Abstract
Background.
Studies of older persons show consumption of light-to-moderate amounts of alcohol is positively associated with cognitive function and, separately, is negatively associated with total brain volume (TBV). This is paradoxical as generally, cognitive function is positively associated with TBV. We examined the relationships of TBV, global cognitive function (GCF), and alcohol consumption in a population-based cohort of 3,363 men and women (b. 1907–1935) participating in the Age Gene/Environment Susceptibility-Reykjavik Study (2002–2006) and who were free of dementia or mild cognitive impairment
Methods.
Drinking status (never, former, and current) and current amount of alcohol consumed were assessed by questionnaire. GCF is a composite score derived from a battery of cognitive tests. TBV, standardized to head size, is estimated quantitatively from brain magnetic resonance imaging.
Results.
Among women and not men, adjusting for demographic and cardiovascular risk factors, current drinkers had significantly higher GCF scores than abstainers and former drinkers (p < .0001); and GCF was associated with amount consumed. TBV was not associated with drinking status or amount consumed in men or women. GCF and TBV did significantly differ in their associations across alcohol categories (p interaction < .001). Within categories of alcohol intake, GCF and TBV were positively associated.
Conclusions.
The difference in associations of alcohol intake to brain structure and function suggests there may be unmeasured factors that contribute to maintaining better GCF relative to TBV. However, at higher levels of reasonable alcohol consumption, there may be factors leading to reduced brain volume.
Key Words: Alcohol consumption, Brain aging, Epidemiology, Imaging, Cognitive aging.
Heavy alcohol consumption and alcoholism are detrimental to cardiovascular health as well as to cognitive and neurological functioning (1). However, there are reports of a J-shaped relationship between alcohol consumption and the risk for a number of adverse health outcomes (2), suggesting light-to-moderate alcohol intake may provide health benefits. These benefits may include a decrease in the risk of Alzheimer’s disease and dementia, as reported in several population-based studies of older persons (3,4).
To better understand these cognitive benefits, other studies have examined the association of alcohol intake to cognitive function (5,6) and separately to brain structure (7–9). When reviewing these studies, the expectation is, given cognitive function and total brain volume (TBV) are positively associated (10,11), that both will have similar relationships to alcohol intake. Studies on cognition in nondemented populations find moderate alcohol intake is associated with better function (12–14), although the relationship is less clear in studies that include participants with dementia (5,6,15,16). In contrast, studies of magnetic resonance imaging (MRI) show no association, or an inverse association, of TBV, to increasing alcohol consumption (7,8). When combined, these two sets of studies suggest that some alcohol intake has either no effect or a positive association with cognitive function, and a negative association with TBV. Thus, these findings create a paradox (Supplementary Figure).
Generally, the alcohol and cognitive analyses are reported in separate articles from the alcohol and brain volume studies. By studying these relationships in the same cohort and thus minimizing methodological issues that cloud comparisons across studies (15–20), we may be able to better understand this inconsistency and advise older patients on alcohol consumption.
Here, we investigate the relationships among alcohol consumption, cognitive function, and TBV in a large and well-characterized population-based cohort of older persons participating in the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik Study).
Materials and Methods
Study Population
The AGES-Reykjavik Study, described elsewhere (21), is a follow-up of the Reykjavik Study initiated in 1967 by the Icelandic Heart Association to monitor the cardiovascular health of the Icelandic population. The Reykjavik Study (1967–1997) examined a random sample of 18,569 men and women born in 1907–1935 who were living in the Reykjavik area during 1967. Of the 11,549 participants still alive in 2002, 5,764 participated in the AGES-Reykjavik Study (2002–2006). All participants signed a written informed consent form. The study was approved by the National Bioethics Committee (VSN00-063) in Iceland and by the institutional review board of the Intramural Research Program of the National Institute on Aging.
Measurement of Alcohol Consumption
Alcohol consumption data were derived from health questionnaire responses. Drinking status categories (abstainer, former drinker, current drinker) are based on the following closed questions: “Do you drink (alcohol) now?” “Did you drink between the ages of 40–59 years old?” and “Was there any other time in your life during which you drank?”
Among current alcohol drinkers, the following questions were asked about amount consumed: “How often do you drink (alcohol) in a month?” and “How many drinks do you consume on a single occasion?” We multiplied the number of occasions by amount consumed on each occasion to calculate the approximate number of drinks consumed per month. We multiplied the product by the standard 14 grams of ethanol per drink and then divided this value by 3.25 to determine grams of ethanol consumed per week.
Measurement of Cognition
As described previously (22), cognitive scores were derived from a battery of six neuropsychological tests from which three domain scores were calculated: memory, speed of processing, and executive function. The composite memory score included the immediate and delayed recall portion of a modified California Verbal Learning Test (23). The speed of processing score included the Figure Comparison Test (24), the Digit Symbol Substitution Test (25), and the modified Stroop Test parts 1 and 2 (26). The executive function score included the Cambridge Neuropsychological Test Automated Battery Spatial Working Memory Test (shortened version) (27), the Digits Backward Test (25), and the Stroop Test part 3 (26).
Measurement of Brain Volumes
As described previously (28), MRI scans were performed on a 1.5-T signa Twinspeed system (General Electric Medical Systems, Waukesha, WI) and included an axial T1-weighted 3-dimensional, a proton-density/T2-weighted fast-spin echo (PD/T2), and a fluid-attenuated inversion recovery sequences. Cerebrospinal fluid, gray matter, white matter, and white matter lesion volumes were segmented automatically with the AGES/Montreal Neurological Institute pipeline, an automatic brain tissue segmentation computer algorithm (29). Intracranial volume was calculated by summing all segmented areas. Percentage of TBV (%TBV) was calculated as a percentage of the sum of gray matter, white matter, and white matter lesions relative to the intracranial volume. %TBV was also transformed into a Z-score so it could be compared with cognitive scores.
Measurement of Covariates
We adjusted our analyses for previously described variables (21,22,28) that are potentially confounding or are known to be associated with alcohol consumption or cognitive function (30,31). These included age, education level, and current depression status measured by the short version of the Geriatric Depression Scale, and self-reported overall health status; mid-life body mass index, and systolic blood pressure; and late-life reported smoking status, coronary calcium load measured by computed tomography, high-density lipoprotein cholesterol concentrations, and presence of the apolipoprotein E ε4 allele, a marker for genetic susceptibility to Alzheimer’s disease. We additionally considered several other variables that may confound the association of interest including mid-life occupation status and physical activity, as well as late-life leisure activity, presence of hypertension, coronary heart disease, type 2 diabetes, statin use, low-density lipoprotein and triglyceride concentrations, and MRI-detected cerebral infarcts and microbleeds. However, adding these additional measurements to the model only slightly attenuated relationships and so they were not included in the final analyses.
Analytical Sample
The AGES-Reykjavik Study included 5,764 participants of whom 4,614 had a MRI scan and 4,224 also had cognitive test scores. Of the remaining participants, we excluded 33 individuals from analyses because their responses to the alcohol questions were incomplete. Furthermore, we excluded persons with dementia, mild cognitive impairment, or unknown cognitive status to reduce unreliability in the responses to the alcohol questions. Of the remaining 3,622 participants, we excluded 232 due to missing covariate data.
Among this analytical subsample, 30.2% (n = 600) of women and 10.0% (n = 138) of men reported never consuming alcohol and were considered abstainers, 7.6% (n = 150) of women and 15.9% (n = 218) of men reported being former drinkers, and the remaining 1,240 (62.4%) women and 1,019 (74.1%) men reported currently consuming alcohol. As the definition of moderate drinking differs between women and men, we created separate consumption categories for each and stratified our analyses by sex, similar to previous studies (7,8,12). We chose the strategy of sex-standardized variables so we could validly compare outcomes within sex, and relationships between men and women. Further, binge drinking (5 drinks on any occasion within the past 30 days) was coded as heavy drinking (32). Female current drinkers were classified as very light (<1 drink per week; n = 866; 43.6% of current female drinkers), light-to-moderate (1–7 drinks per week; n = 372; 18.7%), and heavy (>7 drinks per week; n = 15) drinkers. Current male drinkers were categorized as very light (<1 drink per week; n = 470; 34.2% of current male drinkers), light (1–7 drinks per week, n = 444; 32.3%), moderate (7–14 drinks per week; n = 105; 7.6%), and heavy (>14 drinks per week; n = 12) drinkers. The 27 heavy drinkers were excluded due to small sample size, resulting in a final sample of 3,363 subjects (women: n = 1,988; men: n = 1,375). As expected, subjects included in the analyses were younger, overall healthier, and had higher levels of education, global cognitive function (GCF), and %TBV compared with those excluded due to dementia, mild cognitive impairment, or missing an adjudicated cognitive status.
Statistical Analysis
We transformed the cognitive and MRI variables into sex-specific Z-scores ([individual value of “x” − group mean “x”]/standard deviation of “x”). A GCF score was created by standardizing all three cognitive domains by sex (22) and summing the three standardized domain scores. Likewise, we transformed %TBV into Z-scores stratified by sex. These Z-score transformations rank individuals in the same units so it is possible to compare a person’s rank in the cognitive distributions compared with the %TBV distributions. For instance, if an individual had a cognitive Z-score of 1 and a %TBV Z-score of −0.5, this would suggest the person performs relatively better on the cognitive test compared with the others, but has a relatively smaller %TBV compared with the others.
Analysis of variance and logistic regressions were used to compare baseline characteristics, adjusting for age, by drinking status and across current drinker categories. To better understand the relationships among alcohol, cognition, and brain volume, we examined the question from three different perspectives: First, we examined the association of alcohol intake to cognitive function and %TBV Z-scores, separately, using analysis of variance models. Second, we wanted to see if the association of cognitive function across alcohol categories was similar to that of TBV and alcohol intake. Here, we hypothesized the slopes of GCF scores and %TBV would be similar across categories, as they should be if cognition and brain volume are related. This was tested in one model where we compared the slopes of the relationships of cognitive and %TBV Z-scores across levels of alcohol consumption categories. This was implemented in a generalized mixed model and tested by the interaction between GCF scores and %TBV as they relate to alcohol intake (33). Third, to further investigate whether the cognitive–%TBV associations differed depending on the alcohol category, we hypothesized similar slopes within categories of alcohol consumption. This question was also tested with a mixed model.
For these three questions, we estimated three models: model 1 was adjusted for age, model 2 was further adjusted for education and depression status, and model 3 was adjusted for all covariates. All analyses of %TBV were further adjusted for intracranial volume as an indicator of head size. Statistical tests were performed in SAS version 9.1.3 service pack 3 (SAS Institute Inc., Cary, NC); all tests were two-sided and α = .05
Results
In both women and men, age, high-density lipoprotein cholesterol, and education levels were significantly different across drinking status and alcohol consumption categories (Tables 1 and 2). Approximately half the former and current drinkers were also former smokers. For the most part, both male and female current drinkers were overall healthier than abstainers.
Table 1.
Baseline Characteristics Between Alcohol Consumption Groups in Women
| Characteristics, % (N) | Drinking Status | Alcohol Consumption of Current Drinkers | |||||
|---|---|---|---|---|---|---|---|
| Abstainer | Former | Current Drinker | p-Value* | Very Light | Light-Moderate | p-Value* | |
| (n = 600) | (n = 150) | (n = 1,240) | (n = 866) | (n = 372) | |||
| Age,† y | 76.8 (5.4) | 75.0 (4.7) | 75.0 (5.1) | <.001 | 75.5 (5.1) | 74.0 (4.9) | <.001 |
| Education level | |||||||
| Primary | 34.0 (204) | 33.3 (50) | 18.8 (233) | 21.1 (183) | 12.6 (47) | ||
| Secondary | 49.0 (294) | 41.3 (62) | 51.7 (641) | 51.5 (446) | 52.7 (196) | ||
| College | 13.7 (82) | 18.7 (28) | 21.8 (270) | <.001 | 19.9 (172) | 26.3 (98) | .073 |
| University | 3.3 (20) | 6.7 (10) | 7.7 (96) | 7.5 (65) | 8.3 (31) | ||
| Residence as an adolescent | |||||||
| Farm | 40.0 (240) | 20.0 (30) | 23.4 (294) | 25.6 (222) | 18.5 (72) | ||
| Fish village | 18.5 (111) | 19.3 (29) | 15.5 (195) | 16.1 (140) | 14.1 (55) | ||
| Village | 10.0 (60) | 16.7 (25) | 15.2 (191) | <.001 | 16.1 (140) | 13.1 (51) | .001 |
| City | 31.5 (189) | 44.1 (66) | 46.0 (578) | 42.2 (366) | 54.4 (212) | ||
| Self-reported health status | |||||||
| Poor | 5.5 (33) | 10.0 (15) | 4.1 (52) | 5.1 (44) | 2.1(8) | ||
| Fair | 31.5 (189) | 32.7 (49) | 25.8 (324) | 28.1 (244) | 20.5 (80) | ||
| Good | 31.7 (190) | 30.0 (45) | 31.4 (395) | .038 | 31.5 (273) | 31.3 (122) | .005 |
| Very good | 12.8 (77) | 8.7 (13) | 14.0 (176) | 12.8 (111) | 16.7 (65) | ||
| Excellent | 18.5 (111) | 18.7 (28) | 24.7 (311) | 22.6 (196) | 29.5 (115) | ||
| Smoking status | |||||||
| Nonsmoker | 75.8 (455) | 32.0 (48) | 44.6 (553) | 47.8 (414) | 36.8 (137) | ||
| Former smoker | 18.8 (113) | 50.7 (76) | 41.0 (508) | <.001 | 38.8 (336) | 46.2 (172) | .097 |
| Current smoker | 5.3 (32) | 17.3 (26) | 14.4 (179) | 13.4 (116) | 16.9 (63) | ||
| BMI at midlife†, kg/m2 | 25.5 (4.2) | 24.5 (3.6) | 24.5 (3.3) | <.001 | 24.7 (3.5) | 24.1 (3.0) | .026 |
| Depression, GDS ≥ 6 | 7.0 (42) | 10.7 (16) | 5.8 (73) | .24 | 5.8 (50) | 5.9 (23) | .81 |
| Systolic BP at midlife†, mm Hg | 131 (17) | 127 (15) | 128 (16) | .045 | 129(17) | 126 (16) | .175 |
| Coronary calcium†, Agatston/100 | 3.6 (5.8) | 3.8 (6.3) | 4.1 (7.2) | .003 | 4.4 (7.6) | 3.5 (6.3) | .55 |
| ApoE-ε4 allele present | 29.7 (178) | 20.0 (30) | 26.4 (332) | .102 | 25.8 (224) | 27.7 (108) | .62 |
| HDL-C†, mmol/L | 1.68 (0.42) | 1.69 (0.43) | 1.76 (0.45) | <.001 | 1.73 (0.44) | 1.84 (0.47) | <.001 |
| Intracranial volume†, cm3 | 1411 (107) | 1415 (98) | 1426 (104) | .009 | 1421 (106) | 1437 (99) | .017 |
Notes: ApoE-ε4 = apolipoprotein E ε4; BMI = body mass index; BP = blood pressure; GDS = Geriatric Depression Scale; HDL-C = high-density lipoprotein cholesterol.
*Adjusted for age.
†Mean (SD).
Table 2.
Baseline Characteristics Between Alcohol Consumption Groups in Men
| Characteristics, % (N) | Drinking Status | Alcohol Consumption of Current Drinkers | ||||||
|---|---|---|---|---|---|---|---|---|
| Abstainer | Former | Current Drinker | Very Light | Light | Moderate | |||
| (n = 138) | (n = 218) | (n = 1,019) | p-Value* | (n = 470) | (n = 444) | (n = 105) | p-Value* | |
| Age,† y | 77.0 (5.4) | 76.2 (5.0) | 75.6 (5.0) | .004 | 76.3 (4.9) | 75.0 (4.8) | 75.3 (5.4) | <.001 |
| Education level | ||||||||
| Primary | 16.7 (23) | 14.7 (32) | 9.7 (86) | 13.8 (65) | 6.1 (27) | 6.7 (7) | ||
| Secondary | 50.0 (69) | 51.8 (113) | 54.0 (558) | 58.1 (273) | 54.5 (242) | 38.1 (40) | ||
| College | 16.7 (23) | 15.6 (34) | 15.1 (156) | .023 | 13.2 (62) | 13.7 (61) | 25.7 (27) | <.001 |
| University | 16.7 (23) | 17.9 (39) | 21.2 (219) | 14.9 (70) | 25.7 (114) | 29.5 (31) | ||
| Residence as an adolescent | ||||||||
| Farm | 28.3 (39) | 22.8 (50) | 28.6 (295) | 32.7 (154) | 23.7 (105) | 30.5 (36) | ||
| Fish village | 21.0 (29) | 16.9 (37) | 16.2 (167) | 15.7 (74) | 15.3 (68) | 21.2 (25) | ||
| Village | 17.4 (24) | 12.8 (28) | 14.7 (152) | .51 | 15.3 (72) | 14.9 (66) | 11.9 (14) | .039 |
| City | 33.3 (46) | 47.5 (104) | 40.6 (419) | 36.3 (171) | 46.2 (205) | 36.4 (43) | ||
| Self-reported health status | ||||||||
| Poor | 3.6 (5) | 8.7 (19) | 3.0 (31) | 2.3 (11) | 3.2 (14) | 5.1 (6) | ||
| Fair | 21.7 (30) | 24.2 (53) | 19.2 (198) | 23.4 (110) | 16.4 (73) | 12.7 (15) | ||
| Good | 31.2 (43) | 31.1 (68) | 32.8 (339) | .140 | 33.1 (156) | 32.7 (145) | 32.2 (38) | .108 |
| Very good | 20.3 (28) | 16.9 (37) | 18.2 (188) | 16.6 (78) | 20.1 (89) | 17.8 (21) | ||
| Excellent | 23.2 (32) | 19.2 (42) | 26.8 (277) | 24.6 (116) | 27.7 (123) | 32.2 (38) | ||
| Smoking status | ||||||||
| Nonsmoker | 70.3 (97) | 23.4 (51) | 21.7 (221) | 24.0 (113) | 23.9 (106) | 1.9 (2) | ||
| Former smoker | 26.1 (36) | 68.3 (149) | 65.9 (672) | <.001 | 64.7 (304) | 64.4 (286) | 69.5 (82) | .060 |
| Current smoker | 3.6 (5) | 8.3 (18) | 12.4 (126) | 11.3 (53) | 11.7 (52) | 17.8 (21) | ||
| BMI at midlife,† kg/m2 | 25.9 (3.0) | 25.6 (3.3) | 25.4 (3.1) | .096 | 25.6 (3.2) | 25.2 (3.0) | 25.2 (2.9) | .21 |
| Depression, GDS ≥ 6 | 2.2 (3) | 7.8 (17) | 3.9 (40) | .74 | 4.9 (23) | 2.3 (10) | 5.9 (7) | .071 |
| Systolic BP at midlife,† mm Hg | 135 (16) | 134 (16) | 134 (15) | .90 | 134 (16) | 134 (15) | 134 (15) | .99 |
| Coronary calcium,† Agatston/100 | 8.5 (12.2) | 11.5 (12.5) | 10.5 (13.2) | .048 | 10.0 (13.3) | 10.2 (12.4) | 13.6 (15.3) | .011 |
| ApoE-ε4 allele present | 32.6 (45) | 30.1 (66) | 28.2 (291) | .20 | 28.0 (132) | 27.7 (123) | 30.5 (36) | .83 |
| HDL-C,† mmol/L | 1.32 (0.35) | 1.34 (0.32) | 1.46 (0.40) | <.001 | 1.38 (0.36) | 1.48 (0.39) | 1.66 (0.50) | <.001 |
| Intracranial volume,† cm3 | 1601 (127) | 1617 (113) | 1617 (121) | .37 | 1617 (116) | 1616 (123) | 1624 (130) | .81 |
Notes: ApoE-ε4 = apolipoprotein E ε4; BMI = body mass index; BP = blood pressure; GDS = Geriatric Depression Scale; HDL-C = high-density lipoprotein cholesterol.
*Adjusted for age.
†Mean (SD).
Cognition and Brain Volume Separately
For both women and men, the GCF score significantly varied across drinking status categories, whereby current drinkers had the highest cognitive function scores, and former drinkers had the lowest scores (Supplementary Table 2). Among men and women, consumption categories differed significantly after adjusting for age, but full adjustment (Model 3; Supplementary Table 2) attenuated the association. For both men and women, standardized %TBV scores did differ across drinking status categories when adjusted for age, but when fully adjusted (values of %TBV shown in Supplementary Table 1) only males with moderate intake had significantly lower %TBV compared with lighter drinkers.
Association of Alcohol Intake Categories to Cognition and TBV Compared Concurrently
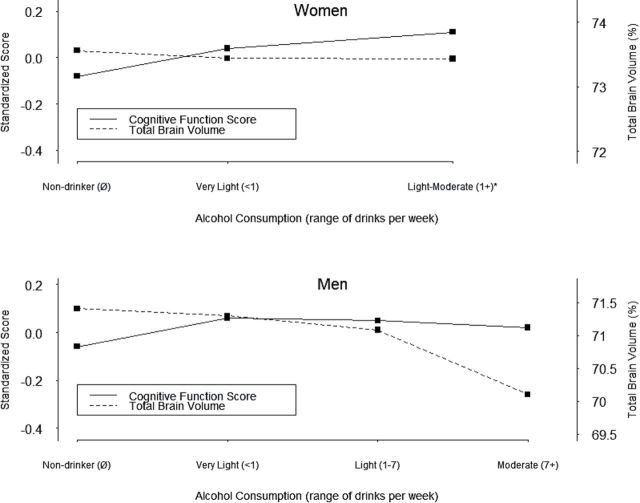
The associations to alcohol of GCF and %TBV differed (Figure 1; test for interaction, p < .001 in both women and men). Upon further inspection, among women, the mean %TBV Z-score was lower than the mean cognition Z-score in light-to-moderate current drinkers (Supplementary Table 1). Among men, the mean %TBV Z-score and cognition did not differ by alcohol consumption level until moderate consumption, where the %TBV Z-score measure was lower than the cognitive Z-score.
Figure 1.
Fully adjusted mean Z-scores of %TBV and global cognitive function scores by alcohol consumption categories. Former drinkers are not included. %TBV = percentage of the total brain volume, relative to the intracranial volume.
Cognition and TBV Association Within Alcohol Groups
Among women, the relationship between GCF and %TBV was positive in all alcohol groups (abstainers [β = 0.033, p = .003], former drinkers [β = 0.065, p = .007], very light drinkers [β = 0.045, p < .0001], light drinkers [β = 0.032, p = .04]; Figure 2). There were no statistically different slopes among the alcohol categories, but there was among the intercepts (p = .004), such that light-to-moderate drinkers had higher cognitive function scores than light drinkers, who had higher scores than abstainers, who had higher scores than former drinkers. Among men, the pattern was similar to women, with their respective slopes being: abstainers (β = 0.064, p = .02), former drinkers (β = 0.049, p = .004), very light drinkers (β = 0.059, p < .001), light drinkers (β = 0.047, p = .0003), and moderate drinkers (β = 0.048, p = .03). Among moderate drinkers, the relationship of brain volume to cognitive function was somewhat attenuated, but there were no significant differences between any of the slopes. The differences between intercepts were significant (p < .02). Adjustment for the presence of the apolipoprotein E ε4 allele did not change these results.
Figure 2.
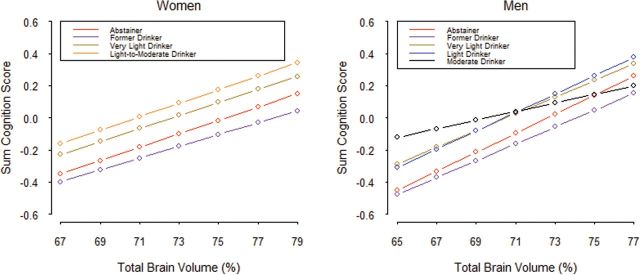
Total brain volume predicting global cognitive function scores by alcohol consumption categories.
Discussion
Cognitive function is positively associated with the underlying brain structure, and risk factors associated with one of these measures is expected to be similarly associated with the other. This analysis was motivated by the seeming paradox in the literature about positive and negative associations of light-to-moderate alcohol intake on the brain. The results for our first question were similar to other studies in that former drinkers had the lowest GCF compared with abstainers and current drinkers; there was no association with amount consumed. We also found no association of %TBV to alcohol categories. For our second question, we found the alcohol–brain and alcohol–cognition association significantly differed, suggesting individuals were not ranked similarly in tissue volume and cognitive function. Overall, we found higher alcohol intake (but still at light levels of intake) was associated with better cognitive function, but those who consumed alcohol did not have higher %TBV as would be expected. Our third question showed similar cognition–TBV slopes within each alcohol category. We conclude from the total set of findings that the paradox exists to the extent individuals are not ranked the same in measures of cognition and brain volume.
Our findings are based on a study with a number of strengths. AGES-Reykjavik is a large cohort with well-characterized cognitive function and brain structure measures. We were able to adjust for a number of potentially confounding factors that could explain associations of alcohol intake to cognitive function and %TBV. We separated life-long abstainers from former drinkers, who, as has been found previously, may have stopped drinking alcohol for health reasons (17,34). Furthermore, we conducted these analyses simultaneously and in one cohort, so methodological or study-specific characteristics do not confound the comparisons of the associations of alcohol intake to brain structure and function.
There are some issues that need to be taken into account when interpreting these findings. These analyses are based on cross-sectional data so the temporal relationships among change in cognitive function and %TBV cannot be studied. We also use current intake as a proxy for long-term drinking habits. Further, alcohol intake was self-reported. Although studies show self-reported alcohol consumption to be generally reliable (35), we attempted to reduce recall error by excluding those with dementia or mild cognitive impairment from all analyses. Excluding these participants means our results cannot be generalized to this group. Further, the participants who were included in these analyses were slightly healthier compared with those not included. However, the extent to which our results are affected by bias depends on whether the associations between the independent and dependent variables differ between included and excluded participants.
We found some gender differences in the alcohol associations, which may be due to amounts consumed or possible differences in the way men and women metabolize alcohol (7). On average, however, we note that compared with other cohorts (3,6,36), the distribution of alcohol consumption is lower among AGES-Reykjavik participants. Other studies show heavy drinkers make up approximately 5%–25% of the total cohort, whereas in the AGES-Reykjavik cohort, they comprise less than 1% of all participants. This difference in levels may explain why a negative association of TBV to alcohol intake only starts to emerge among the men, who had relatively the highest level of alcohol consumption. However, the low sample sizes for moderate male drinkers may contribute to the lack of statistically significant findings in our first and third analytical approach. Longitudinal studies that are able to measure the loss of cognitive function and brain atrophy over time in one cohort should provide stronger evidence concerning the effects of moderate alcohol consumption on the aging brain.
This discrepancy between the cognitive and brain structure findings may reflect several factors. Light-to-moderate alcohol consumption is often associated with a lower risk of coronary heart disease and several putative biologic mechanisms have been proposed. Moderate alcohol consumption may increase the concentration of high-density lipoprotein, lower the oxidation rate of low-density lipoproteins, reduce blood clotting and platelet aggregation, and can reduce blood pressure (30). A recent study suggested that these mechanisms could reduce arterial stiffness and thus mediate the coronary heart disease pathway (37). Reducing the risk of coronary heart disease and atherosclerosis also lowers the risk of cognitive impairment (38). Although we could not control for all the proposed mechanisms, we believe including high-density lipoprotein and blood pressure in our analyses shows that these mechanisms alone do not explain the observed differences in cognitive function and %TBV.
Cognitive reserve may explain the discrepancy between cognitive function and %TBV across alcohol consumption levels. The concept of cognitive reserve suggests that among individuals who live in “enriched environments,” neural plasticity will be sustained as the brain ages, and neural networks can be remapped to maintain cognitive function despite the loss of brain volume (39). It is possible that moderate alcohol consumption, or the psychological and biologic benefits associated with such consumption (40), contribute to compensatory mechanisms in the aging brain. Differences in education, head size, or engagement in cognitively stimulating activities have been suggested as proxies for cognitive reserve and were included in our analyses; our results though were not changed. However, measures of cognitive reserve are still being developed and ours may not fully capture the concept. Further, several measures of brain structure and brain physiology that are not evaluated by structural MRI sequences may explain our observed discrepancy.
In conclusion, our findings suggest the relationship between brain volume and cognitive function may provide some benefit, but be diminished in persons drinking at the upper limit of “moderate” intake. In the context of exploring the effects of alcohol on brain structure and function, unmeasured differences in the general level of literacy, engagement in physical or cognitively stimulating activities, morbidity, and cultural and economic differences across study populations may play a role in the discrepancies between alcohol and different brain characteristics.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by National Institutes of Health (N01-AG-12100), the National Institutes of Health/National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), the Althingi (the Icelandic Parliament), and the University of Iceland Research Fund.
Conflict of Interest
The authors report no conflict of interest.
Supplementary Material
Acknowledgment
B.J.K.D. and L.J.L. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Author Contributions: Study concept and design B.J.K.D., J-S.V., and L.J.L. Acquisition of data: M.G., T.A., M.A.B., M.K.J., and S.S. Analysis and interpretation of data: B.J.K.D. and J-S.V. Drafting of the manuscript: B.J.K.D. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: B.J.K.D. and J-S.V. Obtained funding: T.B.H., V.G., and L.J.L. Administrative, technical, and material support: M.G. and S.S. Study supervision: L.J.L.
References
- 1. Harper C. The neurotoxicity of alcohol. Hum Exp Toxicol. 2007;26:251–257. 10.1177/0960327107070499 [DOI] [PubMed] [Google Scholar]
- 2. O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. 10.1016/j.jacc.2007.04.089 [DOI] [PubMed] [Google Scholar]
- 3. Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. J Am Med Assoc. 2003;289:1405–1413. 10.1001/jama.289.11.1405 [DOI] [PubMed] [Google Scholar]
- 4. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. 10.1016/S0140-6736(02)07493-7 [DOI] [PubMed] [Google Scholar]
- 5. Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. 10.1093/ageing/afn095 [DOI] [PubMed] [Google Scholar]
- 6. Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–253. 10.1056/NEJMoa041152 [DOI] [PubMed] [Google Scholar]
- 7. Paul CA, Au R, Fredman L, et al. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65:1363–1367. 10.1001/archneur.65.10.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mukamal KJ, Longstreth WT, Jr, Mittleman MA, Crum RM, Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke. 2001;32:1939–1946. 10.1161/ hs0901.095723 [DOI] [PubMed] [Google Scholar]
- 9. den Heijer T, Vermeer SE, van Dijk EJ, et al. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. Am J Clin Nutr. 2004;80:992–997. [DOI] [PubMed] [Google Scholar]
- 10. Longstreth WT, Jr, Arnold AM, Manolio TA, et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group. Neuroepidemiology. 2000;19:30–42. 10.1159/000026235 [DOI] [PubMed] [Google Scholar]
- 11. MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:169–174. 10.1212/WNL.59.2.169 [DOI] [PubMed] [Google Scholar]
- 12. Lang I, Wallace RB, Huppert FA, Melzer D. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing. 2007;36:256–261. 10.1093/ageing/afm001 [DOI] [PubMed] [Google Scholar]
- 13. Corley J, Jia X, Brett CE, et al. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 study. Neuropsychology. 2011;25:166–175. 10.1037/a0021571 [DOI] [PubMed] [Google Scholar]
- 14. Lindeman RD, Wayne SJ, Baumgartner RN, Garry PJ. Cognitive function in drinkers compared to abstainers in the New Mexico elder health survey. J Gerontol A Biol Sci Med Sci. 2005;60:1065–1070. 10.1093/gerona/60.8.1065 [DOI] [PubMed] [Google Scholar]
- 15. Au Yeung SL, Jiang C, Zhang W, et al. Moderate alcohol use and cognitive function in the Guangzhou Biobank Cohort study. Ann Epidemiol. 2010;20:873–882. 10.1016/j.annepidem.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 16. Leroi I, Sheppard JM, Lyketsos CG. Cognitive function after 11.5 years of alcohol use: relation to alcohol use. Am J Epidemiol. 2002;156:747–752. 10.1093/aje/kwf107 [DOI] [PubMed] [Google Scholar]
- 17. Lobo E, Dufouil C, Marcos G, et al. Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol. 2010;172:708–716. 10.1093/aje/kwq187 [DOI] [PubMed] [Google Scholar]
- 18. Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–555. 10.1097/JGP.0b013e3181a2fd07 [DOI] [PubMed] [Google Scholar]
- 19. Espeland MA, Gu L, Masaki KH, et al. Association between reported alcohol intake and cognition: results from the Women’s Health Initiative Memory Study. Am J Epidemiol. 2005;161:228–238. 10.1093/aje/kwi043 [DOI] [PubMed] [Google Scholar]
- 20. Pfefferbaum A. Alcoholism damages the brain, but does moderate alcohol use? Lancet Neurol. 2004;3:143–144. 10.1016/S1474-4422(04)00676-3 [DOI] [PubMed] [Google Scholar]
- 21. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. 10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delis D, ed. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 24. Salthouse TA, Kersten AW. Decomposing adult age differences in symbol arithmetic. Mem Cognit. 1993;21:699–710. 10.3758/BF03197200 [DOI] [PubMed] [Google Scholar]
- 25. Weschler D, ed. Administration and Scoring Manual for the Weschler Adult Intelligence Scale-III. London, UK: Psychological Corporation; 2008. [Google Scholar]
- 26. Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. 10.1037/0096-3445.121.1.15 [Google Scholar]
- 27. Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. 10.1159/000106735 [DOI] [PubMed] [Google Scholar]
- 28. Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke. 2010;41:891–897. 10.1161/STROKEAHA.110.579581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. 10.1016/j.neuroimage.2011.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37:409–415. 10.1093/alcalc/37.5.409 [DOI] [PubMed] [Google Scholar]
- 31. Verbaten MN. Chronic effects of low to moderate alcohol consumption on structural and functional properties of the brain: beneficial or not? Hum Psychopharmacol. 2009;24:199–205. 10.1002/hup.1022 [DOI] [PubMed] [Google Scholar]
- 32. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacqmin-Gadda H, Sibillot S, Proust C, Molina J-M, Thiébaut R. Robustness of the linear mixed model to misspecified error distribution. Comput Stat Data An. 2007;51:5142–5154. 10.1016/j.csda.2006.05.021 [Google Scholar]
- 34. Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol. 2007;17:S16–S23. 10.1016/j.annepidem.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 35. Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. [DOI] [PubMed] [Google Scholar]
- 36. Elias PK, Elias MF, D’Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150:580–589. [DOI] [PubMed] [Google Scholar]
- 37. Mattace-Raso FU, van der Cammen TJ, van den Elzen AP, et al. Moderate alcohol consumption is associated with reduced arterial stiffness in older adults: the Rotterdam study. J Gerontol A Biol Sci Med Sci. 2005;60:1479–1483. 10.1093/gerona/ 60.11.1479 [DOI] [PubMed] [Google Scholar]
- 38. Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4:363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug Alcohol Depend. 2000;60:221–247. 10.1016/S0376-8716(00)00112-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.