Abstract
Frailty is a clinical syndrome associated with the aging process and adverse outcomes. The purpose of this short report was to initiate the development of a Frailty Index in 27- to 28-month-old C57BL/6 mice that matched the clinical criteria used in humans (weakness, slow walking speed, low activity level, poor endurance). The selected criteria included grip strength, walking speed, physical activity, and endurance. The criteria in mice were evaluated by the inverted-cling grip test, rotarod test, voluntary wheel running, and derived endurance scores. Each criterion had a designated cutoff point (1.5 SD below the cohort mean) to identify the mice with the lowest performance. If a mouse presented with three of the criteria scores below the cutoff points, it was identified as frail. Mild frailty was designated if two criteria were below the cutoff points. In this mouse cohort, one mouse was identified as frail and one was mildly frail. This prevalence of 9% frailty is consistent with the prevalence of frailty in humans at the same survival age. Collectively, our selected criterion, cutoff point, and Frailty Index provide a potential standardized definition for frailty in mice that is consistent with the operational definition of frailty in humans.
Key Words: Muscle, Frailty, Sarcopenia.
Frailty is a clinical syndrome associated with the aging process, increased vulnerability, decreased physical function, and adverse outcomes (mortality, disability, and hospitalization) (1–3). The prevalence of frailty in older populations (65 years or older) is about 9.9% (4), and this portion increases to 25%–50% in people aged 85 or older (3). Fried and colleagues developed a physical phenotype of frailty according to a combination of weakness, slowness, physical inactivity, increased subjective exhaustion, and weight loss, where frailty was defined as the presence of three or more of the criteria (1). This frailty definition was shown to be valid and a powerful predictor for adverse outcomes (1). Further studies report measurements of frailty using ‘Frailty Indices’ focused on clinical phenotype definitions of frailty or multiple deficits (5).
Besides the clinical studies discussed above, animal models are useful for the investigation of frailty and the mechanisms underlying frailty. Parks and coworkers proposed a Frailty Index in mice, using a well-established mouse model, C57BL/6. The Frailty Index was based on accumulated deficits calculated from 31 invasive and noninvasive measures (6). The invasive tests required anesthetizing the animal before procedures, including body composition (fat mass and lean mass) and basic metabolic status (level of electrolytes and blood components). The noninvasive assessments included the activity analyses: move distance duration and velocity. A frailty score was generated for each mouse in order to quantify the level of frailty, which was higher in the older mice and positively correlated to the age-related cardiac myocyte changes.
Although Park and coworkers recognized the importance of identifying frailty in rodents and provided a Frailty Index score, an index that is closely related to the clinically accepted frailty criteria and is simple to use (noninvasive) may facilitate frailty research in animal models. Moreover, the results of these animal studies would have potential to translate to clinical settings. Another advantage of a Frailty Index in mice lies in the definition of cutoff points for distinguishing between frail and nonfrail mice within each criterion. The cutoff definition provides standardized assessment that has the potential to be used in every research laboratory. As a result, the purpose of this short report is to initiate the development of a Frailty Index including the identification of four criteria of frailty, the definition of the cutoff points for each criterion, the designation of frailty, and the evaluation of the Frailty Index with old mice.
Specifically, in this short report, the Frailty Index matches the physical frailty indicators from the Fried and coworkers study, including weakness, slow walking speed, low activity level, and poor endurance (four noninvasive criteria), and each criterion has a designated cutoff point (1.5 SD below the mean of the cohort). If a mouse presents with three frailty criteria, it is identified as frail.
Methods
Animals
Eleven 27- to 28-month-old male C57BL/6 mice were purchased from the National Institute on Aging Colony and housed in specific pathogen-free facilities at the University of Minnesota. This age of mice was selected because this age represents 50% survival rate in this species (7). The mice were fed ad libitum and maintained on a 12-hour light/dark cycle at 20°C. The mice underwent an acclimation period for 7 days before any experimental procedures. All procedures were performed in agreement to the University of Minnesota’s guidelines on experimental animal research. Each mouse was designated a letter from A to K.
Frailty Criteria
In order to develop a Frailty Index, we identified four frailty criteria including grip strength, walking speed, physical activity, and endurance. These criteria were selected because they were similar to the human clinical criteria described by Fried and coworkers (Table 1).
Table 1.
Frailty Criteria
| Clinical Frailty Criteria* | Mouse Frailty Criteria | Approach |
|---|---|---|
| Weakness | Grip strength | Grip test (s) |
| Slowness | Walking speed | Rotarod test (revolution/min) |
| Low activity | Physical activity | Voluntary wheel running (km/d) |
| Poor endurance | Endurance | Grip test + rotarod (s) |
Note: Each of the four frailty criteria identified by *Fried and coworkers (2001) is matched to a criterion for the mouse with the corresponding experimental approach.
Criterion 1: grip strength.
The inverted-cling grip test was used for evaluating the grip strength of limbs and the endurance of muscles in the mouse (8). The cage-like device had a top lid with a wire grid and a padded bottom. After placing the mouse on the top lid, the lid was immediately closed, so that the mouse was inverted inside of the cage. The outcome measurement was the total time (seconds) the mouse could support itself before falling (latency to fall). Two trials were performed, with a 20-minute rest between trials. The final score (seconds) of grip strength was the average score of the two trials. In addition, this score was used for calculating the endurance score, as described in Criterion 4.
Criterion 2: walking speed.
The walking speed was evaluated using the rotarod test (Rota-Rod R/S; LSi Letica, Cornella, Spain). The rotarod test is widely used for determining overall motor function in mice (8,9). The rod cylinder rotates at a speed of 4rpm and accelerates to 40rpm over 5 minutes. The mouse is placed on the cylinder and the rotating speed (rpm) is recorded when the mouse falls. In the current study, the mice practiced on the device for 3 days (three trials per day of various protocols) before the actual testing procedure. On the testing day (the fourth day), three trials were performed with 20-minute rest between each trial. The last three trials were averaged, and the average was used as the final score of walking speed. The time the mouse remained on the rotarod before falling, latency to fall, was used for the endurance score, which will be discussed in Criterion 4.
Criterion 3: physical activity.
Voluntary wheel running was used for evaluating the daily physical activity level of each mouse. The Lafayette Activity Wheel Cages (model #80820F; Lafayette Instruments, Lafayette, IN) were used for the voluntary running procedure. The mice were individually housed in the wheel-running cages. Mice ran ad libitum in the wheel cages for 1 week. The running distance, in revolutions, was recorded daily at the same time (8–9 am). The unit of revolutions was converted into kilometers for data analysis (1 revolution = 0.4 m). The average daily running distance in the week was calculated (km/d) and used as the score of physical activity.
Criterion 4: endurance.
Both the grip test and the rotarod test were used to assess overall endurance (8). In this study, an endurance score was derived from the two tests. The time of the grip test was recorded as described in Criterion 1. The duration (latency to fall), in seconds, the mouse remained on the rotarod was recorded as outlined in Criterion 2. The score of endurance was calculated by determining the mean time of grip test and rotarod test: [Endurance score (seconds) = (Time of grip test + Time of rotarod test)/2].
Determination of Frailty Index
After evaluating and designating a score to each animal, the cohort mean and the SD were calculated for each criterion. Next, we determined a cutoff point at 1.5 SD below the cohort mean to identify the mice with the lowest performance. Frailty was defined if three or more of the criteria measures were below the cutoff point, whereas, mild frailty was designated if two fell below the cutoff. Table 2 summarizes the Frailty Index.
Table 2.
Determination of Frailty
| Cutoff Points of Each Criterion | Number of Criteria Below the Cutoff Point in Each Mouse | ||
|---|---|---|---|
| Primary (1.5 SD below the mean) |
≥3 | 2 | None |
| Degree of frailty | Frail | Mildly frail | Nonfrail |
Notes: The performance of each mouse is tested across the four criteria (described in Table 1) and designated a score for each criterion. For each criterion, a cutoff point, at 1.5 SD below the mean, was set to define frailty. A mouse was identified as frail if three or more of the criteria measures were below the cutoff point, whereas, mild frailty was designated if two fell below the cutoff.
Results
Scores of Criteria
Grip strength.
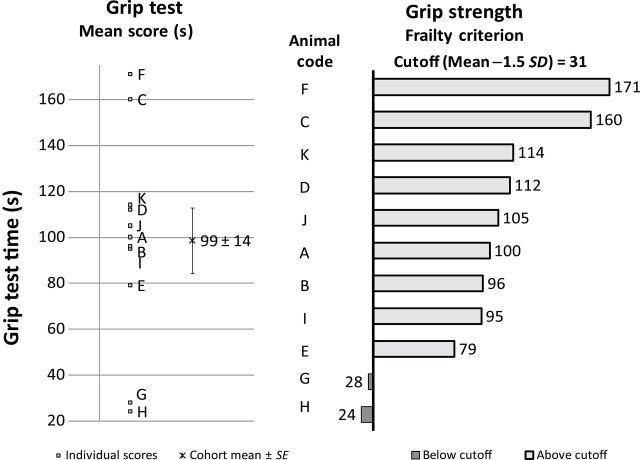
Grip test is a measurement that assesses strength and endurance (Figure 1). The cohort mean of the grip test was 99±14 seconds with a cutoff point at 31 seconds. Animal H (24 seconds) and G (28 seconds) were ranked below the 1.5 SD cutoff, which indicated weaker strength and endurance.
Figure 1.
Criterion 1: grip strength. Left: A–K were individual grip test scores, in seconds, for each animal. The cohort mean of the grip test was 99±14 seconds. Right: the frailty criterion of the grip strength. Animals A–K were ranked from high to low (top to the bottom). The cutoff point was 31 seconds, 1.5 SD below the mean. The grip strength scores of animal H (24 seconds) and G (28 seconds) were below the 1.5 SD cutoff point (dark bars), whereas the remaining animals were above the cutoff point (light bars).
Walking speed.
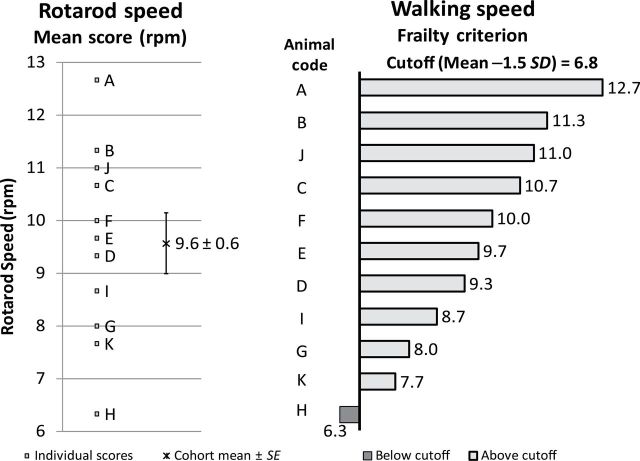
Rotarod test (speed) is a measurement that identifies the slowness in the mouse (Figure 2). The scores of the walking speed for the aged mice (n = 11) had a mean of 9.6±0.6rpm and a cutoff point of 6.8rpm. Animal H showed a slower walking speed at 6.3rpm, which was below the cutoff point.
Figure 2.
Criterion 2: walking speed. Left: A–K were individual rotarod speed, in rpm, for each animal. The cohort mean of the rotarod test was 9.6±0.6rpm. Right: the frailty criterion of walking speed. Animals A–K were ranked from high to low (top to the bottom). The cutoff point was 6.8rpm, 1.5 SD below the mean. The walking speed score of animal H (6.3rpm) was below the 1.5 SD cutoff point (dark bar), whereas for the remaining animals, it is above the cutoff point (light bars).
Physical activity.
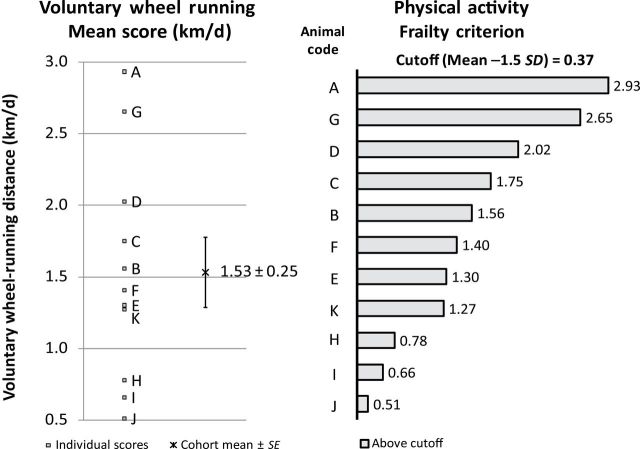
Voluntary wheel running assesses physical activity level (Figure 3). The cohort (11 mice) mean of the daily voluntary wheel-running distance for 1 week was 1.53±0.25 km/d. None of the 11 mice were below the cutoff point (0.37 km/d).
Figure 3.
Criterion 3: physical activity. Left: A–K were individual voluntary wheel-running distances, in km/d, for each animal. The cohort mean of the voluntary wheel running was 1.53±0.25 km/d. Right: the frailty criterion of physical activity. Animals A–K were ranked from high to low (top to the bottom). The cutoff point was 0.37 km/d, 1.5 SD below the mean. The physical activity score of each mouse within our cohort was above the cutoff point (light bars).
Endurance.
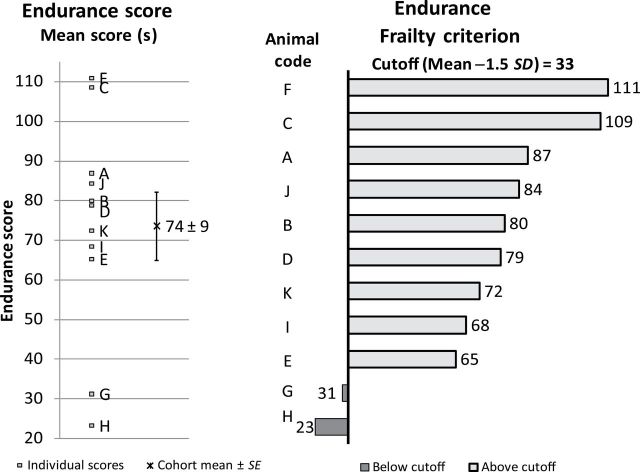
The endurance was determined from the mouse’s performance on the grip test and the rotarod test (Figure 4). The average endurance score in the cohort was 74±9 seconds. Animals H (23 seconds) and G (31 seconds) were identified with poor endurance because they were below the cutoff point (33 seconds).
Figure 4.
Criterion 4: endurance. Left: A–K were individual endurance scores, in seconds, for each mouse. The cohort mean of the endurance score was 74±9 seconds. Right: the frailty criterion of endurance score. Mice A–K were ranked from high to low (top to the bottom). The cutoff point was 33 seconds, 1.5 SD below the mean. The endurance scores of mouse H (23 seconds) and mouse G (31 seconds) were below the 1.5 SD cutoff point (dark bars), whereas the remaining mice were above the cutoff point (light bars).
Identification of frail mice.
Table 3 shows all the rankings under the four criteria in the 28-month-old male C57BL/6 mouse cohort. According to the identification of Frailty Index described previously, animal H was identified as a frail mouse (more than three criteria below the cutoff points). The mouse had overall physical weakness, including weaker grip strength, slower walking speed, and less endurance in comparison to the other mice in the cohort. Animal G was identified as a mildly frail mouse (two criteria below the cutoff; Table 2). The main weaknesses of animal G were strength and endurance.
Table 3.
Frailty Identification
| Criteria | Grip Strength (s) |
Walking Speed (rpm) | Physical Activity (km/d) | Endurance (s) |
|---|---|---|---|---|
| Ranked from high to low | F | A | A | F |
| C | B | G | C | |
| K | J | D | A | |
| D | C | C | J | |
| J | F | B | B | |
| A | E | F | D | |
| B | D | E | K | |
| I | I | K | I | |
| E | G | H | E | |
| G* | K | I | G* | |
| H* | H* | J | H* |
Notes: The animals were ranked, by the scores of each criterion, from high to low. Animals were coded A–K.
*The score of the animals that fell below the 1.5 SD of the cohort specific criterion mean. These animals were identified as frail or mildly frail based on the determination of frailty (Table 2). Specifically, animal H was frail with three criteria below the cutoff points (grip strength, walking speed, and endurance). Animal G was mildly frail with two criteria below the cutoff points (grip strength and endurance).
Discussion
The purpose of this short report was to initiate the development of a Frailty Index in mice that is closely related to the clinically accepted frailty criteria in humans. The Frailty Index was composed of four frailty criteria based on the clinical frailty indicators described by Fried and coworkers (1): weakness, slow walking speed, low activity level, and poor endurance. Frailty was defined in a mouse if three or more of the criteria measures were below the cutoff point, whereas, mild frailty was designated if two fell below the cutoff point. The cutoff point was set at 1.5 SD below the cohort mean of each criterion. Using this Frailty Index, one mouse was frail and one was mildly frail in the 27- to 28-month-old male C57BL/6 mouse cohort (n = 11).
To our knowledge, there is one published study that reports a Frailty Index in mice, and this index was based on the accumulative deficits model (6). In order to quantify frailty, Parks and coworkers determined health related variables associated with the function of vary physiological systems that change with age (muscle, bone, and cardiovascular). In particular, 31 invasive and noninvasive variables were measured including activity levels (distance moved, duration of movement, velocity, meander, and rearing frequency), body composition (weight, bone mass density, body mineral content, body surface area, lean and fat body mass, percent body fat, and total body tissue), and hemodynamic (blood pressures, pulse pressure, heart rate, tail blood flow, and tail blood volume) and metabolic measures (the levels of sodium, potassium, chloride, pH, glucose, hematocrit, bicarbonate, hemoglobin, and urea) to generate a unique score for each mouse.
Using this Frailty Index of deficit accumulation, mice with higher frailty scores had prominent age-related cellular changes when compared with younger mice. Indeed, this Frailty Index identified the older mice; however, these measures do not coincide to the accepted measures used clinically to define frailty, and the measures required a multitude of tools. As a result, categorizing frailty by a more clinical relevant, approachable noninvasive assessment in mice is still desired.
Foremost, the criteria selected in the current short report mimicked the established clinical indicators of frailty, so that future studies would have translational significance (tool to identify risk for disability and mortality in mice), and secondly, the criteria use simple noninvasive measures. Finally, the four selected criteria and cutoff points identify the weak individuals in the cohort.
The Four Selected Criteria
We found a 9% prevalence of frailty using the selected criteria in 27- to 28-month-old males, an age group that is comparable with an 80-year-old humans (10). This finding is consistent with the 11% prevalence of frailty using Fried’s criteria of frailty in age at 76–84 years (1). This finding suggests that the selected criteria used in these old mice (grip strength, walking speed, physical activity, and endurance) are good choices for the Frailty Index.
Grip strength.
Grip strength of the dominant hand is one of the criteria used in identifying frailty (1) because it is able to predict adverse health outcomes when combined with other outcome measures (11). The equivalent criterion in the mouse is the inverted-cling grip test, a functional test for assessing the strength and the endurance. In this test, the mouse is required to activate the limb muscles to hold their body weight (strength) as long as possible (endurance). Based on the individual values, which range from 24 to 171 seconds (4-fold), it is likely the inverted-cling grip test alone would not be able to predict adverse function. However, because it is an easy, reliable assessment, and the potential of combining this assessment with the other frailty criteria, the inclusion of the inverted-cling grip test in the Frailty Index is necessary.
Walking speed.
In the human Frailty Index, the criterion of walking speed is determined by the 15′ walking test (1). This test is selected because walking speed is an assessment of physical function, especially mobility (12). Slower walking speed represents a functional limitation (13,14), and it has been used as a predictor of poor geriatric outcomes, such as risk of fall, institutionalization, and mortality (15,16). In mice, rotarod is a commonly used device for testing overall motor function (balance, coordination, endurance), seems to be a good match to the 15′ walking test, and performance on the rotarod decreases with age (8,9,17,18). Specifically, the rotarod test measures two parameters: speed and latency to fall. Although both parameters are valid, we selected the speed of the rotarod test for the frailty criterion because it is a direct measurement of maximal walking speed before the mouse falls.
Physical activity.
The frailty criterion for activity levels is evaluated by the short version of the Minnesota Leisure Time Activity questionnaire, which covers most of the daily activities and estimates the calorie expenditure during 1 week (1). To match this criterion, the physical activity of the mouse was determined by the daily voluntary wheel-running distance because it is an assessment widely used for quantifying the locomotor activity level (17,19). Moreover, this measurement is a close match to the human physical activity measure because when a running wheel is provided in a mouse cage, the main energy expenditure is attributed to the voluntary running (19,20). In this short report, this criterion did not independently identify an animal as frail using the designated cutoff point. Further evaluation of this criterion within the Frailty Index is needed because the activity levels were very low (0.51–2.93 km/d) in these 27- to 28-month-old mice compared with 19 months old (3.9 km/d) (21). Moreover, it may be necessary to adjust the cutoff points such that each criterion has its own specific cutoff point, which is consistent with Fried’s Frailty Index.
Endurance.
Another criterion in the Frailty Index by Fried’s group is related to exhaustion or poor endurance. Exhaustion is determined by the self-reported CES-D Depression Scale (a valid questionnaire and an indicator for VO2 max level and cardiovascular diseases) (1). To match the clinical study, this short report used a derived endurance score from rotarod (duration in seconds the mouse stayed on the rotarod) and grip test, which is objective and measurable (impossible to ask a mouse if it is exhausted). Indeed, a biasing may exist in this criterion because the grip test is the same as the strength criterion. To avoid the potential for bias in the future, other behavioral measures might be considered such as running to exhaustion.
In summary, the four criteria we selected for the mouse Frailty Index functioned well because they are established measures of mouse physical functions and also match corresponding human criteria.
Frailty Index
Strengths of the identified cutoff points.
In the study of Fried and coworkers, the lowest 20% in the cohort (bottom quintile) was used for most of the criteria (grip strength, walking speed, and activity level). These cutoff points are reasonable for the population investigated because they have been shown to predict hospitalization, mortality, first fall, worsening activities of daily living disability, and worsening morbidity disability. Because of our small sample size, using 20% of the cohort as our cutoff point was not reasonable and would likely identify a mouse as frail incorrectly. Therefore, we chose a more conservative primary cutoff point, 1.5 SD below the mean, because 1 SD is usually considered within the normal range (the main population, 68%). The 1.5 SD identifies the lowest 7%, a much more stringent percentile than the clinical measure (20%).
Definition of frail.
In the human Frailty Index designed and tested by Fried and coworkers, the frail identification (three or more criteria presented) has high predictive power for physical limitation, first hospitalization, first fall, worsening activities of daily living disability, and worsening mobility disability. In contrast, the presence of two criteria is identified as prefrail with significant prediction of outcomes, but with lower association (1).
Consistent with the human Frailty Index, the Frailty Index for mice included the presence of three or more of the criteria as frail and two criteria as mildly frail. However, this definition of frail is more conservative than that provided by Fried and coworkers because there are only four criteria (with a much lower cutoff point of the bottom 7%) and may underestimate the true number of frail or mildly frail mice. In order to improve the identification of frail mice, the inclusion of another criterion would be beneficial. In summary, the selection of the cutoff point as 1.5 SD below the mean and the method of identifying frail or mildly frail mice match the human study, although being more conservative.
The frailty criteria demonstrated in this short report used the classic clinical frailty indicators; however, other frailty criteria require consideration, such as impaired cognition, balance, and gait patterns. These indicators have established protocols such as objective recognition tests (cognition), raised beam test (balance), foot print analysis (gait), and dual-energy X-ray absorptiometry (body composition) (6,22,23). This report is the beginning of Frailty Indices for mice. In order to test the validity of this Frailty Index, a longitudinal study is required with different ages, both genders, and short-lived and long-lived mouse strains. Moreover, the molecular and cellular mechanisms underlying frailty also need to be investigated in the future study.
Collectively, our Frailty Index provides a potential standardized definition for frailty in mice that is consistent with the operational definition of frailty in humans. The Frailty Index, thus, has potential use in future studies in molecular and cellular mechanisms underlying the process of frailty.
Funding
This study was supported by the National Institute of Aging at National Institutes of Health (R0AG017768 to L.V.T. and T32AG029796 and F31AG044108 to T.G.G.) and the University of Minnesota Center for Excellence in Critical Care Center grant (to L.V.T.).
Acknowledgements
The authors thank Dr. Walter C. Low of the Department of Neurosurgery and Briana K. Jones of the Program in Physical Therapy (DPT) at University of Minnesota. L.F.-S. is now an Assistant Professor of Biology at Hamline University in St Paul, Minnesota.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 2. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. 10.1016/S0140-6736(98)04402-X [DOI] [PubMed] [Google Scholar]
- 3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. 10.1111/ j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 5. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67(3):217–227. 10.1093/gerona/glr193 [DOI] [PubMed] [Google Scholar]
- 7. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. 10.1093/gerona/54.11.B492 [DOI] [PubMed] [Google Scholar]
- 8. Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68:1326–1336. 10.1093/gerona/glt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12:155–162. 10.1080/03610738608259454 [DOI] [PubMed] [Google Scholar]
- 10. Arias E. National Vital Statistics Reports. National Vital Statistics System. 2011;59(9):1–60 http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_09.pdf Accessed June 24, 2013. [PubMed] [Google Scholar]
- 11. Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32(6):650–656. 10.1093/ageing/afg111 [DOI] [PubMed] [Google Scholar]
- 12. Whetstone LM, Fozard JL, Metter EJ, et al. The physical functioning inventory: a procedure for assessing physical function in adults. J Aging Health. 2001;13(4):467–493. 10.1177/089826430101300402 [DOI] [PubMed] [Google Scholar]
- 13. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 14. Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. J Am Geriatr Soc. 1999;47(10):1257–1260. [DOI] [PubMed] [Google Scholar]
- 15. Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60(8):1478–1486. 10.1111/j.1532-5415.2012.04074.x [DOI] [PubMed] [Google Scholar]
- 16. Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59(5):887–892. 10.1111/ j.1532-5415.2011.03336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10(7):519–529. 10.1038/nrn2652 [DOI] [PubMed] [Google Scholar]
- 18. Ingram DK, Archer JR, Harrison DE, Reynolds MA. Physiological and behavioral correlates of lifespan in aged C57BL/6J mice. Exp Gerontol. 1982;17:295–303. [DOI] [PubMed] [Google Scholar]
- 19. Harri M, Lindblom J, Malinen H, et al. Effect of access to a running wheel on behavior of C57BL/6J mice. Lab Anim Sci. 1999;49(4):401–405. [PubMed] [Google Scholar]
- 20. Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–1014. 10.1016/j.neubiorev.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. 10.1523/JNEUROSCI.1731-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11):2129–2138. 10.1111/j.1532 [DOI] [PubMed] [Google Scholar]
- 23. Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32(10):1868–1880. 10.1016/j.neurobiolaging.2009.11.003 [DOI] [PubMed] [Google Scholar]