the sections were incubated in primary antibody against AR and TonEBP and in secondary antibody, Alexa 488 goat anti-rabbit IgG (Molecular Probes). The sections were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted in ProLong Gold antifade reagent (Invitrogen). The number of immunostained RGC cells was counted in the GCL (within 100 µm) in three different fields from 5-6 different retinas for each group.
To detect cell death, we used terminal deoxynucleodityl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay kits with TMR red (In situ Cell Death Detection kits, Roche, Germany). Cell death was analyzed according to the manufacturer's directions. To confirm whether the TUNEL-positive cells are RGCs and TonEBP-immunopositive cells, we sequencially performed immunofluorescent staining as described previously for NeuN (Chemicon, mouse monoclonal antibody, Germany), a ganglion cell marker, and TonEBP with TUNEL assay according to the manufacturer's directions on the same cryosections. The total numbers of TUNEL-positive RGCs that colocalized with TonEBP were counted in the GCL (within 100 µm) in three different fields from 5-6 different retinas for each group.
Image capture and analysis
All retinal images were captured at a diatance of approximately 0.8-1.0 mm from the optic nerve head using a disk scanning confocal microscope (Olympus, JP IX2-DSU, Germany). The excitation source was a 490 nm and 590 nm lasers and all images were taken under the same experimental conditions to minimize variations in fluorescence intensity. Quantitative analysis was performed using the Metamorph Image Analysis software (Molecular Devices) and bar graphs were constructed using GraphPad Prism 5. All data are representative of three independent experiments.
RESULTS
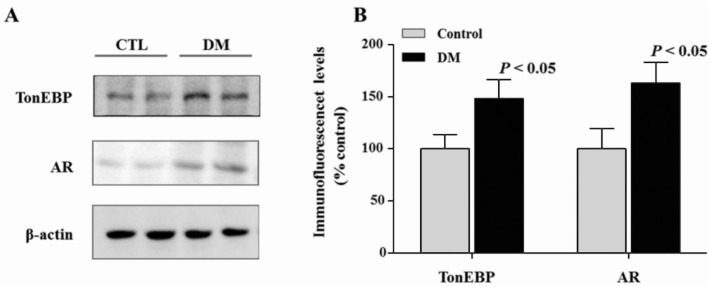
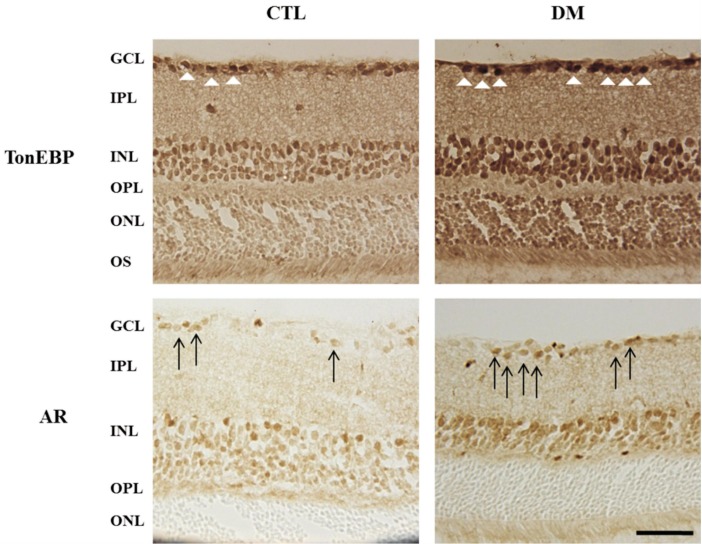
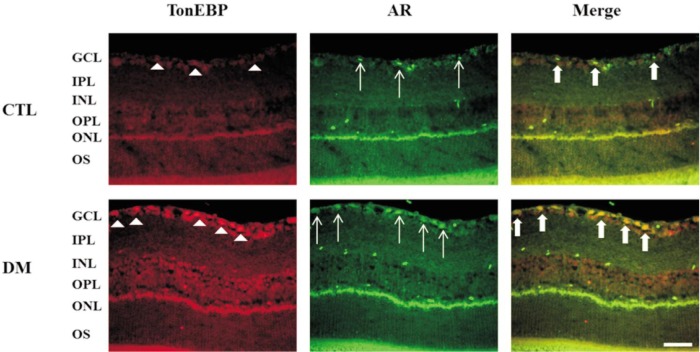
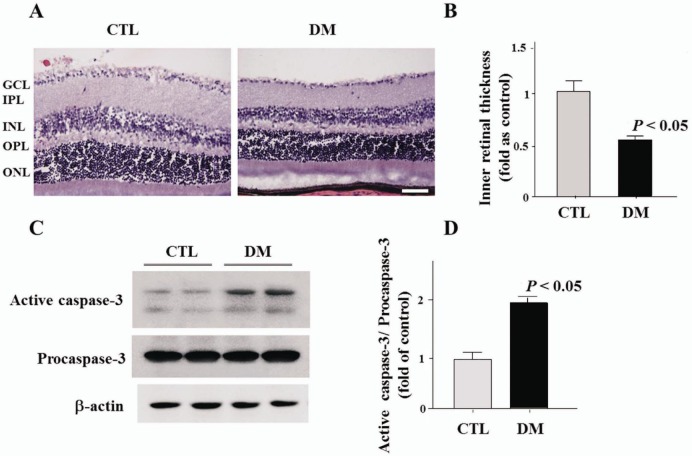
Protein levels of AR and TonEBP were significantly increased in diabetic retinas compared to control retinas (Figure 1A, 1B). Increased level of AR and TonEBP were confirmed by immunostaining in control and diabetic retinas. TonEBP and AR immunoreactivities were increased in diabetic retina compared to control retina, and these were observed especially in the GCL and INL (Figure 2). Moreover, immunofluorescence study showed retinal TonEBP and AR immunoreactivities were dramatically increased and colocalized in diabetic retinas compared to controls, and the colocalization of TonEBP with AR immunoreactivity was mostly observed in GCL and INL (Figure 3). To demonstrate neurodegeneration in the DR, we examinined the changes of inner retinal thickness and the expression of active caspase-3. In H & E staining, there was showed a significant decrease in the inner retinal thickness of diabetic mice compared with those of controls (Figure 4). No diabetes-induced changes were observed in the thickness of the outer retinal regions (data not shown here). And protein levels of active caspase-3 were highly increased in the retinas of diabetic mice compared with controls (Figure 4C, 4D).
Figure 1. Expression of TonEBP and AR in the diabetic retina.
A: TonEBP and AR protein levels were measured by Western blot analysis in the control and diabetic retina; B: Note the increased levels of AR and TonEBP protein in diabetic retinas compared to control retinas.
Figure 2. Immunohistochemical staining in the diabetic retina. Note the increased retinal TonEBP and AR immunoreactivities in diabetic mice compared to controls, and these were observed especially in GCL and INL.
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; OS: Outer segment; AR: Aldose reductase. Scale bar, 50 µm. White arrow head: Increased retinal TonEBP; Black arrow: Increased retinal AR.
Figure 3. Colocalization of TonEBP and AR expression in the retinas of diabetic mice. Note that there was a dramatic increase in TonEBP and AR expression in GCL of diabetic retina and their colocalization was shown in the merged image.
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; OS: Outer segment; AR: Aldose reductase. Scale bar, 50 µm. White arrow head: Increased in TonEBP, White arrow: Increased in AR, Bold arrow: GCL of diabetic retina and their colocalization.
Figure 4. Changes of inner retinal thickness and Western blot analysis of active caspase-3 in the retinas of diabetic mice.
A, B: In H & E staining, diabetic mice showed a significant decrease in the inner retinal thickness, from the GCL to the tip of the INL, compared with those of controls; C, D: In Western blot analysis of active caspase-3 to confirm cell death, protein levels were highly increased in the retinas of diabetic mice compared with controls. GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer. Scale bar, 50 µm.
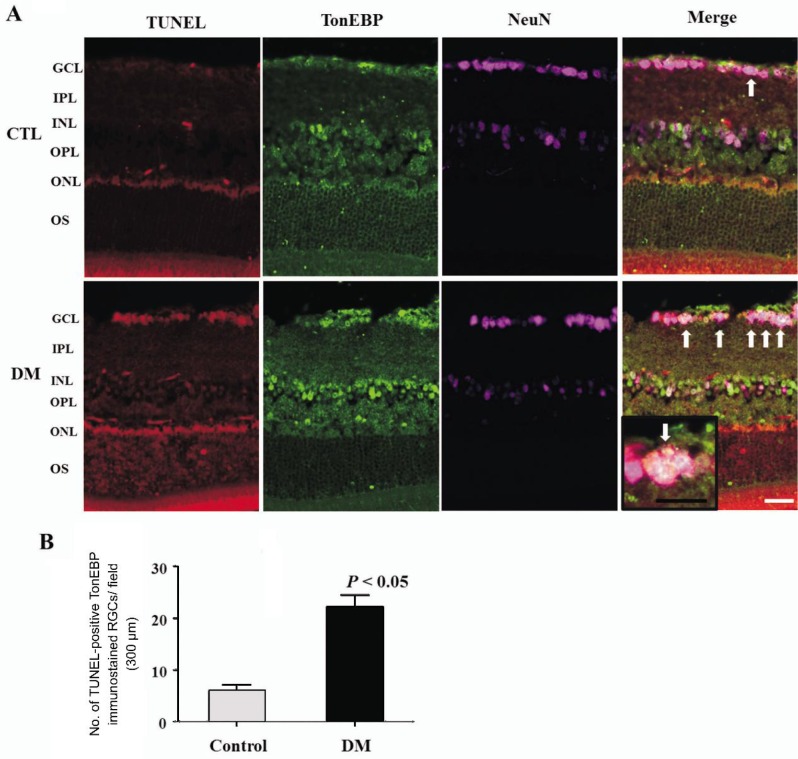
To confirm the death of RGCs, triple immunofluorescent staining of TonEBP (green) and NeuN (violet), the GC marker, with TUNEL (red) staining was performed on retinal sections from control and diabetic mice. The total number of TUNEL-positive RGCs (white arrow, Figure 5A) which were colocalized with TonEBP significantly increased in the diabetic mice compared with controls (Figure 5B).
Figure 5. Triple immunofluorescent staining of TonEBP, TUNEL, and NeuN in retinas of mice 2mo after induction of diabetes compared with controls.
A: Triple immunofluorescent staining of TonEBP (green) and NeuN (violet) with TUNEL (red) showed increased expression of TonEBP colocalized with TUNEL-positive RGCs in diabetic mice (white arrow); B: The total number of TUNEL-positive RGCs which were colocalized with TonEBP significantly increased in the diabetic mice compared with controls. GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; OS: Outer segment. Scale bar, 50 µm.
DISCUSSION
In the present study, we firstly found that increased expression of TonEBP in the DR, especially in the GCL. Moreover, TonEBP colocalized with AR and TUNEL signals in diabetic retinas. These results suggest that TonEBP may contribute to apoptosis of retinal neurons in the DR via AR pathway.
Several clinical studies suggest that neuronal changes occur prior to clinically detectable microvascular complications. And, Studies have suggested that retinal neurodegeneration is a critical component of DR, which is typically characterized by a reduced number of RGCs and a decreased retinal thickness. Barber et al[12] reported a 22% and 14% decrease in the thickness of the inner plexiform layer (IPL) and INL of retinal from STZ-induced diabetic rats. Oshitari et al[13] showed the number of apoptotic RGCs of diabetic retinas was significantly increased compared to that in control retinas. Consistently with previous studies, our result showed significantly decrease in inner retinal thickness and a prominent increase in apoptotic RGCs in the retinas of diabetic mice.
The mechanism of apoptotic processes in RGCs are unclear in DR. Hyperglycemia is one of the important risk factors and causing increasing metabolic flux through several biochemical pathways linked to abnormalities such as increased osmotic and oxidative stress factors, protmoters of DR[4],[14]. Oshitari et al[13] reported that increased cellular apoptosis may be associated with Bax overexpression, which may be responsible for neuronal cell loss in the GCL of diabetic rats. Kim et al[15] suggested Ca2+/calmodulin-dependent protein kinase II (CaMKII) involved in the cell death pathway of RGCs in the STZ-induced diabetic retinas. In our study, we found that the total number of TUNEL-positive RGCs which were colocalized with TonEBP significantly increased in the diabetic mice compared with controls. Consequently, these results suggest that TonEBP might play a role in the apoptosis of retinal neurons via AR pathway during the course of DR.
The AR pathway has been studied in relation to all complications of diabetes including DR. It is well known the polyol pathway may lead to early changes in the retinal vasculature including loss of vascular pericytes and thickening of the basement membrane. And, pro-oxidant effects of the polyol pathway and the fact that reactive oxygen species induced by hyperglycemia beget inflammation, points to the combination of oxidative stress and inflammation as the distinct signature of the polyol pathway on retinal vessel[16],[17]. Drel et al[18] reported the number of TUNEL-positive cell in flat mounted retinas was increased in diabetic rats compared with the control group, and this increase was partially corrected by AR inhibitor (fidarestat). Also, some investigators suggested that neural apoptosis in the diabetic retina was mediated by increased activity of the AR pathway and an inhibitor of AR (Sorbinil) prevented this abnormality[19]. Taken together, AR pathway may play a role in the neurodegeneration as well as vascular abnormalities during the development of DR.
The mechanism that initiate and promote neurodegeneration by upregulation of TonEBP in the DR remains unclear. TonEBP was initially known for its role in the hypertonic inner medulla of the kidney for controlling a genetic program to keep the cellular homeostasis[20]. Recently, many studies suggest that TonEBP might play diverse roles. Some reports suggested that TonEBP contributes to the pathogenesis of diabetic complications, especially diabetic nephropathy. Especially, TonEBP binds to the tonicity-reponsive enhancer element in the promoters of AR genes[9]. So, TonEBP increases the AR enzyme activity which is associated with abnormalities in osmotic and oxidative stress factors that have been cited as promoters of diabetic complications, such as neurodegeneration[9],[21]. Also, since increased activity of the AR pathway is an important pathogenic factor in diabetic complications and several inhibitors of AR have been developed, studies about transcriptional factor that control AR expression, like TonEBP, may provide insight into the mechanisms underlying initiation or progression of DR.
To our knowledge, this is the first experimental investigation suggested that TonEBP contributes to apoptosis of RGC in the DR. However, this experiment was pilot study. Further studies are needed to more precisely define the pathophysiological role of TonEBP and its downstream target in DR. And, in the present study, TonEBP expression significantly increased in INL and ONL of the diabetic retinas as well as GCL (Figures 2, 3). So, future studies should also include in vitro and in vivo studies to investigate whether TonEBP contributes to the microvasculopathy or apoptosis of other neuronal cells and pericytes.
In conclusion, we confirmed that high glucose increased expression of AR and TonEBP in the streptozotocin-induced diabetic retinopathy, especially in the GCL. Also, TonEBP-immunopositive RGCs colocalized with TUNEL signals in DR. These results suggest that increased expression of TonEBP may contribute to the apoptosis of retinal neurons in DR.
Acknowledgments
The abstract of this paper had been presented as e-poster on 2014 ARVO meeting.
We would like to thank Ae-Jin Gu for her technical assistance.
Foundations: Supported by the Basic Research Program of the Korea Science & Engineering Foundation (No.2009-0068732); the Basic Research Program of the National Research Foundation of Korea (No.2011-0020163); the Bio-Industry Technology Development Program funded by the Korea Institute of Planning & Evaluation for Technology in Food, Agriculture Forestry & Fisheries (No.112005-3); the BK21 Program and by the MRC program of KRF (R13-2005-012-01001-1).
Conflicts of Interest: Kim SJ, None; Kim H, None; Park J, None; Chung I, None; Kwon HM, None; Choi WS, None; Yoo JM, None.
REFERENCES
- 1.Hazin R, Colyer M, Lum F, Barazi MK. Revisiting Diabetes 2000: challenges in establishing nationwide diabetic retinopathy prevention programs. Am J Ophthalmol. 2011;152(5):723–729. doi: 10.1016/j.ajo.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 3.Lieth E, Gardner TW, Barber AJ, Antonetti DA, Penn State Retina Research Group Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28(1):3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 5.Hattori T, Matsubara A, Taniguchi K, Ogura Y. Aldose reductase inhibitor fidarestat attenuates leukocyte-endothelial interactions in experimental diabetic rat retina in vivo. Curr Eye Res. 2010;35(2):146–154. doi: 10.3109/02713680903447918. [DOI] [PubMed] [Google Scholar]
- 6.Rosas-Rodríguez JA, Valenzuela-Soto EM. Enzymes involved in osmolyte synthesis: how does oxidative stress affect osmoregulation in renal cells? Life Sci. 2010;23(17-18):515–520. doi: 10.1016/j.lfs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Irarrazabal CE, Gallazzini M, Schnetz MP, Kunin M, Simons BL, Williams CK, Burg MB, Ferraris JD. Phospholipase C-gamma1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc Natl Acad Sci U S A. 2010;107(2):906–911. doi: 10.1073/pnas.0913415107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung CY, Ko BC. NFAT5 in cellular adaptation to hypertonic stress-regulations and functional significance. J Mol Signal. 2013;8(1):5. doi: 10.1186/1750-2187-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Hodgkinson AD, Oates PJ, Kwon HM, Millward BA, Demaine AG. Elevated activity of transcription factor nuclear factor of activated T-cells 5 (NFAT5) and diabetic nephropathy. Diabetes. 2006;55(5):1450–1455. doi: 10.2337/db05-1260. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Kim YS, Kang SS, Noh HS, Kim HJ, Cho GJ, Choi WS. Expression of 14-3-3 zeta and interaction with protein kinase C in the rat retina in early diabetes. Diabetologia. 2005;48(7):1411–1415. doi: 10.1007/s00125-005-1774-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Kim YS, Noh HS, Kang SS, Cheon EW, Park SK, Lee BJ, Choi WS, Cho GJ. Changes in rhodopsin kinase and transducin in the rat retina in early-stage diabetes. Exp Eye Res. 2005;80(6):753–760. doi: 10.1016/j.exer.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshitari T, Hata N, Yamamoto S. Endoplasmic reticulum stress and diabetic retinopathy. Vasc Health Risk Manag. 2008;4(1):115–122. doi: 10.2147/vhrm.2008.04.01.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta N, Kawamori R, Fukuda M, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29(12):1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Kim YS, Kang SS, Cho GJ, Choi WS. Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes. 2010;59(7):1825–1835. doi: 10.2337/db09-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obrosova IG, Kador PF. Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12(3):373–385. doi: 10.2174/138920111794480642. [DOI] [PubMed] [Google Scholar]
- 17.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 18.Drel VR, Pacher P, Ali TK, Shin J, Julius U, El-Remessy AB, Obrosova IG. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21(6):667–676. [PMC free article] [PubMed] [Google Scholar]
- 19.Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52(2):506–511. doi: 10.2337/diabetes.52.2.506. [DOI] [PubMed] [Google Scholar]
- 20.Halterman JA, Kwon HM, Wamhoff BR. Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5) Am J Physiol Cell Physiol. 2012;302(1):C1–8. doi: 10.1152/ajpcell.00327.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]