Abstract
Background/Aim:
Phospholipase C epsilon 1 (PLCE1) plays a crucial role in carcinogenesis and progression of several types of cancers. A single nucleotide polymorphism (SNP, rs2274223) in PLCE1 has been identified as a novel susceptibility locus. The aim of the present study was to investigate the role of three potentially functional SNPs (rs2274223A > G, rs3765524C > T, and rs7922612C > T) of PLCE1 in gastric cancer patients from Kashmir Valley.
Patients and Methods:
The study was conducted in 108 GC cases and 195 healthy controls from Kashmir Valley. Genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism method. Data were statistically analyzed using χ2 test and logistic regression models. A P value of less than 0.05 was regarded as statistically significant.
Results:
The frequency of PLCE1 A2274223C3765524T7922612, G2274223C3765524T7922612, and G2274223T3765524C7922612 haplotypes were higher in patients compared with controls, conferred high risk for GC [odds ratio (OR) =6.29; P = 0.001; Pcorr = 0.003], (OR = 3.23; P = 0.011; Pcorr = 0.033), and (OR = 5.14; P = 0.011; Pcorr = 0.033), respectively. Smoking and salted tea are independent risk factors for GC, but we did not find any significant modulation of cancer risk by PLCE1 variants with smoking or excessive consumption of salted tea.
Conclusion:
These results suggest that variation in PLCE1 may be associated with GC risk in Kashmir Valley.
Keywords: Gastric cancer, Kashmir Valley, haplotype, PLCE1 and PCR-RFLP
Gastric cancer (GC) is one of the most common cancers worldwide, and it ranks as the second leading cause of cancer-related deaths after lung cancer. Almost two-thirds of GC cases and deaths occur in less developed regions.[1] The incidence of GC varies widely by country and population, with higher rates among the lower socioeconomic groups. Within the Indian subcontinent, the valley of Kashmir presents a strikingly different picture where the incidence of GC has been reported to exceed 40% of all cancers and incidence is 3-6 times higher than various metropolis cancer registries in India.[2,3] Some of the genetic and environment factors have been reported to be associated with an increased risk of GCs in Kashmir Valley.[4,5,6]
GC is a complex, multifactorial disease where there is a strong interplay between genetic and environmental factors. In the past few years, the wave of genome-wide association studies (GWASs) provided a more robust tool to find novel susceptibility loci or genes for cancer susceptibility. In 2010, two large-scale GWASs simultaneously reported that notable low penetrance susceptibility locus rs2274223 was strongly associated with the risk of esophageal squamous cell carcinoma (ESCC) and gastric cardia adenocarcinoma (GCA) in a Chinese population.[7,8] The PLCE1 gene, located on chromosome 10q23, is a member of the phospholipase family. PLCE1 encodes the phospholipase C epsilon 1 (PLCE1) that catalyzes the hydrolysis of phosphatidylinositol-4,5-bisphosphate into the secondary messengers inositol 1,4,5-trisphosphate and diacylglycerol (DAG), which participate in cell growth, differentiation, and gene expression.[9] Recent studies have reported that PLCE1 plays crucial roles in carcinogenesis and progression of several types of cancers, including cancers of esophagus, stomach, gallbladder, head and neck, and colorectum.[8,9,10,11,12,13,14,15] Interestingly, another study proved that rs2274223 was associated with a protective effect against colorectal cancer (CRC) in a Chinese population.[16] However, there are reports of null association of PLCE1 rs2274223 SNP with esophageal adenocarcinoma (EAC) or ESCC in Dutch and South African populations, respectively.[17,18] Now it appears that association of PLCE1 variants may be population specific. Therefore, the main aim of the present study was to investigate the role of PLCE1 (rs2274223A > G, rs3765524C > T, and rs7922612C > T) polymorphisms in the GC in Kashmir Valley.
PATIENTS AND METHODS
Population characteristics
Sample size calculations by QUANTO 1:1 showed that the study had <80% power with GC patients. This case–control study comprised histopathologically confirmed cases with GC (108) and healthy controls (195) from the population of Kashmir Valley. The sample size of the present study was adequate to provide 80% power. All subjects were unrelated ethnic Kashmiri residents, referred from the Departments of Gastroenterology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar from May 2006 to July 2008. Patients were excluded if they had nonmalignant conditions such as corrosive esophageal injury, Achalasia, Barrett's esophagus, gastroesophageal reflux disease (GERD), and nonulcer dyspepsia. The controls were also recruited from Sher-i-Kashmir Institute of Medical Sciences, Srinagar, who came for their routine checkup and were diagnosed as having non-severe ailment and no malignancy. All the individuals were personally interviewed for their age, occupation, demographic features, dietary habits (Haak = Brassica oleraceae; Wur = red chillies), usage of hot noon chai (salted tea), and smoking habits. Tobacco usage included smoking of cigarette or hukka (water pipe). Written informed consent was obtained from all participants in the study. The research protocol was approved by the ethical committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow (project number: 5/13/48/2002-NCDIII). Sample collection, storage, and transport, complied with guidelines of the committee. Blood samples were collected in ethylenediaminetetraacetic acid and the genomic DNA was extracted from peripheral blood leukocytes pellet using the standard salting-out method.[19] The quality and quantity of DNA was checked by gel electrophoresis and spectrophotometry using Nanodrop ND-1000 spectrophotometer (Thermo-Fisher, Inc., Wilmington, DE, USA). The ratio of absorbance at 260 and 280 nm of DNA was approximately 1.7-1.9. The isolated DNA was stored at −70°C.
Genotyping
The genotyping of all three PLCE1 gene polymorphisms were carried through polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The genotypes of PLCE1 genetic variants were assigned on the basis of band sizes. In case of PLCE1 rs2274223 A > G polymorphism, A allele showed a band of 242 bp and G allele yielded two fragments of 155 bp and 87 bp. PLCE1 C > T rs3765524 C allele resulted in 326 bp fragment and the variant allele, designated T allele resulted in 200 bp and 126 bp fragments. The wildtype C allele of PLCE1 C > T rs7922612 polymorphism on restriction digestion showed two fragments of 197 bp and 143 bp; however, the variant T allele was resistant to digestion and yielded a single fragment of 340 bp. More than 15% of the samples were randomly selected for confirmation, and the results were 100% concordant.
Statistical analysis
Demographic characteristics of patients and controls were described as frequencies and percentages, whereas descriptive statistics of patients and controls were presented as mean and standard deviations for continuous measures. Statistical significance of frequency differences between patients and control groups was evaluated using the χ2 test. Deviation from the Hardy–Weinberg equilibrium in controls was assessed using the χ2 test; P value was considered significant at <0.05 level. The same controls were used for analyzing two sets of cancer cases. Association was expressed as odds ratios (OR) for risk estimation with 95% confidence intervals (95% CI). All analyses were performed using the SPSS statistical analysis software, version 15.0 (SPSS, Chicago, IL, USA). Construction of the PLCE1 haplotypes and frequency calculation were performed using the SNP Analyzer Version 1.0 (ISTECH Inc., York, PA, USA) by expectation–maximization algorithm. Also comparison of multiple corrections was done using Bonferroni corrections.
RESULTS
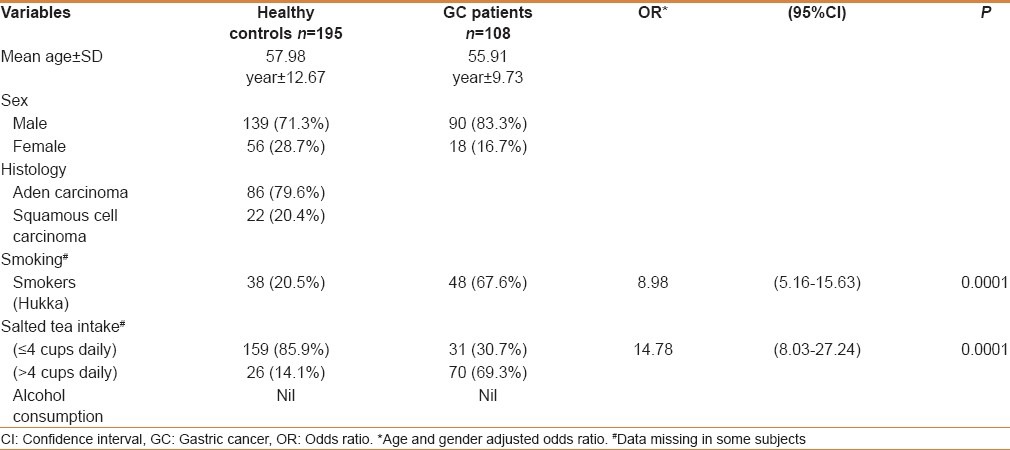
The mean age of healthy subjects (controls) and patients with GC was 57.98 years ± 12.67 and 55.91 years ± 9.73, respectively (t-test P = ns). Cancer was highly prevalent in males (83.3%) than in females. In patients with GC, most of the cases were with adenocarcinoma (ADC, 79.6%). Smoking habit (Hukka) showed significantly higher risk in GC (8.98; 95%CI = 5.16-15.63; P = 0.0001) patients. Individuals consumed salted-tea in a range of 2-8 cups per day; and median consumption of tea was 4 cups per day. So, we grouped individuals into ≤4 cups or >4 cups per day and individuals consuming salted tea >4 cups per day were regarded as high salted tea consumers. Higher consumption of salted tea was also found to be associated with increased risk of GC (OR = 14.78; 95%CI = 8.03-27.24; P = 0.0001) [Table 1]. None of the patients or controls reported consumption of alcohol, so interaction of alcohol intake with genetic variations could not be analyzed.
Table 1.
Demographic profile of study subjects
Association of PLCE1 polymorphism with susceptibility to GC
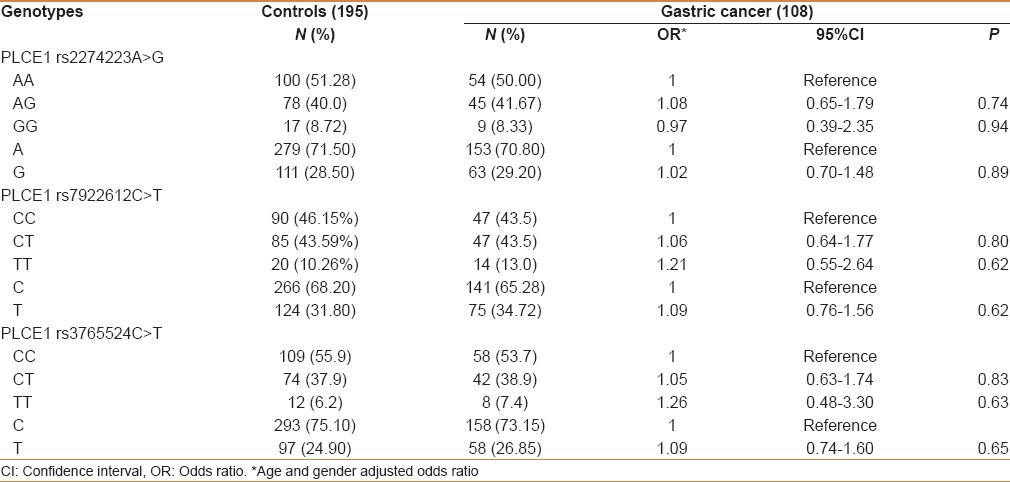
The genotype and allele distributions of three SNPs (rs2274223A > G, rs3765524C > T, and rs7922612C > T) in cases and controls are shown in Table 1. The observed genotype frequencies for these three polymorphisms were all in Hardy–Weinberg equilibrium in the controls (P = 0.74, 0.99, and 0.90, respectively). The single locus analyses revealed that genotype distributions of these three polymorphisms were not significantly different between overall cases and controls. Also at the allele level, we did not find any significant association [Table 2].
Table 2.
Overall frequency distribution of PLCE1 genotypes in gastric cancer patients and controls
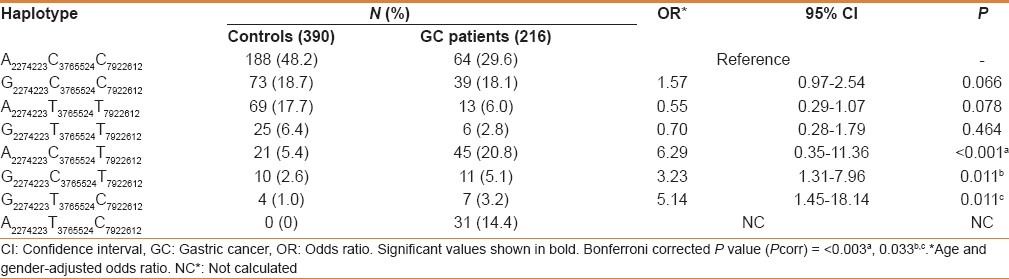
PLCE1 haplotype analysis and risk of GC
A total of eight haplotypes were observed in the study subjects. The frequency of A2274223C3765524T7922612, G2274223C3765524T7922612, and G2274223T3765524C7922612 haplotype was higher in patients as compared with controls, conferred high risk for GC (OR = 6.29; 95%CI = 0.35–11.36; P = 0.001; Pcorr = 0.003), (OR = 3.23; 95%CI = 1.31–7.96; P = 0.011; Pcorr = 0.033), and (OR = 5.14; 95%CI = 1.45–18.14; P = 0.011; Pcorr = 0.033), respectively [Table 3].
Table 3.
Frequency distribution of PLCE1 haplotypes in GC patients and healthy controls
Association of PLCE1 genotypes with tumor histopathology and environmental factors such as smoking and salted tea
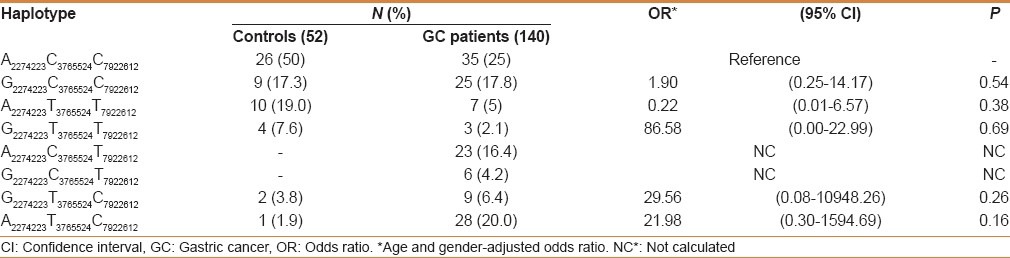
While analyzing the genotypes with histology (squamous cell carcinoma and adenocarcinoma), none of the PLCE1 genetic variants were associated with risk in GC (data not shown). We examined the possible interactions of PLCE1 (rs2274223, rs7922612, and rs3765524) genotypes with smoking and salted tea consumption and risk of GC in study subjects. Although both smoking and salted tea were independent risk factors for GC in the Kashmir Valley, but in gene–environment interaction we did not find any significant modulation of cancer risk by PLCE1 genotypes with smoking or excessive consumption of salted tea [Tables 4 and 5]. Also interaction of PLCE1 haplotypes with smoking and salted tea did not show any modulation of GC risk [Tables 6 and 7].
Table 4.
Interaction of PLCE1 genotypes and smoking in modulation of gastric cancer risk
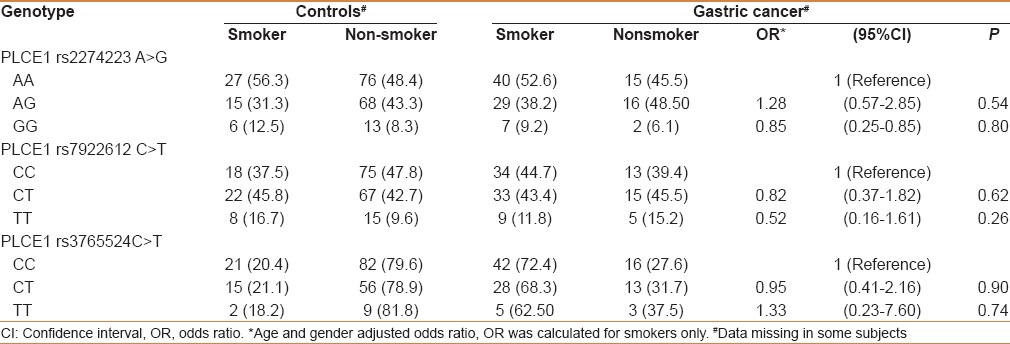
Table 5.
Interaction of PLCE1 genotypes and salted tea in modulation of gastric cancer risk
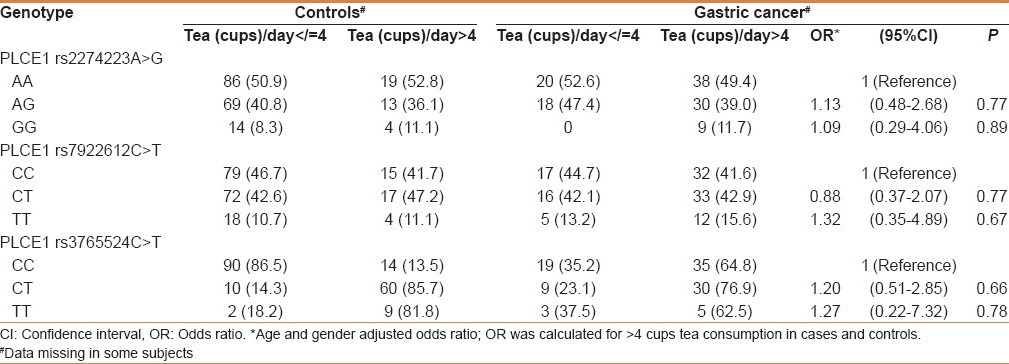
Table 6.
Interaction of PLCE1 haplotypes and smoking in modulation of GC risk
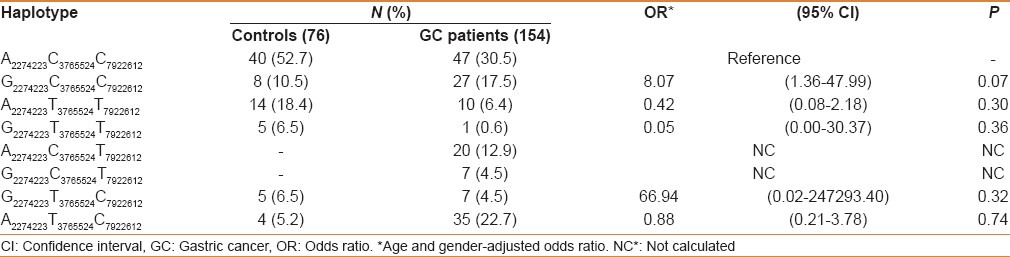
Table 7.
Interaction of PLCE1 haplotypes and salted tea in modulation of GC risk
DISCUSSION
In the present study, although PLCE1 genotypes did not independently modulate risk of GC; however, three PLCE1 A2274223C3765524T7922612, G2274223C3765524T7922612, and G2274223T3765524C7922612 haplotypes were found to be associated with significant increased risk of GC in Kashmiri population. Our findings for PLCE1 and the risk of GC are in agreement with some previous reports from Chinese[10,20] and Caucasian populations.[21] Recently two meta-analysis reports, especially among Asian populations also confirmed the association of PLCE1 with the risk of GC.[22,23]
The PLCE1 belongs to the phospholipase family that catalyzes the hydrolysis of polyphosphoinositides to generate secondary messengers, such as inositol-1,4,5-trisphosphate and diacylglycerol. Therefore, PLCE1 is involved in cell growth and differentiation.[24] Epidemiological studies have shown that PLCE1 functions as an effector of Ras and is a major factor in progression of various cancers such as intestine,[25] skin,[26] bladder,[27] colorectal,[28] and head and neck.[29] These findings suggest that single nucleotide polymorphisms (SNPs) in PLCE1, may affect the risk of some cancers due to their effects on gene expression or protein function.
In colorectal cancer (CRC), PLCE1 expression is significantly downregulated compared with the normal colonic mucosa, with increasing suppression of PLCE1 correlated with advancing tumor stage.[30] In a CRC cell line, overexpression of PLCE1 was found to inhibit cell proliferation and promote apoptosis.[31] In the APC min/+ mouse, knockout of PLCE1 was associated with resistance to tumor development through attenuation of angiogenesis and tumor-associated inflammation.[32] Similar pro-inflammatory actions have been observed in skin carcinogenesis.[26] These findings suggest that PLCE1 functions as a tumor suppressor gene; however, tumor-promoting effects have also been reported for PLCE1.
In addition to possible exposure to well-known risk factors (such as smoking and salted tea) for GCs, people of the valley have many unique social, cultural, and dietary features, which are different from the rest of the world. Salted tea used by people is prepared by using baking soda (sodium bicarbonate) and common salt (sodium chloride) and boiled for few hours before consuming. It has been suspected that the salts might cause thermal injury to gastric epithelium.[2] Several previous studies have attributed high incidence of GCs in Kashmir to considerable amount of nitroso compounds in raw foodstuffs and use of hot salted tea.[2,3] Salted tea used in Kashmir Valley has considerable amounts of N-nitrosoproline (NPRO) (360 μg/kg) and N-nitrosopipecolic acid (5870 μg/kg), which may impart risk for GC in this area.[4] In the present study, salted tea was significantly associated with increased high risk for GC (OR = 8.89; P = 0.0001). Also our results show significant independent association of smoking (Hukka) with GC (OR = 14.78; P = 0.0001). However, modulation of GC risk due to these environmental factors was not noticed in the present study.
CONCLUSION
Our study suggests that PLCE1 haplotypes are better predictors of susceptibility to GC in Kashmir Valley. However, due to the small sample size, there is a need to perform similar studies in a larger number of samples.
ACKNOWLEDGMENT
The study was supported by a fellowship grant from Indian Council of Medical Research, New Delhi.
Footnotes
Source of Support: The study was supported by a fellowship grant from Indian Council of Medical Research, New Delhi
Conflict of Interest: Authors declared that there is no conflict of interest.
REFERENCES
- 1.Wang YC, Wei LJ, Liu JT, Li SX, Wang QS. Comparison of Cancer Incidence between China and the USA. Cancer Biol Med. 2012;9:128–32. doi: 10.3969/j.issn.2095-3941.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khuroo MS, Zargar SA, Mahajan R, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut. 1992;33:11–5. doi: 10.1136/gut.33.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqi M, Kumar R, Fazili Z, Spiegelhalder B, Preussmann R. Increased exposure to dietary amines and nitrate in a population at high risk of oesophageal and gastric cancer in Kashmir (India) Carcinogenesis. 1992;13:1331–5. doi: 10.1093/carcin/13.8.1331. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi M, Tricker AR, Preussmann R. The occurrence of preformed N-nitroso compounds in food samples from a high risk area of esophageal cancer in Kashmir, India. Cancer Lett. 1988;39:37–43. doi: 10.1016/0304-3835(88)90038-9. [DOI] [PubMed] [Google Scholar]
- 5.Malik MA, Upadhyay R, Mittal RD, Zargar SA, Modi DR, Mittal B. Role of xenobiotic-metabolizing enzyme gene polymorphisms and interactions with environmental factors in susceptibility to gastric cancer in Kashmir Valley. J Gastrointest Cancer. 2009;40:26–32. doi: 10.1007/s12029-009-9072-0. [DOI] [PubMed] [Google Scholar]
- 6.Malik MA, Sharma KL, Zargar SA, Mittal B. Association of matrix metalloproteinase-7 (-181A>G) polymorphism with risk of esophageal squamous cell carcinoma in Kashmir Valley. Saudi J Gastroenterol. 2011;17:301–6. doi: 10.4103/1319-3767.84480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–7. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–63. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 9.Bunney TD, Baxendale RW, Katan M. Regulatory links between PLC enzymes and Ras superfamily GTPases: Signalling via PLCepsilon. Adv Enzyme Regul. 2009;49:54–8. doi: 10.1016/j.advenzreg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Jin G, Li H, Ren C, Ding Y, Zhang Q, et al. Genetic variants at 1q22 and 10q23 reproducibly associated with gastric cancer susceptibility in a Chinese population. Carcinogenesis. 2011;32:848–52. doi: 10.1093/carcin/bgr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma KL, Umar M, Pandey M, Misra S, Kumar A, Kumar V, et al. Association of potentially functional genetic variants of PLCE1 with gallbladder cancer susceptibility in north Indian population. J Gastrointest Cancer. 2013;44:436–43. doi: 10.1007/s12029-013-9537-z. [DOI] [PubMed] [Google Scholar]
- 12.Ma H, Wang LE, Liu Z, Sturgis EM, Wei Q. Association between novel PLCE1 variants identified in published esophagealcancer genome-ide association studies and risk of squamous cell carcinoma of the head and neck. BMC Cancer. 2011;11:258. doi: 10.1186/1471-2407-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Z, Yuan H, Ma H, Chu M, Wang Y, Hu Z, et al. Genetic variants at 10q23 are associated with risk of head and neck cancer in a Chinese population. Oral Oncol. 2013;49:332–5. doi: 10.1016/j.oraloncology.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Zhou C, Qiu G, Yang Y, Yan D, Xing T, et al. Phospholipase C epsilon plays a suppressive role in incidence of colorectal cancer. Med Oncol. 2012;29:1051–8. doi: 10.1007/s12032-011-9981-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Chen P, Chen D, Liu F, Pan W. Association between phospholipase C epsilon gene (PLCE1) polymorphism and colorectal cancerrisk in a Chinese population. J Int Med Res. 2014;42:270–81. doi: 10.1177/0300060513492484. [DOI] [PubMed] [Google Scholar]
- 16.Li FX, Yang XX, He XQ, Hu NY, Wu YS, Li M. Association of 10q23 with colorectal cancer in a Chinese population. Mol Biol Rep. 2012;39:9557–62. doi: 10.1007/s11033-012-1820-8. [DOI] [PubMed] [Google Scholar]
- 17.Dura P, Bregitha CV, Te Morsche RH, Roelofs HM, Kristinsson JO, Wobbes T, et al. GWAS-uncovered SNPs in PLCE1 and RFT2 genes are not implicated in Dutch esophageal adenocarcinoma and squamous cell carcinoma etiology. Eur J Cancer Prev. 2013;22:417–9. doi: 10.1097/CEJ.0b013e32835c7f53. [DOI] [PubMed] [Google Scholar]
- 18.Bye H, Prescott NJ, Lewis CM, Matejcic M, Moodley L, Robertson B, et al. Distinct genetic association at the PLCE1 locus with oesophageal squamous cell carcinoma in the South African population. Carcinogenesis. 2012;33:2155–61. doi: 10.1093/carcin/bgs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1998;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–7. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer AJ, Lochhead P, Hold GL, Rabkin CS, Chow WH, Lissowska J, et al. Genetic variation in C20orf54, PLCE1 and MUC1 and the risk of upper gastrointestinal cancers in Caucasian populations. Eur J Cancer Prev. 2012;21:541–4. doi: 10.1097/CEJ.0b013e3283529b79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang Y, Gu D, Cao C, Zhang Q, Xu Z, et al. Increased risk of developing digestive tract cancer in subjects carrying the PLCE1 rs2274223 A>G polymorphism: Evidence from a meta-analysis. PLoS One. 2013;8:e76425. doi: 10.1371/journal.pone.0076425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mai R, Cheng Y, Huang Y, Zhang G. Esophageal squamous cell carcinoma and gastric cardia adenocarcinoma shared susceptibility locus in PLCE1: A meta-analysis. PLoS One. 2013;8:e69214. doi: 10.1371/journal.pone.0069214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wing MR, Bourdon DM, Harden TK. PLC-epsilon: A shared effector protein in Ras-, Rho-, and G alpha beta gamma-mediated signaling. Mol Interv. 2003;3:273–80. doi: 10.1124/mi.3.5.273. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Edamatsu H, Kitazawa R, Kitazawa S, Kataoka T. Phospholipase Cepsilon promotes intestinal tumorigenesis of Apc (Min/+) mice through augmentation of inflammation and angiogenesis. Carcinogenesis. 2009;30:1424–32. doi: 10.1093/carcin/bgp125. [DOI] [PubMed] [Google Scholar]
- 26.Bai Y, Edamatsu H, Maeda S, Saito H, Suzuki N, Satoh T, et al. Crucial role of phospholipase Cepsilon in chemical carcinogen-induced skin tumor development. Cancer Res. 2004;64:8808–10. doi: 10.1158/0008-5472.CAN-04-3143. [DOI] [PubMed] [Google Scholar]
- 27.Ou L, Guo Y, Luo C, Wu X, Zhao Y, Cai X. RNA interference suppressing PLCE1 gene expression decreases invasive power of human bladder cancer T24 cell line. Cancer Genet Cytogenet. 2010;200:110–9. doi: 10.1016/j.cancergencyto.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Zbou C, Qiu G, Fan J, Tang H, Peng Z. Screening of new tumor suppressor genes in sporadic colorectal cancer patients. Hepatogastroenterology. 2008;55:2039–44. [PubMed] [Google Scholar]
- 29.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–40. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 30.Danielsen SA, Cekaite L, Ågesen TH, Sveen A, Nesbakken A, Thiis-Evensen E, et al. Phospholipase C isozymes are deregulated in colorectal cancer--insights gained from gene set enrichment analysis of the transcriptome. PLoS One. 2011;6:e24419. doi: 10.1371/journal.pone.0024419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhou C, Qiu G, Yang Y, Yan D, Xing T, et al. Phospholipase C epsilon plays a suppressive role in incidence of colorectal cancer. Med Oncol. 2012;29:1051–8. doi: 10.1007/s12032-011-9981-1. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Edamatsu H, Kitazawa R, Kitazawa S, Kataoka T. Phospholipase Cepsilon promotes intestinal tumorigenesis of Apc (Min/+) mice through augmentation of inflammation and angiogenesis. Carcinogenesis. 2009;30:1424–32. doi: 10.1093/carcin/bgp125. [DOI] [PubMed] [Google Scholar]


