Abstract
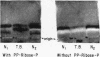
Among affected members of a family (B. family) with excessive purine production and gout, activity of phosphoribosylpyrophosphate synthetase (EC 2.7.6.1) is 2.5- to 3.0-fold higher than among normal people. The molecular basis for this increased enzyme activity was studied. Antibody to purified human phosphoribosylpyrophosphate synthetase of erythrocytes was obtained from immunized rabbits. Studies with the IgG fraction of this antiserum show the presence of normal quantities of immunoreactive enzyme, but 2.5- to 3.0-fold higher activity per molecule in affected members of the B. family. In addition, by use of a stain for phosphoribosylpyrophosphate synthetase activity, a difference in electrophoretic mobility was demonstrated on cellulose acetate gel between the partially purified enzyme from normal people and an affected member of the B. family. These studies suggest that the enzyme aberration responsible for purine overproduction and gout in the B. family results from a structurally altered enzyme with increased activity per molecule.
Keywords: structural mutation, genetic variation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. A., Meyer L. J., Seegmiller J. E. Gout with purine overproduction due to increased phosphoribosylpyrophosphate synthetase activity. Am J Med. 1973 Aug;55(2):232–242. doi: 10.1016/0002-9343(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Meyer L. J., Wood A. W., Seegmiller J. E. Purine overproduction in man associated with increased phosphoribosylpyrophosphate synthetase activity. Science. 1973 Mar 16;179(4078):1123–1126. doi: 10.1126/science.179.4078.1123. [DOI] [PubMed] [Google Scholar]
- DEWEY M. M., CONKLIN J. L. Starch gel electrophoresis of lactic dehydrogenase from rat kidney. Proc Soc Exp Biol Med. 1960 Dec;105:492–494. doi: 10.3181/00379727-105-26153. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971 Sep 25;246(18):5739–5748. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Phosphoribosylpyrophosphate in man: biochemical and clinical significance. Ann Intern Med. 1971 Mar;74(3):424–433. doi: 10.7326/0003-4819-74-3-424. [DOI] [PubMed] [Google Scholar]
- Greene M. L., Boyle J. A., Seegmiller J. E. Substrate stabilization: genetically controlled reciprocal relationship of two human enzymes. Science. 1970 Feb 6;167(3919):887–889. doi: 10.1126/science.167.3919.887. [DOI] [PubMed] [Google Scholar]
- Katzen H. M. The multiple forms of mammalian hexokinase and their significance to the action of insulin. Adv Enzyme Regul. 1967;5:335–356. doi: 10.1016/0065-2571(67)90025-8. [DOI] [PubMed] [Google Scholar]
- Pehlke D. M., McDonald J. A., Holmes E. W., Kelley W. N. Inosinic acid dehydrogenase activity in the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jun;51(6):1398–1404. doi: 10.1172/JCI106935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Sperling O., Boer P., Persky-Brosh S., Kanarek E., De Vries A. Altered kinetic property of erythrocyte phosphoribosylpsyrophosphate synthetase in excessive purine production. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):703–706. [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A. Amino acid substitution (histidine to tyrosine) in a glucose-6-phosphate dehydrogenase variant (G6PD Hektoen) associated with over-production. J Mol Biol. 1970 Sep 28;52(3):483–490. doi: 10.1016/0022-2836(70)90414-6. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Motulsky A. G. A pseudocholinesterase variant (E Cynthiana) associated with elevated plasma enzyme activity. Am J Hum Genet. 1969 Sep;21(5):486–498. [PMC free article] [PubMed] [Google Scholar]