Abstract
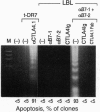
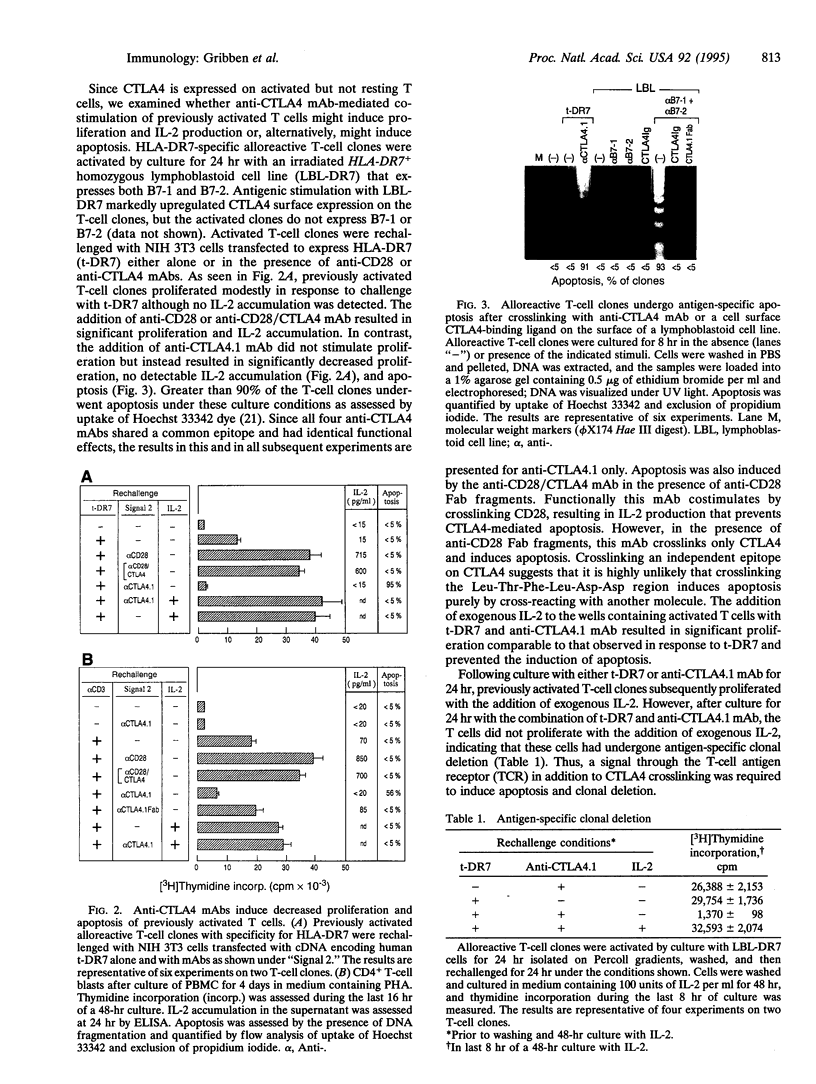
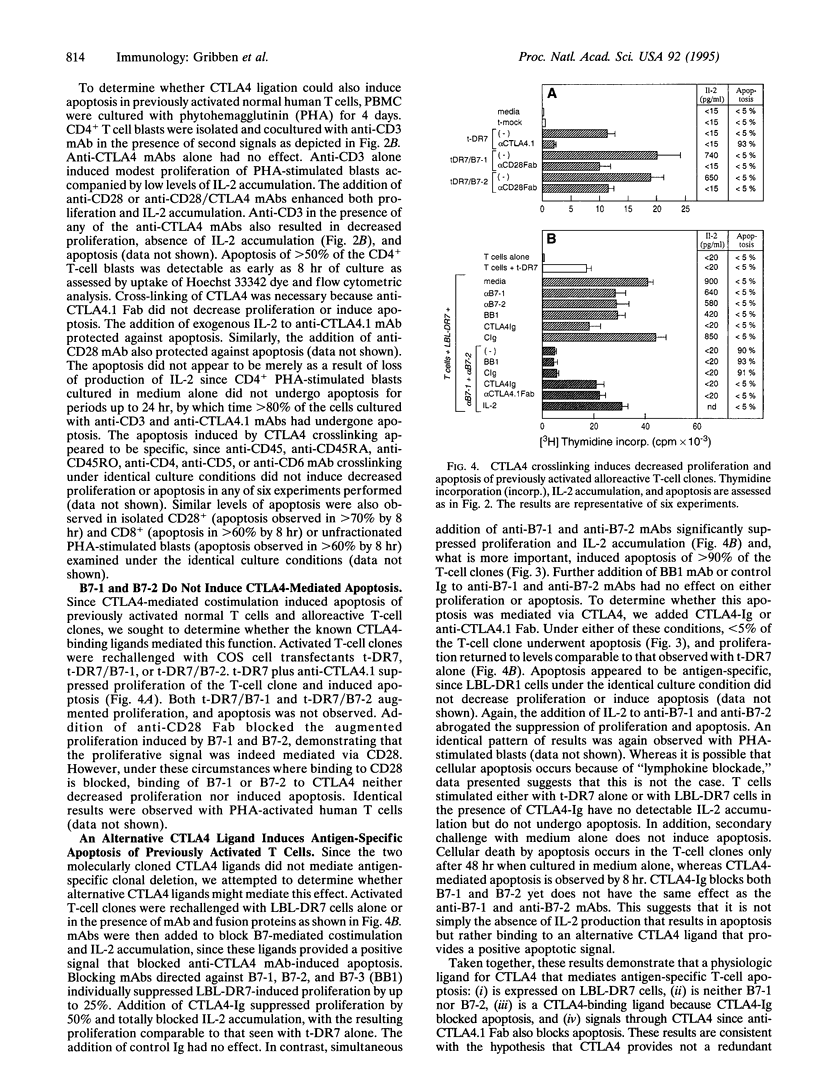
The regulation of T cell-mediated immune responses requires a balance between amplification and generation of effector function and subsequent selective termination by clonal deletion. Although apoptosis of previously activated T cells can be induced by signaling of the tumor necrosis factor receptor family, these molecules do not appear to regulate T-cell clonal deletion in an antigen-specific fashion. We demonstrate that cross-linking of the inducible T-cell surface molecule CTLA4 can mediate apoptosis of previously activated human T lymphocytes. This function appears to be antigen-restricted, since a concomitant signal T-cell receptor signal is required. Regulation of this pathway may provide a novel therapeutic strategy to delete antigen-specific activated T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Griffin J. D., Gray G. S., Gribben J. G., Nadler L. M. CD2 is involved in maintenance and reversal of human alloantigen-specific clonal anergy. J Exp Med. 1994 Nov 1;180(5):1665–1673. doi: 10.1084/jem.180.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Leytze G., Myrdal S., Aruffo A., Ledbetter J. A., Linsley P. S. Costimulation of T lymphocytes with integrin ligands intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 induces functional expression of CTLA-4, a second receptor for B7. J Immunol. 1994 Mar 15;152(6):2686–2697. [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Gray G., Nadler L. M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt M. S., Elias L. The type B receptor for tumor necrosis factor-alpha mediates DNA fragmentation in HL-60 and U937 cells and differentiation in HL-60 cells. Blood. 1992 Sep 1;80(5):1339–1346. [PubMed] [Google Scholar]
- Hardin J. A., Sherr D. H., DeMaria M., Lopez P. A. A simple fluorescence method for surface antigen phenotyping of lymphocytes undergoing DNA fragmentation. J Immunol Methods. 1992 Sep 18;154(1):99–107. doi: 10.1016/0022-1759(92)90217-h. [DOI] [PubMed] [Google Scholar]
- Harper K., Balzano C., Rouvier E., Mattéi M. G., Luciani M. F., Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991 Aug 1;147(3):1037–1044. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jellis C. L., Cradick T. J., Rennert P., Salinas P., Boyd J., Amirault T., Gray G. S. Defining critical residues in the epitope for a HIV-neutralizing monoclonal antibody using phage display and peptide array technologies. Gene. 1993 Dec 27;137(1):63–68. doi: 10.1016/0378-1119(93)90252-x. [DOI] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Lau L. L., Jamieson B. D., Somasundaram T., Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994 Jun 23;369(6482):648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Bradshaw J., Urnes M., Grosmaire L., Ledbetter J. A. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J Immunol. 1993 Apr 15;150(8 Pt 1):3161–3169. [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Tan P., Bradshaw J., Ledbetter J. A., Anasetti C., Damle N. K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992 Dec 1;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Russell J. H., White C. L., Loh D. Y., Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Gaur A., Chan P. Y., Springer T. A. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J Immunol. 1992 May 15;148(10):3271–3274. [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]