Abstract
Backgrounds/Aims:
To study the efficacy and safety of drotaverine hydrochloride (HCl) 80 mg tablet given thrice a day in the symptomatic relief of patients with irritable bowel syndrome (IBS).
Patients and Methods:
The study was a multicentric, randomized, double-blind, placebo-controlled parallel group study performed at three centers. The patients who fulfilled Rome II Criteria of IBS were included in the study. A total of 180 patients with IBS were randomized to drotaverine and placebo treatment groups. Abdominal pain and stool frequency were measured every week in both the groups for all the 4 weeks of treatment duration. Subject Global Assessment of Relief (SGA) of IBS symptoms was assessed at the end of the study. Appropriate statistical analysis was done using SPSS software.
Statistical Analysis Used:
Mann–Whitney U-test (two-tailed), Wilcoxon signed ranks test, and McNemar tests.
Results:
Pain frequency decreased significantly (P < 0.01) in 22 (25.9%), 51 (60%), and 66 (77.7%) patients in the drotaverine group, at the end of 2nd, 3rd, and 4th weeks, respectively, as compared with 8 (9.4%), 18 (21.2%), and 26 (30.6%) in the placebo group. Pain severity scores also decreased significantly in the drotaverine group 66 (77.7%) as compared with placebo 26 (30.6%) after 4 weeks. Drotaverine HCl was shown to provide significant improvement (P < 0.01) in global relief in abdominal pain as perceived by the patient (85.9% vs 39.5%) and the clinician (82.4% vs 36.5%) in the drotaverine group as compared with placebo. There is significant (P < 0.01) improvement in stool frequency in drotaverine HCl treatment group as compared with placebo. The drug is well tolerated without any major side effects.
Conclusions:
A 4-week treatment with drotaverine significantly improves abdominal symptoms in patients with IBS.
Keywords: Drotaverine, irritable bowel syndrome, Subject global assessment of relief
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by abdominal pain and altered bowel habits in the absence of specific and unique organic pathology. Studies in Asia estimate a prevalence of 3.7%–22% using Rome II diagnostic criteria.[1,2,3] However, the data reported from developed countries such as United States estimate the prevalence of IBS at 10%–20% and the incidence of IBS at 1%–2% per year. The incidence is markedly different among countries depending on the race, food habits, diagnostic criteria used, and so on. Of people with IBS, approximately 10%–20% seek medical care.[4]
The most common symptoms of IBS are abdominal pain or discomfort often reported as cramping, bloating, gas, diarrhea, and/or constipation. Four bowel patterns may be seen with IBS. These patterns include IBS-D (diarrhea predominant), IBS-C (constipation predominant), IBS-M (mixed diarrhea and constipation), and IBS-A (alternating diarrhea and constipation). Abdominal pain is one of the most common reasons why people seek medical care and is the most bothersome symptom in patients of IBS.[5] A report by Indian Society of Gastroenterology Task Force revealed that as high as 70% of IBS patients have abdominal pain or discomfort significantly affecting the patient's quality of life.[6] Treatment for IBS may include medicine, stress relief, and changes in eating habits.[3] The cause of abdominal pain has traditionally been ascribed to smooth muscle spasm. Therefore, antispasmodics have been and remain the main stay of therapy, but clinical evidence supporting the use is limited. Most of the clinical trials done to assess their effectiveness are dated and only three trials have been conducted in the last 10 years. Most of these are small studies and are fraught with methodological flaws, including diagnostic criteria used, inclusion criteria used, dosing schedules, duration of therapy, and study end points used to assess the response. Antispasmodics, namely, hyoscyamine, dicyclomine, propantheline, and mebeverine are commonly used, but they are associated with lot of anticholenergic side effects, which restricts their use. Drotaverine, an antispasmodic, has a good relaxing effect on intestinal smooth muscle, which helps in alleviating pain and does not have side effects like anticholinergics.
There is paucity of data regarding uses of drotaverine in alleviating the pain and overall symptoms of IBS. Two abstracts[7,8] have shown beneficial effects in patients of IBS. The present study has been undertaken, to systematically evaluate the effects of drotaverine, with regards to relief of pain (frequency and severity), stool frequency and overall global improvement in patient's complaints. All the symptoms have been quantified using visual analog scale (VAS).
These data reveal that any therapy that relieves the abdominal pain or discomfort improves the patient's Quality of Life.
PATIENTS AND METHODS
A total of 180 patients from all the three centers, namely, Jaipur (S.M.S. Medical College), Allahabad (M.L.N. Medical College), and Delhi (St. Stephens Hospital) were randomized by the computer-generated randomization numbers. The concealment was ensured by keeping the results of number allotted in a sealed envelope, in the custody of Ethics committee of SMS Medical College, Jaipur, which was not opened till the end of the analysis of the study. Patients were assigned to drotaverine and placebo treatment groups.
Patients with symptoms indicative of IBS that met the Rome II diagnostic criteria for IBS were investigated. A stool test for ova and/or parasites, occult stool blood, blood test for full count, liver function tests (serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase) had to be in the normal range. The Exclusion Criteria were: pregnancy, age under 18 and over 80 years, any history of fever, passage of blood in stool, loss of weight in the recent past, any organic disease of the gastrointestinal tract, malignancy of any other organ, patient on any other concomitant medication for abdominal pain, bowel disturbance, or altering gastrointestinal motility.
The study was a randomized, double-blind, placebo-controlled study. Each patient admitted to the study was randomly given a tablet of drotaverine HCl (80 mg, Walter Bushnell Pvt Ltd) or placebo thrice a day, for 4 weeks. Patients were asked to visit every week. The visits were as follows:
Visit 0 (at enrolment), visit 1 (1 week after taking drug), visit 2 (2 weeks after treatment), visit 3 (3 weeks after treatment), visit 4 (4th week, i.e., end of study).
The compliance with medication was ensured by counting the number of tablets not used by the patient, before giving next set of drugs at each visit.
The parameters that were taken into consideration are as follows:
-
(I)
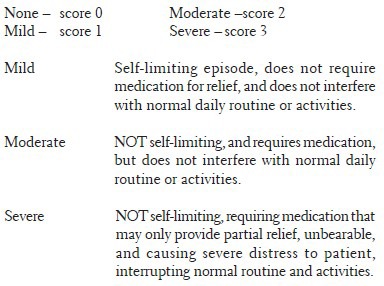
Severity of pain were recorded as: Pain severity score –
Visual analog scale
![]()
Note for VAS: The severity of the pain were asked by the investigator not in terms of severity (eg. mild, moderate, and severe) but were asked as 100 points or paise.
-
(II)
Stool frequency was recorded as follows:
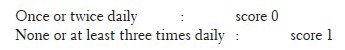
-
(III)
Overall clinical evaluation of response of therapy was scored both by patient and the clinician separately and independently:

Daily symptom data would be collected using patient diary during the treatment and follow-up periods. Physical examination and stool examination were done for each patient during the treatment period and follow-up visits.
A. Pain severity: (a) None – score 0; (b) Mild – score 1; (c) Moderate – score 2; (d) Severe – score 3. B. Stool frequency was recorded as follows: (a) Once or twice daily – score 0; (b) None or at least three times daily – score 1. C. Global assessment questionnaire: (a) Improved – score 2; (b) Same – score 1; (c) Worsened – score 0. The collection of symptoms was performed at entry and at the end of every week till the completion of 4 weeks of treatment.
The study was approved by the institutional ethical committee from all the three centers.
Statistical analysis
The Mann–Whitney U-test (two-tailed) was used to compare the changes in symptom score between drotaverine and the placebo groups at 2nd, 3rd, and 4th weeks. Wilcoxon signed ranks test and McNemar tests were used for paired data to test the changes in the symptoms score between baseline and 2nd, 3rd, and 4th weeks. A P ≤ 0.05 was considered statistically significant.
RESULTS
One hundred and eighty patients, who satisfied the inclusion criteria, were studied: 87 taking drotaverine and 93 taking a placebo. At the end of the study, two patients in the drotaverine group and eight patients in the placebo group did not return for the final examination. These patients were excluded from the study. The data from 170 patients, therefore, was available for comparison. Eighty-five patients were in the drotaverine group and 85 patients in the placebo group. The two groups were well balanced regarding the baseline symptoms in terms of the percentage of patients with the presence or absence of a specific symptom [Table 1].
Table 1.
Demographic data of patients in drotaverine and placebo groups
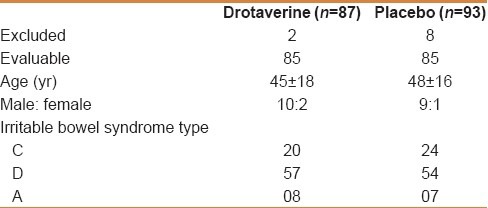
In IBS C group, the stool frequency was less than three stools per week (20% of patients had a stool frequency of one or two passages of hard stools per day). The constipation was not severe in these patients.
Ninety percent of the patients had undergone sigmoidoscopy to exclude any organic cause.
In none of the patients tissue diagnosis was required, as endoscopic examination showed normal mucosa.
Most of the patients used Psyllium two teaspoons per day, after night meal.
No other drug was used in all these patients for 4 weeks. In those patients with IBS D, use of loperamide 2 mg was allowed as rescue medication, 12 patients in treatment group and 26 patients in control group used loperamide 1–2 times during the 4-week study period.
The Mann–Whitney U-test used to compare the drotaverine group with the placebo group, showed a statistically significant (P < 0.01) lower pain frequency and severity scores at the end of 2nd, 3rd, and 4th weeks [Figure 1].
Figure 1.
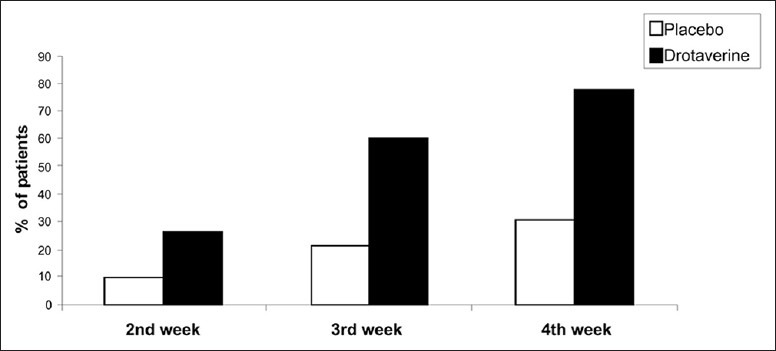
Relief in pain frequency and severity
Pain frequency decreased significantly (P < 0.01) in 22 (25.9%), 51 (60%), and 66 (77.7%) patients in the drotaverine group, at the end of 2nd, 3rd, and 4th week, respectively, as compared with 8 (9.4%), 18 (21.2%), and 26 (30.6%) in the placebo group. Pain severity scores also decreased significantly in the drotaverine group [66 (77.7%)] as compared with placebo [26 (30.6%)] after 4 weeks.
There was a statistically significant (P < 0.01) global relief in abdominal pain as perceived by the patient (85.9% vs 39.5%) and the clinician (82.4% vs 36.5%) in the drotaverine group as compared with placebo group [Figure 2].
Figure 2.
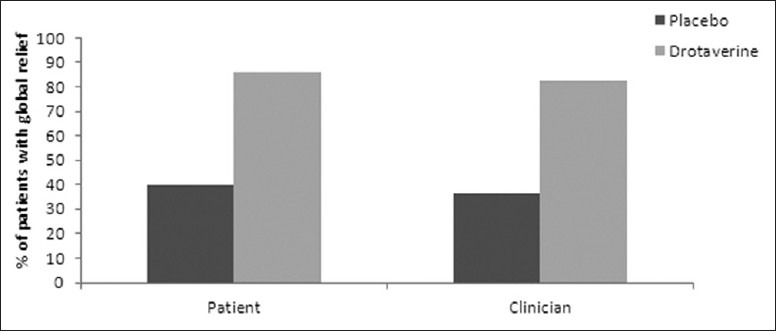
Global assessment of relief
Significant improvement in stool frequency also occurred in 26 (30.6%) and 38 (44.7%) patients in the drotaverine group at the end of 3rd and 4th weeks, respectively, as compared with decrease in 10 (11.8%) and 13 (15.3%) patients in the placebo group (P < 0.01) [Figure 3].
Figure 3.
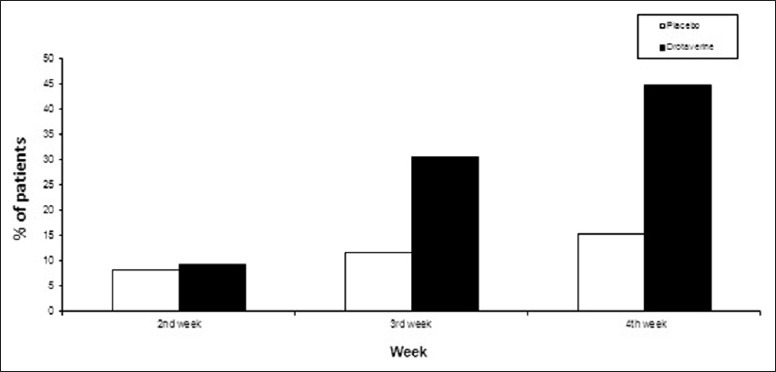
Improvement in stool frequency
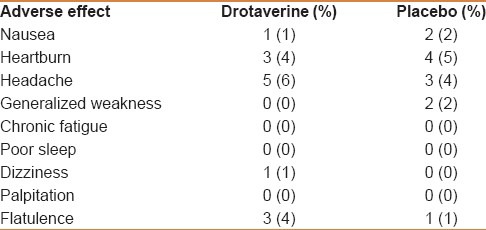
Four percent of the patients in the drotaverine group and 3% in the placebo group experienced mild adverse effects [Table 2], which did not warrant discontinuation of therapy.
Table 2.
Incidence of adverse events in drotaverine and placebo groups
DISCUSSION
IBS is a common condition, which occurs in approximately 11.5% of the population,[9] with a tendency toward youth and the female gender.[10] The condition generates a substantial workload in primary and secondary care. IBS has a considerable impact and causes reduced quality of life.[11] People with IBS are more likely to be unable to work and to have visited their doctor than the general population. Abdominal pain is one of the most common reasons why people seek medical care (after headaches, backaches, and dizziness).[12]
This study shows that, in patients with IBS, treatment with drotaverine HCl 80 mg given three times daily for 4 weeks is more effective than placebo in reducing abdominal symptoms related to IBS.
Because no objective markers for improvement of IBS exist, determination of efficacy of drotaverine is based on somewhat arbitrary rating scales. Changes in abdominal pain, bowel habits, and overall well-being are the main outcome measurements recommended in these studies. In our study, we used VAS for assessing the pain response to the treatment.
Several types of therapy are available for IBS treatment and include bulking agents, prokinetics, antispasmodics, 5-HT agonists and antagonists, smooth muscle relaxants, and antidepressants. However, most studies are hampered by poor methodology and inconclusive findings.[13] The absence of truly randomized placebo-controlled trials for many of these therapies has limited meaningful progress in this area.[14]
To quote Klein “Not a single study offers convincing evidence that any therapy is effective in treating the IBS symptom complex. The only method that can reliably evaluate IBS therapies is the randomized, double-blind, placebo-controlled treatment trial.”[15]
Out of all the clinical trials conducted to evaluate various treatment options, only smooth muscle relaxants consistently decreased abdominal pain, the most frequent and disabling symptom of the IBS.[16]
So far, only a few studies have shown the role of drotaverine on symptoms of IBS. In a preliminary study, Pap et al.[7] had shown the efficacy of this drug in IBS in a double-blind placebo-controlled trial. They found that the decrease of pain was 47% in the IBS group as compared with 3% in the placebo group. This study, however, did not clearly define the main outcome measures used and if there was any global benefit in pain of IBS. Mishra and colleagues[8] found that drotaverine decreased pain frequency and severity significantly in 31.4% and 71.4% of IBS patients as compared with placebo at the end of 2nd and 4th weeks, respectively. Our results were found to be in concordance with the previously reported findings (25.9%, 60%, and 77.7% at the end of 2nd, 3rd, and 4th weeks, respectively).
Two patients in the drotaverine group and seven patients in the placebo group left the study due to reasons not linked to the treatment. Four percent of patients in the drotaverine group and 3% of patients in the placebo group experienced mild adverse effects [Table 2]. These effects did not warrant discontinuation of therapy. Drotaverine was well tolerated and found to be safe.
In our study, the improvement in IBS symptoms observed in the group treated with drotaverine may be due to the relaxing effect of drotaverine on the intestinal smooth muscle obtained by the inhibition of PDE (Phosphodiesterase) inhibitory and Ca2+–calmodulin complex.[17] Furthermore, the antispasmodic effect of drotaverine could explain not only the reduced diarrhea present in the majority of our patients through a prolongation of orocecal transit time but also of the constipation present in the rest of the group. In fact, antispasmodics may decrease the functional obstruction caused by increased phasic colonic contractions that may be present in constipation.[18]
The role of a multidimensional therapeutic approach in the management of IBS is well recognized.[19] An effective physician–patient relationship, patient education, reassurance, and judicious dietary modification remain central to treatment. Pharmacologic treatment should be targeted at major symptoms, and treatment should be carefully monitored for adverse effects that can mitigate the benefit of an intervention. We found reasonable evidence to support the use of drotaverine for abdominal pain, which is a predominant and disabling symptom in patients with IBS. More needs to be known about IBS and how to effectively treat it. Focused and sustained research is absolutely crucial.
ACKNOWLEDGMENT
We acknowledge Walter Bushnell for providing the study medication, drotaverine tablet (Drotin DS). We also thank Dr. Seema Jaggi, Senior scientist, Indian Agricultural Statistics Research Institute for her valuable contribution in statistical applications.
Footnotes
Source of Support: Sponsored by Walter Bushnell, New Delhi.
Conflict of Interest: None declared.
REFERENCES
- 1.Rey E, Talley NJ. Irritable bowel syndrome: Novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–80. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Gwee KA, Wee S, Wong ML, Png DJ. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an Asian urban community. Am J Gastroenterol. 2004;99:924–31. doi: 10.1111/j.1572-0241.2004.04161.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman D, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, editors. 2nd ed. McLean, VA: Degnon Associates; 2000. Rome II: The functional gastrointestinal disorders: Diagnosis, pathophysiology and treatment: A multinational consensus. [Google Scholar]
- 4.Irritable Bowel Syndrome: EMedicine Gastroenterology. [Last updated on 2014 Apr 22]. Available from: http://emedicine.medscape.com/article/180389-overview .
- 5.Mangel AW, Northcutt AR. Review article: The safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13(Suppl 2):77–82. doi: 10.1046/j.1365-2036.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: Report of the Indian society of gastroenterology task force. Indian J Gastroenterol. 2008;27:22–8. [PubMed] [Google Scholar]
- 7.Pap A, Hamvas J, Filiczky I, Burai M, Szikszay E. Beneficial effect of drotaverine in irritable bowel syndrome (IBS) Gastroenterology. 1998;114:A818. [Google Scholar]
- 8.Misra SC, Pande R. Efficacy of drotaverine in irritable bowel syndrome: A double blind randomized, placebo controlled clinical trial. Am J Gastroenterol. 2000;95:2544. [Google Scholar]
- 9.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns, and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruigomez A, Wallander MA, Johansson S, Garcia Rodriguez LA. One-year follow-up of newly diagnosed irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:1097–102. doi: 10.1046/j.1365-2036.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 12.Samuels LA. Pharmacotherapy update: Hyoscine butylbromide in the treatment of abdominal spasms. Clin Med Ther. 2009;1:647–55. [Google Scholar]
- 13.Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta-analysis: The treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1253–69. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 14.Hussain Z, Quigley EM. Systematic review: Complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:465–71. doi: 10.1111/j.1365-2036.2006.02776.x. [DOI] [PubMed] [Google Scholar]
- 15.Klein KB. Controlled treatment trials in the irritable bowel syndrome: A critique. Gastroenterology. 1988;95:232–41. doi: 10.1016/0016-5085(88)90319-8. [DOI] [PubMed] [Google Scholar]
- 16.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–47. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 17.Blasko G. Pharmacology, mechanism of action and clinical significance of a convenient antispasmodic agent: Drotaverine. J Am Med Assoc Ind. Physicians’ Update. 1998;1:63–9. [Google Scholar]
- 18.Snape WJ., Jr Role of colonic motility in guiding therapy in patients with constipation. Dig Dis. 1997;15:104–11. doi: 10.1159/000171625. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA, Thompson WG. The irritable bowel syndrome: Review and a graduated multicomponent treatment approach. Ann Intern Med. 1992;116:1009–16. doi: 10.7326/0003-4819-116-12-1009. [DOI] [PubMed] [Google Scholar]


