Abstract
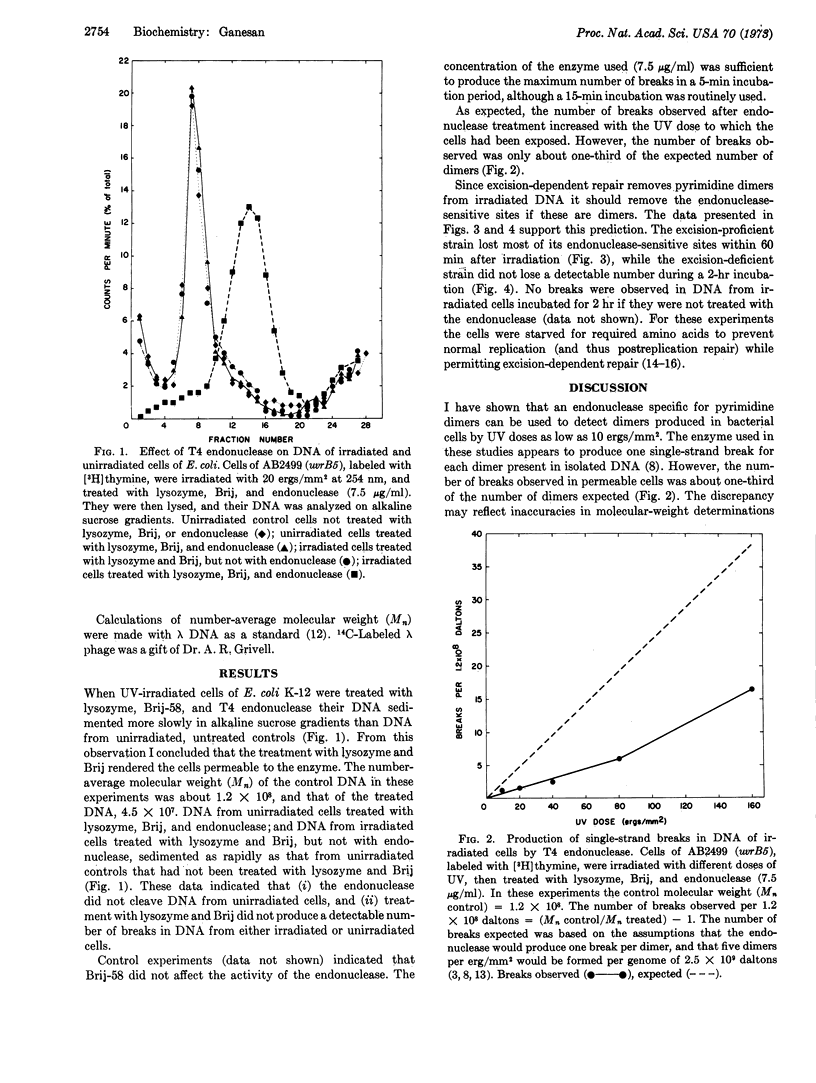
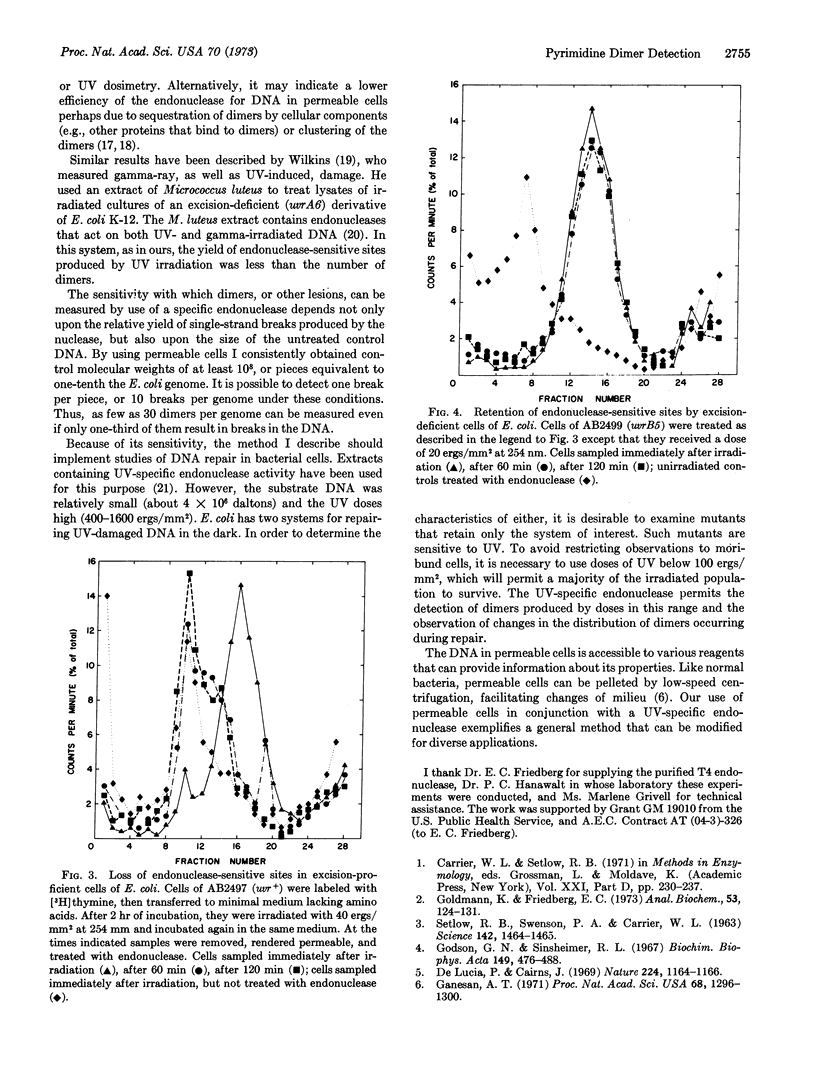
Cells of Escherichia coli treated with lysozyme and Brij-58 become permeable to proteins, but do not release their DNA. I incubated permeable cells with an endonuclease that produces single-strand breaks in DNA-containing pyrimidine dimers. The enzyme entered the permeable cells, and, if they had been irradiated with ultraviolet light, caused breaks in their DNA. The frequency of breaks was estimated from the sedimentation pattern of the DNA in alkaline sucrose gradients. The procedure is sensitive enough to detect the dimers produced by a dose of 10 erg/mm2 at 254 nm, or about 50 dimers per E. coli genome. This method exemplifies and extends the use of permeabilized cells for examining biological processes at the molecular level.
Keywords: permeable cells, dimer-specific endonuclease, E. coli
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Brunk C. F. Distribution of dimers in ultraviolet-irradiated DNA. Nat New Biol. 1973 Jan 17;241(107):74–76. doi: 10.1038/newbio241074a0. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Clayton D. A. Electron microscopic studies on substrate specificity of T4 excision repair endonuclease. Nature. 1972 May 12;237(5350):99–100. doi: 10.1038/237099a0. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. T. Adenosine triphosphate-dependent synthesis of biologically active DNA by azide-poisoned bacteria. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1296–1300. doi: 10.1073/pnas.68.6.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Goldmann K., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. Anal Biochem. 1973 May;53(1):124–131. doi: 10.1016/0003-2697(73)90413-2. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Pettijohn D. E., Pauling E. C., Brunk C. F., Smith D. W., Kanner L. C., Couch J. L. Repair replication of DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:187–194. doi: 10.1101/sqb.1968.033.01.022. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., SWENSON P. A., CARRIER W. L. THYMINE DIMERS AND INHIBITION OF DNA SYNTHESIS BY ULTRAVIOLET IRRADIATION OF CELLS. Science. 1963 Dec 13;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- Shafranovskaya N. N., Trifonov E. N., Lazurkin Y. S., Frank-Kamenetskii M. D. Clustering of thymine dimers in ultraviolet irradiated DNA and the long-range transfer of electronic excitation along the molecule. Nat New Biol. 1973 Jan 10;241(106):58–60. doi: 10.1038/newbio241058a0. [DOI] [PubMed] [Google Scholar]
- Strauss B., Searashi T., Robbins M. Repair of DNA studied with a nuclease specific for UV-induced lesions. Proc Natl Acad Sci U S A. 1966 Sep;56(3):932–939. doi: 10.1073/pnas.56.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins R. J. Endonuclease-sensitive sites in the DNA of irradiated bacteria: a rapid and sensitive assay. Biochim Biophys Acta. 1973 Jun 8;312(1):33–37. doi: 10.1016/0005-2787(73)90049-x. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



