Abstract
AIM
To assess cultured limbal epithelial stem cell transplantation in patients with limbal stem cell deficiency by analyzing and quantifying corneal neovascularization.
METHODS
This retrospective, interventional case series included eight eyes with total limbal stem cell deficiency. Ex vivo limbal epithelial stem cells were cultured on human amniotic membrane using an animal-free culture method. The clinical parameters of limbal stem cell deficiency, impression cytology, and quantification of corneal neovascularization were evaluated before and after cultured limbal stem cell transplantation. The area of corneal neovascularization, vessel caliber (VC), and invasive area (IA) were analyzed before and after stem cell transplantation by image analysis software. Best-corrected visual acuity (BCVA), epithelial transparency, and impression cytology were also measured.
RESULTS
One year after surgery, successful cases showed a reduction (improvement) of all three parameters of corneal neovascularization [neovascular area (NA), VC, IA], while failed cases did not. NA decreased a mean of 32.31% (P=0.035), invasion area 29.37% (P=0.018) and VC 14.29% (P=0.072). BCVA improved in all eyes (mean follow-up, 76±21mo). Epithelial transparency improved significantly from 2.00±0.93 to 0.88±1.25 (P=0.014). Impression cytology showed that three cases failed after limbal epithelial stem cell therapy before 1y of follow-up.
CONCLUSION
This method of analyzing and monitoring surface vessels is useful for evaluating the epithelial status during follow-up, as successful cases showed a bigger reduction in corneal neovascularization parameters than failed cases. Using this method, successful cases could be differentiated from failed cases.
Keywords: limbal stem cell, corneal neovascularization, stem cell therapy, impression cytology, limbal stem cell deficiency
INTRODUCTION
Limbal epithelial stem cell deficiency is defined as loss of corneal epithelial transparency, superficial corneal neovascularization, epithelial irregularity on late-fluorescein staining, and a history of recurrent or persistent epithelial defects[1]. Corneal limbal epithelial stem cell deficiency is associated with corneal epithelium opacification and vision loss. In limbal epithelial stem cell deficiency, the cornea acquires an epithelium from bulbar conjunctival cells resulting in corneal neovascularization, chronic inflammation, and scarring[2]. Different therapeutic strategies have been implemented to treat limbal epithelial stem cell deficiency, such as limbal allograft or autograft transplantation[3]. The limitations of corneal allografting include a shortage of donor tissues and the potential for immune graft rejection; the use of autologous grafts is limited by the need for large limbal cell harvests from the healthy eye that is required for transplantation. Since Pellegrini et al[4] described use of ex vivo cultured limbal epithelial stem cells for transplantation in humans, several studies have reported different methods of cellular expansion, source of donor tissue, surgical approach, and postoperative management[5],[6]. Different studies have reported varying definitions of success after limbal epithelial stem cell therapy. Many of these studies have described improved visual acuity (VA) alone or did not define the parameters used to consider a successful resolution after limbal epithelial stem cell therapy, while others implemented a scoring system based on defined clinical findings[5],[6]. The parameters most often reported were VA; re-establishment of a stable, transparent corneal epithelium; resolved corneal conjunctivalization; and resolved persistent epithelial defects. In the current study, we reported a long-term follow-up of a mean of 76mo of eight patients with total limbal epithelial stem cell deficiency after ex vivo cultured limbal epithelial stem cell transplantation using measurements of corneal neovascularization to determine whether this could be a useful strategy to assess and predict the long-term success in patients undergoing limbal epithelial stem cell transplant.
SUBJECTS AND METHODS
Subjects
Eight patients (6 men, 2 women; mean age: 48.75±18.7y; range: 19-75) were included. The demographic data are shown in Table 1. This case series included consecutive patients with total limbal epithelial stem cell deficiency who were eligible for transplantation and preparation of autologous serum to be used for postoperative treatment and culture medium. Conjunctival epithelial ingrowth was confirmed by goblet cells on impression cytology in the affected eye. All patients had good tear function with Schirmer's test (type I) results exceeding 5 mm and good bilateral eyelid closure; however, seven of eight patients had chronic conjunctival hyperemia in the affected eye despite medical treatment. Patients with hepatitis B or C, syphilis, or human immunodeficiency virus (HIV) were excluded. Ethics statements were in accordance with the tenets of the Declaration of Helsinki; the Institutional Review Board for Human Studies and Ethics Committee, the Regional Review Board for Clinical Trials with Human Subjects, and the Spanish Health Authorities specifically approved this study (PI052074). Written informed consents were required from all included patients and from placenta donors for amniotic membrane preparation. All informed consents were approved by the Institutional Review Board for Human Studies and Ethics Committee of Clínica Universidad de Navarra, University of Navarre.
Table 1. Patient demographics and ocular conditions before treatment.
| Patient | Age1/gender/eye | Previous surgeries | Other ocular diagnoses | LSCD etiology | Source of donor tissue | Limbal zone for biopsy |
| 1 | 75/M/OD | PK | Cataract | Abscess | Fellow eye | Superior |
| 2 | 32/M/OD | AMT, PK | Symblepharon | Alkali burn | Fellow eye | Superior |
| 3 | 43/F/OS | AMT, PK | Amblyopia | Herpetic | Fellow eye | Superior |
| 4 | 19/M/OS | AMT, PK | Blepharitis | Acid burn | Fellow eye | Superior |
| 5 | 69/M/OS | AMT | Cataract | Herpetic | Fellow eye | Inferior |
| 6 | 60/M/OS | PK, cataract | Idiopathic | Fellow eye | Superior | |
| 7 | 48/F/OS | PK | Alkali burn | Fellow eye | Superior | |
| 8 | 44/F/OS | PK (3 times) | Herpetic | Living relative (brother)2 | Superior |
LSCD: Limbal stem cell deficiency; BCVA: Best-corrected VA; OD: Right eye; OS: Left eye; PK: Penetrating keratoplasty; AMT: Amniotic membrane transplantation. 1Age at the time of limbal epithelial stem cell transplantation surgery; 2Patient had bilateral total LSCD after several bilateral keratoplasties.
Methods
Limbal biopsy
Impression cytology confirmed a normal corneal phenotype in the asymptomatic fellow eyes of seven of eight patients before the procedure. In one patient with bilateral limbal stem cell deficiency, allograft tissue was obtained from a related living donor. Cells for culture were obtained during a 2×4-mm2 biopsy of the superior limbus under topical anesthesia, with the exception of one case in which an inferior limbus biopsy was performed. Human amniotic membrane was sutured in place to cover the epithelial defect.
Limbal epithelial cell culture
Human limbal explants were rinsed three times with sterile phosphate buffered saline (PBS) solution containing 50 µg/mL gentamicin and 1.25 µg/mL amphotericin B, and then expanded over a denuded human amniotic membrane fastened onto a culture insert, and cultured in a medium comprised of Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY, USA) and HAMS F-12 (Bio-Whittaker, Inc., Walkersville, MD, USA) (1:1), supplemented with 10 ng/mL epidermal growth factor (E-9644, Sigma, Sigma-Aldrich Corporation, St. Louis, MO, USA), 1 mg/mL human insulin (I0908, Sigma), 0.1 mg/mL cholera toxin (C8052, Sigma), 0.5% dimethyl sulfoxide (Department of Pharmacy, Clínica Universidad de Navarra), 10% human autologous serum, and 1% penicillin/streptomycin (DE17-602E, Sigma). Cultures were incubated at 37°C under 5% carbon dioxide and 95% air, and the medium was changed every 2 to 3d. Explant cultures were maintained for 15 to 20d until the human limbal epithelial tissue reached confluence. Since 2005, cell cultures have been performed under good manufacturing practice (GMP) at the Department of Cell Therapy, Clínica Universidad de Navarra.
Preparation of human amniotic membrane
Human placenta was harvested during cesarean sections after obtaining written informed consent (approved by the Institutional Review Board for Human Studies and Ethics Committee of Clínica Universidad de Navarra, University of Navarre) from donors with negative serologic tests for hepatitis B and C virus, human T-cell lymphotropic virus, cytomegalovirus, syphilis and HIV at the time of cesarean section and 3mo later. Donors are asked to complete a questionnaire about their lifestyle and travel history. Under sterile conditions, the amnion was separated from the chorion by blunt dissection and washed with a PBS solution containing penicillin-streptomycin 1% (DE17-602E). After being flattened onto nitrocellulose paper, the amniotic membrane was stored at -80°C in a sterile vial containing sterile glycerol and DMEM. Immediately before use, the amniotic membrane was thawed, washed three times with sterile PBS, and deprived of their amniotic epithelial cells by incubation with 0.02% ethylenediaminetetraacetic acid at 37°C for 2h to loosen the cellular adhesion, followed by gentle scraping with a cell scraper. Preliminary experiments on hematoxylin-stained, ethanol-fixed tissues confirmed that this protocol effectively removed epithelial cells from the amniotic membrane. Tissues then were washed again twice with sterile PBS.
Surgery and postoperative management
The same surgeon (Moreno-Montañés J) performed all surgical procedures at the Department of Ophthalmology, Clínica Universidad de Navarra. Under retrobulbar anesthesia, a 360-degree periotomy 2 mm from the limbus was performed and fibrotic tissue was removed from the cornea and sclera. Fibrovascular tissue on the corneal surface was stripped by lamellar keratectomy with the help of a polisher to leave a homogeneous stromal bed. The entire cornea and limbal area were covered with a sheet of cultured limbal epithelial cells together with the amniotic membrane substrate, epithelial side up. The graft was attached to the cornea with an interrupted 10-0 nylon running suture and to surrounding episclera and conjunctiva with an interrupted 8-0 Vicryl suture. During the procedure, cultured limbal epithelium was protected by sodium hyaluronate (Healon, AMO, Santa Ana, CA, USA). A silicone hydrogel contact lens was placed onto the eye and covered by a pressure patch overnight. Topical preservative-free prednisolone acetate 1% and preservative-free ceftazidime were started 4 times daily beginning the day after surgery for the first 2wk and twice daily for the next 2wk, followed by 0.1% fluorometholone twice daily for 2mo. The contact lens was removed at 1mo if the tear film was stable.
Follow-up
A complete ophthalmologic examination was performed in all patients every 2wk during the first 2mo, every month for 6mo, and every 3mo thereafter. The examination included measurement of the best-corrected VA (BCVA), slit-lamp biomicroscopy (Takagi slitlamp microscope SM70, Takagi Seiko Co Ltd., Japan) with digital photography (Nikon Coolpix 995, Nikon Corporation, Japan), tonometry and indirect ophthalmoscopy. Impression cytology was performed every 3mo during the first 2y and once annually afterward. To compare the evolution of these patients, we considered a 12mo period of follow-up and the last visit of each patient. Two examiners who were not involved in the procedures and were not informed about the impression cytology results assessed the clinical outcomes.
Main outcome measures
Successful limbal epithelial stem cell therapy was defined as the presence of a normal corneal phenotype on impression cytology, i.e. absence of goblet cells and CK3-positive stain[7].
Epithelial transparency was scored from 1 to 3 by slit-lamp biomicroscopy according to the classification of Rama et al[8], with 1 indicating hazy corneal epithelium and/or recurrent epithelial defects; 2, persistent epithelial defects; and 3, total corneal conjunctivalization. The BCVA levels before and 1y after limbal epithelial stem cell therapy were compared using logarithm of the minimum angle of resolution (logMAR) units.
Quantification of corneal neovascularization was measured using the technique of Dastjerdi et al[9] with a few modifications. The parameters evaluated were the neovascular area (NA), defined as the area of corneal vessels when projected onto the plane of a photograph; vessel caliber (VC), defined as the mean diameter of the vessels; and invasive area (IA), defined as the percentage of the corneal area in which vessels were present. IA is measured at the very ends of all vascular sprouts, and by connecting all these ends, the contour was traced and the measured area was again normalized to the whole corneal area. Briefly, digital slit-lamp pictures were analyzed morphometrically using Adobe Photoshop CS5 (Adobe Systems Incorporated, San Jose, CA, USA) and cropped with an elliptical marquee tool to work within the corneal area inside the limbus. Using different filters in the same software, red vessels were highly saturated for easier identification. Using a freeform pen tool, the centripetal ends of the longest vessels then were connected to each other. This area free of vessels represented the counterpart of IA, so both areas equaled the entire cornea. NA was obtained by duplicating layers and manually drawing the vessels that were not recognized automatically with a magnetic tool. Using ImageJ software (public domain, http://rsb.info.nih.gov/ij/), the images were analyzed in binary mode, so that the black-and-white areas could be calculated automatically. A percentage of an area could be obtained in the neovascular and IA. AutoCAD 17.2 version software (Autodesk, Inc., San Rafael, CA, USA) was used for VC quantification. The two most prominent vessels in each quadrant, totaling eight vessels, were measured at the beginning of the corneal invasion, which is next to the limbal area. The average diameter of the selected vessels was the VC parameter.
Statistical Analysis
After obtaining all measurements, the T-Student test was used (SPSS 16.0.2, IBM Corp., NY, USA) to compare the results of the evaluations of the VC, IA, and NA before and 1y postoperatively, assuming a P<0.05 as statistical significant.
RESULTS
The results of limbal epithelial stem cell therapy after the last follow-up visit are shown in Table 2. There were no intraoperative or early postoperative complications related to the surgical procedure in any case. The overall success rate for this treatment in our cohort was five of eight eyes (62.5%) according to the impression cytology criteria of success. The treatment failed in patients 2, 4, and 7, who had conjunctivalization of the treated cornea, which was confirmed by the presence of goblet cells on impression cytology at 6, 9, and 3mo, respectively. No cases failed after 1y of follow-up. Representative clinical photographs of changes after limbal epithelial stem cell therapy are shown in Figures 1 and 2.
Table 2. Long-term follow-up after LESC therapy.
| Patient | Follow-up (mo) | Impression cytology | 1Time to failure after LESC surgery (mo) | Subsequent surgeries (months after LESC surgery) | Final BCVA (logMAR) | Final BCVA (Snellen) |
| 1 | 100 | Corneal | Cataract (6) | 0.2 | 20/32 | |
| 2 | 96 | Conjunctival | 6 | Symblepharon (27) | 2 | 20/2000 |
| 3 | 94 | Corneal | PK (15) | 0.5 | 20/63 | |
| 4 | 81 | Conjunctival | 9 | - | 1 | 20/200 |
| 5 | 69 | Corneal | PK (4); cataract (22) | 0.4 | 20/50 | |
| 6 | 65 | Corneal | - | 0.1 | 20/25 | |
| 7 | 65 | Conjunctival | 3 | - | 1 | 20/200 |
| 8 | 36 | Corneal | PK (24) | 0.3 | 20/40 |
LESC: Limbal epithelial stem cell; BCVA: Best corrected visual acuity (at last follow-up visit); PK: Penetrating keratoplasty; logMAR: Logarithm of the minimum angle of resolution. 1Month of failure according to impression cytology.
Figure 1. A 60-year-old man (patient 6) with idiopathic limbal stem cell deficiency in his left eye.
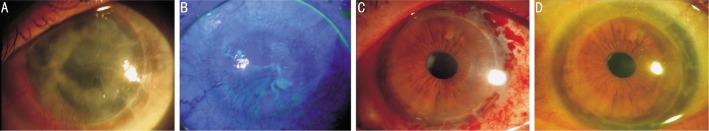
A: conjunctivalization and neovascularization in a pseudophakic eye with a 10-year-old corneal graft; B: Late fluorescein staining with epithelial defects. C: A few weeks after limbal epithelial cell transplantation on amniotic membrane; D: Fully transparent graft with intact epithelium 5y after corneal limbal epithelial stem cell therapy.
Figure 2. Total limbal stem cell deficiency secondary to herpetic keratitis in a 43-year-old woman (patient 3).
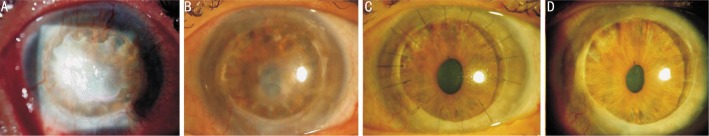
A: Thick fibrovascular tissue with corneal neovascularization 2y after a penetrating keratoplasty; B: 1y after limbal epithelial stem cell transplantation, an intact epithelium with less neovascularization is achieved; C: Penetrating keratoplasty was performed 15mo after limbal epithelial stem cell therapy because a residual stromal leukoma in the original graft affected the visual axis; D: Normal corneal epithelium remains stable after 7y of follow-up.
Overall, the epithelial transparency improved significantly 1y postoperatively, from 2.00±0.93 to 0.88±1.25 (P=0.014). All successful cases achieved a 0 score on epithelial transparency at 1y, while failed cases 2 and 7 marginally improved to a score 2 and failed case 4 did not show improvement (score 3). VA increased significantly (P=0.007) in all patients. The mean±standard deviation improvement in the BCVA was 1.3±0.78 logMAR 1y after treatment.
The vascular parameters evaluated before and 1y after surgery are shown in Table 3. Successful cases showed a reduction (improvement) of all three parameters of corneal neovascularization, while in failed cases at least one parameter (NA, VC or IA) did not reduce (worsening). After surgery, NA decreased a mean of 32.31% (P=0.035), invasion area 29.37% (P=0.018) and VC 14.29% (P=0.072). The changes of the three vascular parameters in successful cases showed a reduction above the mean change of the whole population (n=8). Among failed cases, changes were below the overall mean reduction (Table 4). Some examples of the changes in the NA and IA before and 1y postoperatively are shown in Figures 3 and 4.
Table 3. Quantification of corneal neovascularization before and 1y after surgery.
| Patient | NA pre | NA post | VC pre | VC post | IA pre | IA post |
| 1 | 7.8 | 5.3 | 0.150 | 0.126 | 36.6 | 27.3 |
| 12 | 26.0 | 19.7 | 0.160 | 0.160 | 89.6 | 72.7 |
| 3 | 9.8 | 4.1 | 0.169 | 0.128 | 37.4 | 18.1 |
| 14 | 10.5 | 12.3 | 0.157 | 0.100 | 32.5 | 37.2 |
| 5 | 1.8 | 1.7 | 0.171 | 0.102 | 7.0 | 6.1 |
| 6 | 9.5 | 4.9 | 0.103 | 0.100 | 39.7 | 17.9 |
| 17 | 13.1 | 4.7 | 0.130 | 0.158 | 42.4 | 22.8 |
| 8 | 2.5 | 2.1 | 0.090 | 0.063 | 9.8 | 6.2 |
NA: Neovascular area; VC: Vessel caliber; IA: Invasion area; Pre: Preoperative; Post: 1y postoperatively; NA and IA is a % of the total cornea area; VC expressed as a score provided by the software. 1Failed cases (according to impression cytology results).
Table 4. Statistical analysis of corneal neovascularization parameters before and 1y after surgery.
| Parameters | Before (a) | 1a | 1P | Mean reduction (%) |
| NA | 10.12 (7.51) | 6.85 (6.12) | 0.035 | 32.31 |
| Successful cases | 6.28 (3.85) | 3.62 (1.63) | 0.075 | 42.36 |
| Failed cases | 16.53 (8.30) | 12.23 (7.50) | 0.301 | 26.01 |
| VC | 0.14 (0.03) | 0.12 (0.03) | 0.072 | 14.29 |
| Successful cases | 0.14 (0.04) | 0.11 (0.03) | 0.040 | 21.43 |
| Failed cases | 0.15 (0.02) | 0.14 (0.03) | 0.740 | 06.67 |
| IA | 36.87 (25.25) | 26.04 (21.50) | 0.018 | 29.37 |
| Successful cases | 26.10 (16.23) | 15.12 (9.03) | 0.057 | 42.07 |
| Failed cases | 54.83 (30.51) | 44.23 (25.68) | 0.302 | 19.33 |
NA: Neovascular area; VC: Vessel caliber; IA: Invasion area. 1Student's t-test; NA and IA are a fraction (%) of the total cornea area; VC expressed as a score provided by the software.
x±s
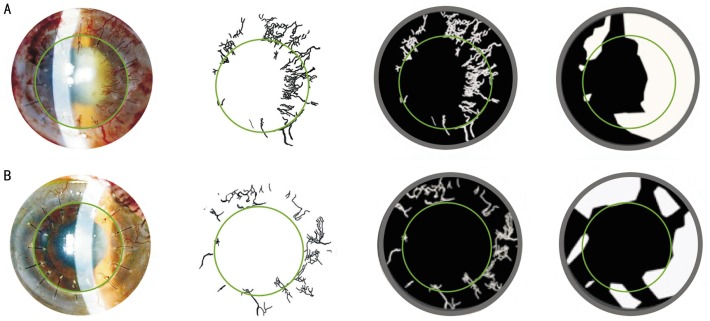
Figure 3. Evaluation of corneal neovascularization parameters using our method.
An image from patient 1. From left to right, the digital image is being processed with filters, selection tools, and layers. The black background indicates the cornea; the white lines indicate the vessels; the inner green circle indicates the corneal graft/host interface; and the outer grey circle indicates the limbus. Before (A) and after (B) limbal epithelial stem cell therapy (at 1y of follow-up).
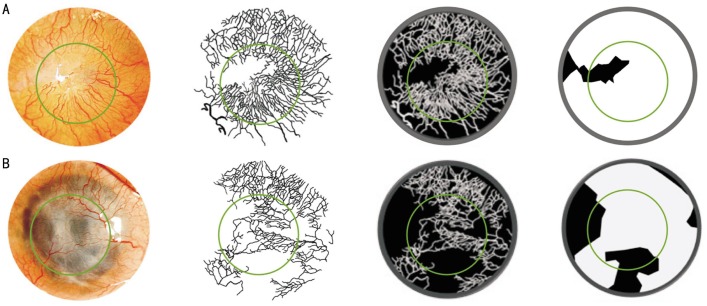
Figure 4. Evaluation of corneal neovascularization parameters using our method.
An image from patient 2. From left to right, the digital image is being processed with filters, selection tools, and layers. The black background indicates the cornea; the white lines indicate the vessels; the inner green circle indicates the corneal graft/host interface; and the outer grey circle indicates the limbus. Before (A) and after (B) limbal epithelial stem cell therapy (at 1y of follow-up).
DISCUSSION
Adult stem cells hold promise for many human diseases in regenerative medicine. The major obstacle of the procedure is obtaining an adequate number of stem cells from the donor tissue. This has been addressed on the ocular surface by ex vivo expansion. Over the past 15y, case series and studies have reported promising results[10]; however, due to variations within and between studies, it is often difficult to make an objective assessment. Many studies have used VA as the main outcome measure to define a successful or failed treatment[6]. Using amniotic membrane as the carrier of limbal epithelial stem cells provides an anti-inflammatory effect[11] and, at least initially, VA might improve because a smoother ocular surface is obtained immediately after surgery, once the fibrovascular tissue is removed by superficial keratectomy. In the current case series, even failed cases had improved VA, so using VA as a measure of success is inadequate in this pathology. Impression cytology is the definitive diagnostic method, besides biopsy, to investigate limbal epithelial stem cell deficiency[12]. Although impression cytology is a simple minimally invasive technique, it has some drawbacks. To increase the number of harvested cell, the ocular surface is usually dried by keeping the eye open before sampling, which might damage the epithelium. There also is a risk of conjunctival cell contamination and misinterpretation of the peripheral rim of conjunctival cells as initial limbal epithelial stem cell deficiency because of the presence of goblet cells. Immunocytochemistry performed to identify CK19-negative and CK3-positive cells on corneal impression cytology is a precise method to investigate limbal epithelial stem cell deficiency, but a good sample with high cellularity is required[7].
Some authors have already reported corneal neovascularization as an outcome measure in limbal epithelial stem cell therapy[13],[14]. In those studies, the corneal vessels were monitored photographically and graded according to extent and intensity. Our modified method of quantification of corneal neovascularization is helped by an automated image analysis process, resulting in a quantitative measurement (score) that can be used easily to statistically compare results within a cohort and between different studies. Similar quantitative techniques have been used previously in animal models with different image acquisition methods, but they were difficult to integrate into a routine follow-up protocol[15],[16]. We consider the current method to be useful during a regular clinical examination using slit-lamp biomicroscopy, where digital pictures can be obtained easily. With high-quality photographs, NA and VCs can be measured with modern image analysis software.
Maintaining corneal avascularity, which is important for the proper optical performance of the cornea, is a complex process under homeostatic conditions and in wound healing. Three clinical entities of corneal neovascularization can be discerned: first, deep corneal neovascularization overlying Descemet's membrane seen in herpetic and luetic interstitial keratitis; second, stromal corneal neovascularization mainly associated with most forms of stromal keratitis; and third, vascular pannus comprised of connective tissue proliferating in the superficial corneal periphery and mainly associated with ocular surface disorders. Experimental studies have suggested an antiangiogenic role of the corneal epithelium[17]. The ability of the stem cells to replenish the corneal epithelium allows the perilimbal zone to prevent invasion of conjunctival epithelium onto the corneal surface, thereby preventing vascular pannus from proliferating under normal circumstances. Because corneal vascularization is the earliest sign of failed limbal epithelial stem cell transplantation, we think its quantification is useful for evaluating the limbal health[18]. The ingrowth of new blood vessels into the cornea can lead to tissue scarring, edema, lipid deposition, and persistent inflammation independent of the underlying disease, leading to corneal neovascularization, and, therefore, the new blood vessels might be direct and indirect risk factors for substantial visual loss, although corneal neovascularization outside the optic zone might still allow good visual function. For this reason, we think VA is not a good outcome measure to be used alone in the follow-up of this patients.
In the current study, the results were similar to those already published[6],[19]. Rama et al[20] reported a case series of 107 eyes with a mean follow-up of 35mo (range: 12-120mo); the 120mo follow-up in one case is the longest reported. The current case series had a small sample of patients with a mean follow-up of 76mo (range: 36-100mo) which is, to the best of our knowledge, the longest mean follow-up published to date; every patient had at least 36mo of follow-up. Initially, all of the current patients responded to limbal epithelial stem cell therapy; however, three cases failed within the first postoperative year. Complete turnover of the corneal epithelium is thought to take 9 to 12mo and at 1y of follow-up our results were definitive[21]. For these reasons, we used 1y of follow-up examination as the time to compare the results of corneal neovascularization.
This current study had limitations. First, our method still requires a subjective step to measure the vessels. Working with two-dimensional pictures can lead to confusion about the plane where the vessels actually are, making it difficult to discern the type of corneal neovascularization: deep, stromal, or superficial[10]. However, only superficial corneal neovascularization was evaluated in our cases, because deep or stromal corneal neovascularization occurs infrequently in limbal epithelial stem cell deficiency. Second, some artifacts such as a light reflex in the picture or reddish tissue such as brown-pigmented iris can obscure corneal vessels. The observer who analyses the photographs might need to evaluate more than one picture of the same eye obtained during the same visit to dispel any doubts. This is both a difficult step to reproduce and time consuming. We realized that it is impractical and not time efficient to manually trace the corneal vessels in every follow-up photograph acquired. However, this is the first study to evaluate the corneal neovascularization characteristics to determine the evolution of limbal epithelial stem cell therapy. Finally, the current case series had a small number of patients, and we think this is the main reason of not achieving statistically significant differences in our work. We think this method should be evaluated in a larger study to reach statistical significance in every measurement compared. Despite these limitations, we think that our method for analyzing corneal neovascularization after limbal epithelial stem cell therapy may provide a valuable tool to identify the progression of these patients, especially if new automated software exists to analyze the superficial vessels. Performing invasive methods such as impression cytology would be less frequently required.
Acknowledgments
Conflicts of Interest: Guarnieri A, None; Moreno-Montañés J, None; Alfonso-Bartolozzi B, None; Sabater AL, None; García-Guzmán M, None; Andreu EJ, None; Prosper F, None.
REFERENCES
- 1.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1(2):110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010;117(6):1207–1213. doi: 10.1016/j.ophtha.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 5.Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, Tuft SJ, Daniels JT. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112(4):993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 7.Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22(6):533–538. doi: 10.1097/00003226-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72(9):1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R, 2nd, Pavan-Langston D, Dana R. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127(4):381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Kinoshita S. New hopes and strategies for the treatment of severe ocular surface disease. Curr Opin Ophthalmol. 2011;22(4):274–278. doi: 10.1097/ICU.0b013e3283477d4d. [DOI] [PubMed] [Google Scholar]
- 11.Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 12.Calonge M, Diebold Y, Sáez V, Enríquez de Salamanca A, García-Vázquez C, Corrales RM, Herreras JM. Impression cytology of the ocular surface: a review. Exp Eye Res. 2004;78(3):457–472. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.IInatomi T, Nakamura T, Koizumi N, Sotozono C, Yokoi N, Kinoshita S. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141(2):267–275. doi: 10.1016/j.ajo.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Sheha H, Fu Y, Giegengack M, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011;152(5):739–747. doi: 10.1016/j.ajo.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Bock F, Onderka J, Hos D, Horn F, Martus P, Cursiefen C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res. 2008;87(5):462–470. doi: 10.1016/j.exer.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Bachmann B, Taylor RS, Cursiefen C. The association between corneal neovascularization and visual acuity: a systematic review. Acta Ophthalmol. 2013;91(1):12–19. doi: 10.1111/j.1755-3768.2011.02312.x. [DOI] [PubMed] [Google Scholar]
- 17.Abdiu O, Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008;40(1):16–18. doi: 10.1159/000111153. [DOI] [PubMed] [Google Scholar]
- 18.Cursiefen C, Colin J, Dana R, Diaz-Llopis M, Faraj LA, Garcia-Delpech S, Geerling G, Price FW, Remeijer L, Rouse BT, Seitz B, Udaondo P, Meller D, Dua H. Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol. 2012;96(1):3–9. doi: 10.1136/bjo.2011.204701. [DOI] [PubMed] [Google Scholar]
- 19.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. doi: 10.1159/000315020. [DOI] [PubMed] [Google Scholar]
- 20.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 21.Daya SM, Watson A, Sharpe JR, Giledi O, Rowe A, Martin R, James SE. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology. 2005;112(3):470–477. doi: 10.1016/j.ophtha.2004.09.023. [DOI] [PubMed] [Google Scholar]