Abstract
The impressive first results of the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) and the adjuvant Tamoxifen To offer more (aTTom) trials both demonstrate that 10 years of tamoxifen is superior to five years of treatment. Tamoxifen is a nonsteroidal antiestrogen that blocks estrogen-stimulated tumor growth. Paradoxically, mortality decreases dramatically only in the decade after long-term tamoxifen is stopped. It is proposed that the evolution and clonal selection of micrometastases that acquire tamoxifen resistance now become increasingly vulnerable to endogenous estrogen-induced apoptosis. Laboratory and clinical studies confirm the concept, and supporting clinical evidence from the estrogen-alone trial in the Women’s Health Initiative (WHI), demonstrate that long-term estrogen-deprived women given exogenous physiologic estrogen have a decreased incidence of breast cancer and decreased mortality. It is proposed that a natural process of apoptosis is recruited to execute the long-term survival benefit of stopping ten years of adjuvant tamoxifen, but only after clonal selection of vulnerable breast cancer cells in an estrogen-deprived environment.
During the 1970s, a strategy of long-term adjuvant therapy with tamoxifen was formulated using a carcinogen-induced rat mammary carcinoma model (1–3), at a time when the Food and Drug Administration (FDA) was only to approve the use of tamoxifen for the treatment of metastatic breast cancer in postmenopausal women on December 29, 1977. Through the clinical trials process and the overview of worldwide randomized adjuvant clinical trials with tamoxifen (4), the standard of care advanced from the original one year of adjuvant therapy to become five years of adjuvant tamoxifen by the mid 1990s (5–14). However, a small clinical trial in node-negative patients, comparing five vs 10 years of tamoxifen, found no evidence of a benefit for patients taking 10 years of adjuvant tamoxifen, but side effects were increased (15). As a result, the standard of care with tamoxifen for the treatment and prevention of breast cancer remained at five years for the next 20 years. But now there is change based on two extremely large randomized clinical trials (16,17).
The recent results of the two evaluations of five vs 10 years of adjuvant tamoxifen clinical trials, Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) (16) and Adjuvant Tamoxifen To offer more (aTTom) (17), both demonstrate statistically significant decreased recurrence rates and decreased mortality for the arm receiving 10 years of tamoxifen, but only in the decade after tamoxifen is stopped. In the ATLAS trial it is evident that there is a modest decrease in disease-free survival (DFS) during treatment (hazard ratio [HR] = 0.95, 95% confidence interval [CI] = 0.79 to 1.02), but this is greater after therapy (HR = 0.75, 95% CI = 0.62 to 0.90) (16). The subsequent response to adjuvant tamoxifen is enhanced with time of treatment. This is excellent news for patient care. However, if tamoxifen and its metabolites (Figure 1) are competitive inhibitors of estrogen-induced tumor growth at the tumor estrogen receptor (ER), then why does mortality decrease in the decade after tamoxifen is stopped (16)? Where does the cytotoxicity come from with tamoxifen, only a palliative therapy in metastatic breast cancer? It is classified a competitive inhibitor of estrogen action (18) that effectively blocks estrogen-stimulated growth at the ER before acquired resistance occurs (19). Stopping adjuvant tamoxifen should allow estrogen binding to ER to cause tumor growth, but it does not.
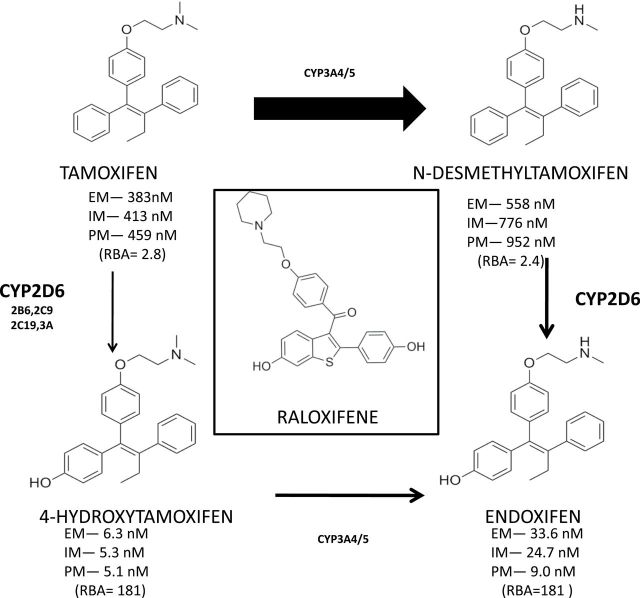
Figure 1.
A diagrammatic representation of relevant tamoxifen metabolism in humans. The thickness of the arrows indicates predominance of pathways with the metabolizing enzymes listed. The relative circulating levels of the parent or the metabolite are reported for extensive metabolizers, intermediate metabolizers, or poor metabolizers by Mürdter et al. (111) based on CYP2D6 genotyping. It can be argued that patients that efficiently metabolize tamoxifen to hydroxylated metabolites (4OHT and endoxifen) with high binding affinity to the estrogen receptor (ER) in an estrogen-rich environment will be better able to block estrogen-stimulated breast cancer cell growth (112). The mix of metabolites will ultimately have an impact on the long-term plasticity of acquired resistant cell populations based on the efficiency of “antiestrogenic pressure” at the tumor ER. Acquired resistance to tamoxifen evolves over five years of retransplantion of ER-positive MCF-7 tumors into athymic mice to become tamoxifen-stimulated but vulnerable to estrogen-induced apoptotic tumor regression (75). The Study of Tamoxifen And Raloxifene showed that during treatment tamoxifen or raloxifene (structure shown center above) produced the same 50% in primary breast cancer (55), but after the five-year treatment was stopped tamoxifen maintained the antitumor action but raloxifene did not and tumor incidence increased (59). Tamoxifen is a long-acting prodrug that maintains high levels of antiestrogen to block the ER; raloxifene is rapidly excreted (57), so lack of compliance will reduce antiestrogenic selection pressure and retard the development of vulnerable populations, sensitive to estrogen-induced apopotosis. The relative binding affinity is based on comparison with E2 as 100. EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer; RBA = relative binding affinity.
The long-term “carry over” effect of tamoxifen is not a new finding, as the biological phenomenon has been noted previously not only in adjuvant clinical trials of tamoxifen (4,20,21) but also in clinical trials used to prevent breast cancer (22–24) and after long-term adjuvant aromatase inhibitor (AI) therapy (25,26). One would predict that once tamoxifen treatment is stopped or an AI stopped and the drug cleared, a women’s own estrogen would reactivate waiting unoccupied tumor ER and cause growth by increasing replication, recurrence rates and death, but it does not! A biological mechanism must come into play to prepare surviving tumor cells for destruction after adjuvant breast cancer therapy with tamoxifen is stopped. Looked at another way, the antiestrogen action of tamoxifen and its metabolites holds the estrogen-stimulated growth of ER+ breast cancer cells during adjuvant therapy and prepares new populations for future sacrifice once tamoxifen is stopped. Cancer cell populations are not static; they must respond to selection pressure to survive. Survival involves a change in chemosensitivity of cells based on new environmental pressures. This has recently been demonstrated to occur rapidly in cell culture with estrogen-deprived breast cancer cells (27).
The hypothesis to be considered here is that long-term treatment selection pressure creates new surviving populations of vulnerable cancer cells that are ready to die when tamoxifen stops. It is proposed that a woman’s own estrogen triggers apoptosis via the tumor ER.
Based on emerging laboratory and clinical evidence, a new unifying concept will be derived that depends on the pharmacology of tamoxifen (28–30), the duration of treatment (31,32), compliance (33,34), and the new biology of estrogen-induced apoptosis that kills correctly prepared estrogen-deprived breast cancer (35).
The Pharmacology of Tamoxifen
Tamoxifen accumulates within the patient’s body and reaches steady state levels in serum within four to six weeks (36). Lien and colleagues (37,38) demonstrated that tamoxifen and metabolites accumulate in tissues 8- to 70-fold above serum levels in patients receiving 40mg daily for at least 30 days, ie, steady state. Daniel et al. (39) measured tamoxifen and metabolites in breast tumors noting results similar to the Lien study (38) when corrections for dosing were made.
In an anecdotal report, intentional noncompliance, by missing increasing numbers of tablets regularly, was noted to result in declining circulating tamoxifen and metabolite levels over a one-year period which eventually resulted in an ER-positive recurrence and subsequent death (40). Recent studies (41,42) build on past work, but further work needs to be done to address intratumor levels of tamoxifen and metabolites. Overall, the fact that tamoxifen and its principal metabolite N-desmethyltamoxifen (Figure 1) have long plasma half-lives of seven and 14 days, respectively, actually plays to the advantage of the medicine to maintain an antiestrogenic environment around the ER-positive micrometastases. Missing a few tablets does not matter. However, tamoxifen is also metabolically activated to 4-hydroxytamoxifen (4OHT) (Figure 1) (43–46). This is important, because the affinity of 4OHT is dramatically increased for the ER. 4-Hydroxytamoxifen became the standard antiestrogen for all future mechanistic studies in vitro and for the discovery of new drugs (47,48) once selective ER modulators (SERMs) emerged a decade later (49). 4-Hydroxy N-desmethyltamoxifen (Figure 1) (50,51), now known as endoxifen, was subsequently shown to have almost identical properties as 4OHT (52–54).
In the 1980s, long-term adjuvant therapy was emerging in the wake of the publication of the Scottish Medical Research Council (MRC) trial of five years of adjuvant tamoxifen vs placebo therapy (but tamoxifen treatment at first recurrence) (14). Early adjuvant therapy saved lives rather than waiting to treat a recurrence after surgery (14). However, there was no strong correlation of response with ER status and a similar conclusion was made following the two-year Nolvadex Adjuvant Trial Organization (NATO) (11,12). It turns out that the problem was the clinical ER assays, but this was solved by the Overview Analysis of all trials (4). A positive outcome for the treatment of breast cancer with tamoxifen depends on the presence of tumor ER (4). There is an interesting déjà vu with the CYP2D6 story of tamoxifen metabolic activation linked to survival and methodologies (29). Unfortunately, this was not completely resolved satisfactorily by overview analysis (30) of studies, as other complex genotyping issues of methodology, ie, loss of heterozygosity in tumor CYP2D6 (29) and treatment issues, are involved, notably patient noncompliance. Nevertheless, a correlation between the presence or absence of a functioning CYP26D genotype was noted with five years of adjuvant therapy (30).
The concept of linking CYP2D6 of genotyped patients with clinical outcomes is simple in theory. It seems obvious that a genotyped population of extensive metabolizer (EM) patients with multiple copies of the CYP2D6 gene will create more endoxifen and an enhanced antiestrogenic environment to block estrogen action at the ER. Tumor growth will be stopped. In contrast, poor metabolizer (PM) patient populations without a competent gene to create the hydroxylating enzyme will be less able to block estrogen binding at the ER. The theory is valid but absolute proof is lacking, probably because of poor compliance; ie, stopping the drug after a year or two is less effective at controlling recurrence and death than full compliance for five years (33). However, if the high affinity of endoxifen for ER was the only factor for treatment success, can pharmacological and clinical evidence be found to support the antiestrogenic “strength” of endoxifen (a hydroxylated high affinity antiestrogen, Figure 1) as the key to a successful outcome after long-term treatment?
Evidence to the contrary comes from the Study of Tamoxifen and Raloxifene (STAR). Tamoxifen and raloxifene (Figure 1) performed equally (50% decrease in tumor incidence) as chemopreventive agents during treatment (55). The pharmacology of tamoxifen and raloxifene is very different. While tamoxifen is a long-acting prodrug, animal studies demonstrate that raloxifene and chemically related compounds are rapidly excreted (56,57). Raloxifene has only 2% bioavailability in patients (58) and is excreted faster than tamoxifen as the former SERM is polyhydroxylated (see Figure 1). Raloxifene is rapidly cleared within 48 hours, especially when the patient is intermittently noncompliant. However, there was a supporting surprise in store for the necessity of cell populations to be driven effectively to a favorable apoptotic state with the reanalysis of STAR after the five-year SERM treatment is terminated (59). Tamoxifen’s antitumor action is maintained when treatment stopped, whereas once raloxifene is stopped antitumor action evaporates. Two years after stopping raloxifene, the effectiveness of breast cancer chemoprevention is only 76% of tamoxifen’s (59). Continuous therapy with raloxifene is recommended to maintain antiestrogen action, as the continuing effect noted with tamoxifen after stopping treatment is not observed with raloxifene. Despite being a SERM with high affinity for ER, raloxifene is different than the prodrug tamoxifen. The durations of treatment are the same, but raloxifene does not create optimal selection pressure clinically in preparation for stopping the drug. In the laboratory, with controlled and consistent daily antihormonal selection pressure with raloxifene used in vitro (60) and in vivo (61), it is possible to drive cell selection to populations that are vulnerable to estrogen-induced apoptosis. Compliance is, therefore, essential to achieve optimal selection pressure for long durations.
Duration of Treatment and Compliance
The ER is the target in the breast tumor that is essential for estrogen-stimulated growth. Tamoxifen and metabolites bind to the ER and block estrogen-stimulated growth (62). Despite the emerging laboratory data in a carcinogen-induced rat mammary carcinoma model (1–3) that longer was going to be superior than shorter therapy, in the 1970s the choice of one to two years of adjuvant tamoxifen (5–12) was sound. Tamoxifen was only effective in metastatic breast cancer for one to two years (19,63-65). Therefore, it was reasoned that adjuvant therapy for more than a year would encourage antihormone resistance, early recurrence, and death. Over the next decade, numerous adjuvant trials crept from one year to five years (4,11–14).
The individual trials of long-term adjuvant tamoxifen treatment established an important fact, ie, the development of resistance to tamoxifen therapy during the treatment of metastatic breast cancer is completely different than when tamoxifen is deployed to treat micrometastases.
The Overview Analysis at Oxford (4,20) precisely defined three important facts in the 1980s and 1990s. Firstly, the ER is the target for effective tamoxifen action: ER-negative tumors do not respond to long-term adjuvant tamoxifen therapy. This clinical conclusion, about 20 years ago, validated tamoxifen’s role as the first targeted therapy in cancer that saves lives (66). Secondly, trials of one, two, and five years of tamoxifen showed (4,20) that longer adjuvant tamoxifen was better at preventing recurrences and decreasing mortality. This was particularly clear with ER-positive tumors in premenopausal patients receiving tamoxifen alone. The increases in lives saved with increasing durations of tamoxifen therapy now provided a database to estimate lives lost from noncompliance with long-term adjuvant tamoxifen therapy (33). In other words, stopping tamoxifen before five years say for one or two years is likely to reduce chances of survival. Thirdly, there is nothing unique about the profound decreases in mortality even in ATLAS (16) or aTTom (17). Five years of adjuvant tamoxifen therapy continues to enhance survival by decreasing mortality after stopping the drug, but not as much as 10 years. Five years is superior to two years of adjuvant tamoxifen, and one year is inferior to two years (4,20). Tamoxifen is not simply a competitive antagonist of estrogen action at the ER. Longer-term treatment improves the chances of survival after tamoxifen stops.
If the key to success with tamoxifen used for the adjuvant treatment of breast cancer is long-term therapy targeted to the tumor ER, then compliance is important. It is axiomatic in therapeutics for the treatment of disease that no drug means no benefit. The recent review by Chlebowski and colleagues (34) elegantly summarizes this substantial clinical problem in adjuvant antihormone therapy of breast cancer and for prevention. In both cases, long-term therapy of at least five years has been shown to have therapeutic benefit for patients. Lives are saved or breast tumor incidence reduced, as demonstrated in randomized clinical trials where adherence is high. By contrast, Chlebowski and colleagues (34) argue that adherence in clinical practice can be as low as 50%. Procedures need to address this issue, because lives are lost by the premature cessation of tamoxifen (33). To understand the critical importance of duration of therapy and compliance, it is necessary to appreciate the evolution of knowledge of how antihormone resistance occurs that has changed over the past three decades.
Evolution of Acquired Antihormone Resistance
Studies with MCF-7 cells inoculated into ovariectomized athymic mice provide a valuable insight into the development of acquired resistance to long-term antihormone therapy. The ER-positive tumors grow despite long-term treatment of the mice with tamoxifen (67). However, the observation that the ER-positive tumors eventually grow in response to tamoxifen (68) described a new form of resistance: tamoxifen stimulated tumor growth. Further study demonstrated that the model was unique to the tumors, as they grew in athymic rats, thereby negating the argument that species-specific metabolism to nonsteroidal estrogens was responsible for tumor-stimulated growth (69). Additional studies (70) showed that tamoxifen derivatives resistant to isomerization to potential estrogenic isomeric metabolites also stimulated tumors to grow, so it was unlikely that estrogenic metabolites were being produced against time to create acquired resistance. This focused attention on discovering new second-line therapies directed at acquired tamoxifen resistance. The pure antiestrogen, ICI 164,384 was noted to prevent tamoxifen-stimulated growth (71) in athymic mice, and a decade later fulvestrant or an aromatase inhibitor (no estrogen) became the second-line treatments of choice, following tamoxifen failure (72,73).
The initial laboratory model (68) replicated clinical experience with tamoxifen for the treatment of metastic breast cancer. The model did not replicate clinical experience, published around the same time in 1987 (14) with long-term adjuvant tamoxifen therapy that effectively suppressed the growth of poorly established micrometastases in the Scottish Trial of five years of adjuvant treatment vs placebo (tamoxifen at recurrence). Long-term adjuvant tamoxifen therapy was effective but another mechanism of resistance must be occurring to benefit patients under adjuvant conditions with micrometastases. Clinical reality trumps a laboratory model in the conversation with nature.
Micrometastases are small clusters of ER-positive cells that may choose to grow, die, or remain static for years. They are suppressed from growth by long-term adjuvant tamoxifen therapy. Only breakaway cell populations, selected for growth in a tamoxifen metabolite environment, will slowly become established into tumors with acquired resistance during long-term adjuvant therapy. Early noncompliance of the patient and a woman’s own estrogen will then advance the tumors through replication and growth, which ultimately results in a recurrence and death. This is why one or two years of adjuvant tamoxifen does not promote the survival advantages of five years of tamoxifen (4,20,21). Longer is better to select early acquired resistant breast cancer cells to evolve into a new population of vulnerable clones.
The initial form of acquired tumor resistance to tamoxifen that grows with either tamoxifen or estrogen is now referred to as Phase I resistance (31). Experience with laboratory models demonstrates that Phase I resistance persists for about three years in a tamoxifen-treated environment in vivo (68). However, laboratory evidence also demonstrates that continuous passage of Phase I resistant tumors into successive generations of tamoxifen-treated athymic mice results in an evolution of the biological characteristics of the resulting cell population (74,75). Tamoxifen continues to support tumor growth and vitality, but physiologic estradiol now causes rapid tumor regression, and small tumors disappear whereas larger tumors regress (74,75). This is called Phase II acquired resistance (31).
The laboratory finding in vivo (75) that breast cancer cells maintained in a long-term estrogen-deprived environment with the antiestrogen tamoxifen can be triggered to die with estrogen treatment reaches back 60 years to the pioneering use of high-dose estrogen therapy as the first successful treatment of breast cancer in postmenopausal women. However, the clinical finding that tumor response depended on the time of treatment from menopause is relevant. The success of estrogen treatment depended on timing and estrogen deprivation.
The Paradox of Physiologic Estrogen Action as Antitumor Agent in Estrogen-Deprived Patients
The pioneering use of high-dose estrogen therapy as the first chemical therapy to treat any cancer in the 1940s is important (76), as the principles noted are consistent with current clinical observations. Haddow (77) conducted perhaps the first multicenter clinical trial through the good offices of the Royal Society of Medicine (at the time of his trial of high-dose estrogen therapy, he was the President of the Oncology Section). His words best state his findings:
When the various reports were assembled at the end of that time, it was fascinating to discover that rather general impression, not sufficiently strong from the relatively small numbers in any single group, became reinforced to the point of certainty; namely, the beneficial responses were three times more frequent in women over the age of 60 years than in those under that age; that oestrogens may, on the contrary, accelerate the course of mammary cancer in younger women, and that their therapeutic use should be restricted to cases 5 years beyond the menopause. Here was an early and satisfying example of the advantages which may accrue from cooperative clinical trial. (77)
Today this is interpreted as meaning that an estrogen-free period is necessary following the menopause to allow cell populations that are in harmony with a replete estrogenic environment to die of estrogen starvation, but a small remaining cell population slowly emerges by selection in the austere estrogen environment after the menopause (Figure 2). Stoll (78) illustrated the point of a menopausal interval being necessary for metastatic breast tumors vulnerable to estrogen-induced regression to emerge. Out of 63 patients within five years of menopause, there was 9% regression. Out of 344 patients who had been menopausal for over five years, there was 35% regression (78). This was based on his own clinical practice before the time of tamoxifen (Table 1). The principle has recently been illustrated in cell culture (79). So if it is necessary to have an appropriate time of estrogen deprivation (which remarkably seems to be five years in models with MCF-7 cells in the laboratory, in Haddow and Stoll studies, and in the overview of clinical trials!), this, by coincidence, appears to be necessary for cell selection pressure to create the evolution of vulnerable populations (Figure 2).
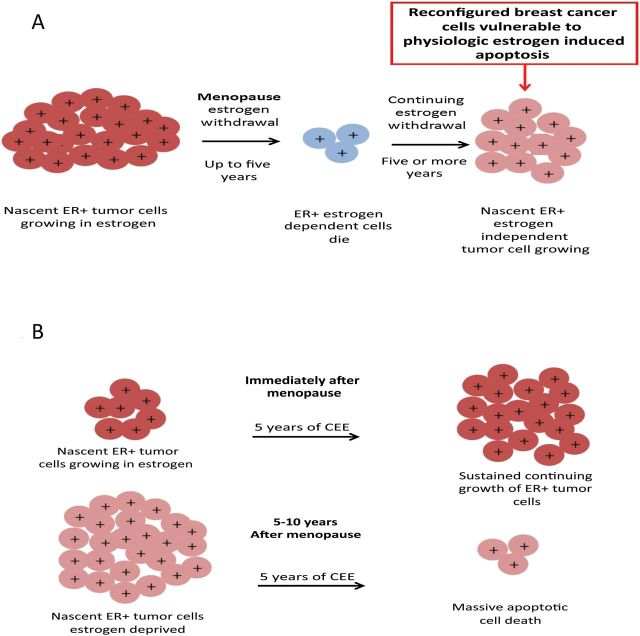
Figure 2.
The success of estrogen replacement therapy to reduce the risk of breast cancer is dependent on the menopausal state of a woman. A) Immediate estrogen withdrawal at menopause in postmenopausal women causes ER-positive estrogen-dependent cells to die but ultimately some cells continue to grow independent of estrogen. B) Treatment of women immediately after menopause with conjugated equine estrogen results in sustained growth of nascent ER-positive tumors, whereas treatment five years after menopause causes apoptotic cell death (reproduced with permission) (79). Additional support for this model comes from the Million Women Study (100); but numbers of breast cancer are too few at the relevant ages to arrive at a firm conclusion. By contrast, the pioneering work of Sir Alexander Haddow, FRS (77), first noted that a gap of five years was necessary for high-dose estrogen therapy to be successful at a 30% response rate. Patients treated nearer the menopause were unresponsive to high-dose therapy. A period of estrogen deprivation was required prior to successful high-dose treatment for metastatic breast cancer. Stoll (78) confirmed this requirement in his own practice with a response rate of metastatic breast cancer to high-dose estrogen of 9% for patients within five years of the menopause but 35% for patients after the five-year gap period. ER = estroger receptor; CEE = conjugated equine estrogen.
Table 1.
Objective response rate to estrogen therapy in 407 patients with advanced breast cancer to age group in relation to menopause (78)
| Age since menopause | Patient, no. | % regression |
|---|---|---|
| Postmenopausal 0–5 y | 63 | 9 |
| Postmenopausal >5y | 344 | 35 |
The importance of these laboratory findings on acquired resistance to tamoxifen (74,75) is evidenced by translation to clinical care. The exhaustive antihormonal treatment of estrogen-driven metastatic breast cancer forces the evolution to Phase II acquired antihormone refractory disease successfully, so that high-dose estrogen therapy now is effective in causing tumor regression (80). The results of a limited series of patients by Lonning (80) are remarkable, with four out of 32 patients having complete remission. The response of one refractory metastatic breast cancer is of note, as the patient had a complete remission on continuous estrogen therapy for five years but remained tumor free for another six years after estrogen therapy was stopped (81). Unfortunately, the application of high-dose estrogen therapy is of limited value because of extensive unpleasant side effects, including life-threatening thrombosis. Recurrences following the failure of adjuvant aromatase inhibitor treatment have been the focus of a small study of high- (30mg) vs low-dose (6mg) estradiol therapy (82). Response rates were again 30% for clinical benefit. The fact that response rates were not more profound may lie in the fact that tumors only responded and failed one or at a most two rounds of antihormone treatment. A more aggressive antihormone environment is necessary to drive antihormone-resistant cell selection and for the larger established recurrences to be destroyed.
With this background of the influence of the pharmacology of SERMs, duration of adjuvant therapy, compliance, and the new knowledge of the acquired resistance to SERMs that leads to a vulnerability, ie, estrogen-induced apoptosis, it is now appropriate to create a hypothesis for future evaluation.
A Consolidated Hypothesis of the Mechanism by Which Tamoxifen Decreases Mortality
There are multiple interconnected dimensions to the development of the correct cellular population that is vulnerable to eradication through the apoptotic action of physiological estrogen. The concept is summarized in Figure 3. It is evident that by creating selection pressure immediately after surgery through adjuvant tamoxifen therapy, the key to the evolution of cell populations is time. Estrogen-induced apoptosis is dependent on compliance, and tamoxifen is a drug where daily compliance is not essential but years of therapy are. Tamoxifen has a long biological half-life; and selection pressure is maintained. But selection pressure is potentially improved to drive Phase I acquired resistance in micrometastases (that occurs in one to two years) through to completion of Phase II acquired resistance by the appropriate CYP2D6 genotyping for the breast cancer patient. Time is essential to evolve to Phase II resistance with the optimal CYP2D6 patient genotype (EM) to create a potent mix of antiestrogenic metabolites, with a depot of constant endoxifen supply to develop vulnerability to trigger apoptosis with physiologic estrogen. Remaining at Phase I acquired resistance and stopping tamoxifen results in estrogen-induced growth, recurrence, and death (33). It is essential to have long-term therapy to get to Phase II resistance; however, an EM patient may achieve that goal earlier for the vulnerable tumor cell population. A PM patient with little of the high affinity metabolite, endoxifen, may need much longer tamoxifen.
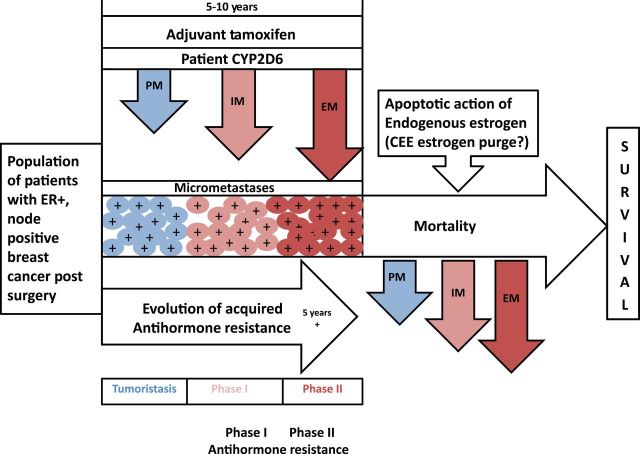
Figure 3.
The evolution of acquired resistance to tamoxifen based on CYP2D6 status for extensive metabolizers (EM), intermediate metabolizers (IM), and poor metabolizers (PM). Long-term tamoxifen (five years) is already complete by the protocol requirements for recruitment to the ATLAS trial. It is suggested that the cellular selection pressure for PM, IM, and EM differs based on the local mixture of metabolites continuing endoxifen (Figure 1). The higher selection pressure brings to completion the selection to a vulnerable population that is triggered to apoptosis by estrogen in the woman’s body (or if appropriate a few weeks of an exogenous “estrogen purge” in future study). Decreases in the mortality may therefore be found to be proportional to the metabolite concentration (genotype), given compliance out to five years (30). The core scientific concept for the evolution of acquired resistance by micrometastatic disease during adjuvant therapy is derived using the ER-positive MCF-7 cell line serially transplanted into successive generations of athymic mice during selective ER modulation therapy (61,75). The rate of evolution to Phase II (vulnerable to estrogen-induced apoptosis) resistance will depend on the “antiestrogen mix” of potent metabolites of tamoxifen created by genotyping PM, IM, and EM. The resulting vulnerable populations will have mortality decreases based on their prepared vulnerability to estrogen-induced apoptosis from a woman’s own estrogen after tamoxifen is stopped. Survival could subsequently be correlated to CYP2D6 status, compliance, estrogen levels in patients, and body mass index as a surrogate of circulating estrogen status. EM = extensive metabolizer; ER = estrogen receptor; IM = intermediate metabolizer; PM = poor metabolizer.
To suggest a clinical model of tamoxifen action that depends on the selection of vulnerable cells, two questions must now be asked. Firstly: Is there evidence that cells from ER-positive primary tumors can be enriched through endocrine therapy to exhibit markers not present in the original tumor? The enrichment of mutant ER in a metastasis can be viewed “generically” as a response to selection pressure. Although this is not evidence of selection pressure to an estrogen-induced apoptotic state, it is evidence of population change, nevertheless. The ER of cells that triggers estrogen-induced apoptosis is wild type, ie, it is the cell context, not the ER. There are two intriguing reports of selection of specific genotype changes in the ER in the literature: 1) The D351Y ER mutation cannot be detected in wild-type MCF-7 cells, but selection pressure in athymic mice during months of tamoxifen therapy produces a tumor with an excess of ER D351Y (83,84) that is known to modulate the estrogenicity of SERMS (85), and it is therefore a growth advantage to have an ER that increases the estrogenicity of SERMs; 2) mutant ERs in clinical metastases have recently been discovered from patients that are resistant to antihormone therapy (86–90). Incidentally, the mutations in the ER need to interact with D351 as an anchor to create the active complex (88), so it is a self-supporting mechanistic story. The same mutant ERs are either below the level of detection or at a low frequency in the primary tumors. If metastatic cell selection is demonstrable (86–90) under therapeutic pressure with aromatase inhibitors, and populations evolve to survive, then what is the second question? The second question is: Is there clinical evidence that physiologic estrogen can be an apoptotic agent to vulnerable nascent breast cancer cells?
The Women’s Health Initiate (WHI) of conjugated equine estrogen (CEE) actually provides the strongest clinical evidence that estrogen administration to menopausal and estrogen-deprived women in their 60s can destroy the correctly prepared populations of ER-positive breast cancer cells. The repeated analysis of the WHI study of CEE vs placebo in hysterectomized women (91–93) showed a paradoxical decrease in the incidence of breast cancer, which was maintained for six years after CEE therapy (six years) was stopped. The substantial antitumor effect on microscopic disease was unanticipated, because estrogen clearly stimulates the growth of ER-positive cells in cell culture (94) and tumors will only growth in athymic mice inoculated with ER-positive tumor cells if estrogen is administered (95). This is the first law of estrogen-dependent breast cancer growth and is the entire basis for the success of antiestrogenic strategies (tamoxifen, aromatase inhibitors) in the treatment of breast cancer (96).
However, as noted previously in the commentary, the work of Haddow (77) and Stoll (78) observed the requirement for a five-year gap period following menopause for postmenopausal women to benefit optimally with a 30% response rate for the treatment of breast cancer. The tumor has adapted to learn to grow without high concentrations of premenopausal estrogens, but now high-dose estrogen caused tumor regression (Figure 2).
In the case of women taking estrogen replacement therapy (ERT), the nascent breast cancer model would depend on the regrowth of estrogen-independent breast cancer cells that after five years of estrogen deprivation would be vulnerable to CEE-induced apoptosis (Figure 2A). This explanation is offered to explain the results of the WHI CEE trial (79,93), based on results with long-term estrogen-deprived breast cancer cells in the laboratory (97,98). However, if the CEE is given at menopause, cell replication rather than apoptosis would be anticipated based on cell culture studies (Figure 2B) (79). Support for the science is available from the Million Women Study (99). The risk of breast cancer for women taking both estrogen and a progestin was greater if therapy was started before or immediately after menopause than after a longer gap (P < .001) (100). Current users of ERT had no increase in breast cancer if begun five years after the menopause, but statistically experience an increase if used less than five years after the menopause (risk ratio [RR] = 1.43) (100).
A model of tamoxifen-mediated cell survival and the evolution of estrogen-deprived populations over time is now proposed based on a synthesis of laboratory and clinical observations (Figure 3) to explain decreases of mortality in ATLAS and aTTom after long-term tamoxifen is stopped. There is a remarkable symmetry in the biology of estrogen in the WHI and the benefits of long-term adjuvant tamoxifen (Figure 4). Understanding of the interlocking dimensions of compliance and the time required to create the selection pressure for the cells to survive and, most importantly, to enter a vulnerable state ready to be killed by estrogen is essential. Tamoxifen must be viewed as a depot drug that saturates the body, slowly producing metabolites with high affinity for ER. These metabolites do not just block ER but are critical to aid cell selection to estrogen vulnerability. The recent overview publication by the International Tamoxifen Pharmacogenomics Consortium (30) modestly illustrates the point that with five years of tamoxifen, the genotyping to EM and PM shows that recurrence and survival is better for EM patients than PM patients. The CEE WHI study supports the mechanistic investigation of estrogen-induced apoptosis in estrogen-deprived patients. Like the overview analysis of adjuvant clinical trials (21) and the chemoprevention trials (22–24), the CEE WHI study shows enduring benefit after CEE is stopped (93). Laboratory evidence (79) illustrates the conclusion that the small nascent estrogen-deprived tumor breast cells are readily destroyed by CEE. The same apoptotic biology can apply to long-term adjuvant tamoxifen with the woman’s own estrogen responsible for the survival advantages in ATLAS and aTTom after tamoxifen stops (16,17).
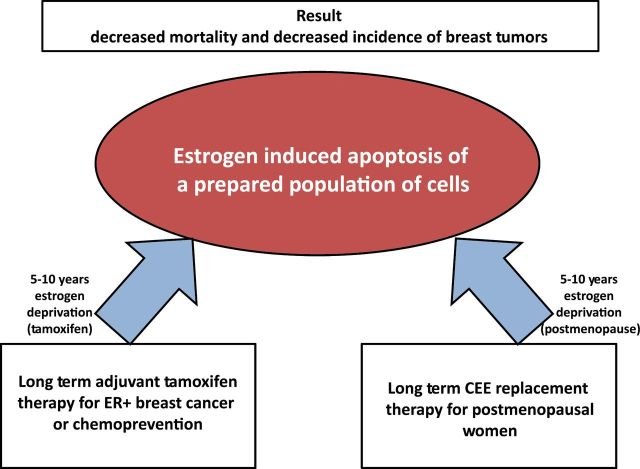
Figure 4.
The symmetry of the results from conjugated equine estrogen (CEE) trial of the Women’s Health Initiative (93) and long-term tamoxifen action (16). The hypothesis to address is that both require at least five years of estrogen deprivation to trigger estrogen-induced apoptosis. In the case of CEE and the historical use of high doses of estrogen therapy for breast cancer, both approaches require an interval of at least five years postmenopause to achieve an optimal 30% tumor response rate. Five years of tamoxifen produces an optimal result during treatment to create estrogen deprivation in the microtumor environment by ER blockade; but both tamoxifen and CEE produced sustained decreases in mortality after treatment. Microfoci of estrogen-deprived cells are killed by estrogen by the woman’s own estrogen or by CEE treatment in a woman during her 60s, respectively. The fact that CEE causes a decrease in incidence and mortality in women in their 60s provides a prima facia case that the estrogen deprivation created by long-term tamoxifen therapy creates vulnerable cell populations that can respond to estrogen with an apoptotic response to affect sustained cures. ER = estrogen receptor; CEE = conjugated equine estrogen.
By Looking Back We Can See the Way Forward
Sir Alexander Haddow, FRS, presented (77) the inaugural David A. Karnofsky Lecture, the highest award from the American Society of Clinical Oncology, in 1970. He stated, “…the extraordinary extent of tumor regression is perhaps 1% of postmenopausal cases (with estrogen) has always been regarded as of major theoretical importance, and it is a matter of some disappointment that so much of the underlying mechanisms continue to elude us...” (77).
Much has now been accomplished to understand the molecular mechanism and modulation of estrogen-induced apoptosis (27,101,102) and how the shape of different classes of estrogens used by Haddow can modulate the apoptotic trigger (103–105) in estrogen-deprived breast cancer cells. However, much must be accomplished in the future to confirm, consolidate, and exploit the link between estrogen-induced apoptosis and long-term antihormone therapy.
The cellular models used to understand the evolution of acquired resistance to tamoxifen are derived from the ubiquitous MCF-7 cell line that has already taught us so much about the general principles of hormone-dependent breast cancer in patients (95). Surprisingly enough, the MCF-7 cell line is from a pleural effusion of a patient treated with high-dose estrogen, before the days of tamoxifen. A large panel of ER-positive metastatic breast cancer cells needs to be created to track the evolution of acquired resistance to estrogen withdrawal and tamoxifen both in vitro and transplanted into athymic mice. A study to document further the importance of time for selection of vulnerable populations will be important with a broad spectrum of ER-positive cell types. Perhaps a signature for estrogen-induced apoptosis could be documented in the laboratory and trials in patients following exhaustive antihormone therapy could be used to validate predictive data. Gene signatures are emerging to discover probabilities of late relapse based on both MA-17 and ATAC trials (106–108) to answer the questions of who would be a candidate for extended antihormone adjuvant therapy.
The Study of Letrozole Extension (SOLE) (35) trial of continuous aromatase inhibition extension for five years following completion of five years of antihormone adjuvant therapy is compared to an arm with three months of an annual aromatase inhibitor (AI) holiday (35). The hypothesis is that the woman’s own estrogen, which is resynthesiszed during the drug holiday, may cause apoptosis in vulnerable AI-resistant breast cancer cells, thereby reducing recurrence rates and improving survival. Depending on the results, it may be that an estrogen “purge” could be evaluated in clinical trial to enhance the success of apoptosis further. The design would be similar to the proposal (109) to use a bazedoxifene/conjugated estrogen formulation to drive the evolution of occult breast cancers to an antihormone-resistant state with the SERM bazedoxifene during a five-year period at menopause and then “purge” vulnerable SERM-resistant occult breast cancer cells with estrogen alone for a few months.
In closing, it is worthy of note that for more than a century, the response to antihormone therapy as first-line treatment has remained at one in three tumors having an objective response (96), and this was enhanced by the identification and selection of patients with the tumor ER target (62). Now multiple agents are being evaluated to enhance antihormone therapy. A major question to be addressed in the future by cellular studies and clinical trials is why only about one third of patients with long-term acquired resistance to estrogen deprivation can have apoptosis triggered with estrogen (76,78,80,82,93). Is there a way to predict and improve estrogen-induced apoptosis response rates after long-term adjuvant antihormone therapy? The clues to progress may be within reach by a study of why ERT causes apoptosis in the occult estrogen-deprived breast cancer in 60-year-old postmenopausal women, but a combination with a synthetic progestin antagonizes the process and increases breast cancer risk (93). Not only would there be an advance in understanding what blocks estrogen-induced apoptosis, but also it may be possible to define a safer hormone replacement therapy that can be used, after an appropriate gap following menopause to reduce the incidence of breast cancer (110). The idea of two linked concepts of long-term estrogen deprivation therapy to exert selection pressure and expose vulnerable cells for estrogen-induced apoptosis (74,75) may have many applications in the future to aid women’s health.
Funding
This work was supported by the Department of Defense Breast Program (award number W81XWH-06-1-0590) Center of Excellence, Susan G. Komen for the Cure Foundation (award number SAC100009), Comprehensive Cancer Center Support Grant (core grant NIH P30 CA051008).
The author takes full responsibility for the content of this commentary, and the decision to submit the commentary for publication. The author declares no potential conflicts of interest.
This commentary is dedicated to the memory and achievements of Sir Alexander Haddow, FRS, whose translational research opened my eyes to the potential of estrogen action to kill breast cancer cells. I would like to thank Russell McDaniel and Fadeke Agboke for their indispensable assistance to complete this manuscript. The views and opinions of the author do not reflect those of the US Army or the Department of Defense.
References
- 1. Jordan VC, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. In: Salmon S, Jones S, eds. Adjuvant Therapy of Cancer II. New York, NY: Grune & Stratton Inc.; 1979:19–26. [Google Scholar]
- 2. Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16(2):239–251. [DOI] [PubMed] [Google Scholar]
- 3. Jordan VC. Laboratory studies to develop general principles for the adjuvant treatment of breast cancer with antiestrogens: problems and potential for future clinical applications. Breast Cancer Res Treat. 1983;3 Suppl:S73–S86. [DOI] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 5. Hubay CA, Pearson OH, Marshall JS, et al. Adjuvant therapy of stage II breast cancer: 48-month follow-up of a prospective randomized clinical trial. Breast Cancer Res Treat. 1981;1(1):77–82. [DOI] [PubMed] [Google Scholar]
- 6. Ribeiro G, Palmer MK. Adjuvant tamoxifen for operable carcinoma of the breast: report of clinical trial by the Christie Hospital and Holt Radium Institute. Br Med J (Clin Res Ed). 1983;286(6368):827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludwig, Group BCS. Randomised trial of chemo-endocrine therapy, endocrine therapy, and mastectomy alone in postmenopausal patients with operable breast cancer and axillary node metastasis. Lancet. 1984;323:1256–1260. [PubMed] [Google Scholar]
- 8. Cummings FJ, Gray R, Davis TE, et al. Adjuvant tamoxifen treatment of elderly women with stage II breast cancer. A double-blind comparison with placebo. Ann Intern Med. 1985;103(3):324–329. [DOI] [PubMed] [Google Scholar]
- 9. Rose C, Thorpe SM, Andersen KW, et al. Beneficial effect of adjuvant tamoxifen therapy in primary breast cancer patients with high oestrogen receptor values. Lancet. 1985;1(8419):16–19. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro G, Swindell R. The Christie Hospital tamoxifen (Nolvadex) adjuvant trial for operable breast carcinoma--7-yr results. Eur J Cancer Clin Oncol. 1985;21(8):897–900. [DOI] [PubMed] [Google Scholar]
- 11. Nolvadex Adjuvant Trial Organisation. Controlled trial of tamoxifen as adjuvant agent in management of early breast cancer. Interim analysis at four years. Lancet. 1983;1(8319):257–261. [PubMed] [Google Scholar]
- 12. Nolvadex Adjuvant Trial Organisation. Controlled trial of tamoxifen as single adjuvant agent in management of early breast cancer. Analysis at six years. Lancet. 1985;1(8433):836–840. [PubMed] [Google Scholar]
- 13. Fisher B, Brown A, Wolmark N, et al. Prolonging tamoxifen therapy for primary breast cancer. Findings from the National Surgical Adjuvant Breast and Bowel Project clinical trial. Ann Intern Med. 1987;106(5):649–654. [DOI] [PubMed] [Google Scholar]
- 14. Scottish Cancer Trials Office (MRC). Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Edinburgh. Lancet. 1987;2(8552):171–175. [PubMed] [Google Scholar]
- 15. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88(21):1529–1542. [DOI] [PubMed] [Google Scholar]
- 16. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The aTTom Collaborative Group. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31 18 Suppl. [Google Scholar]
- 18. Jordan VC. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984;36(4):245–276. [PubMed] [Google Scholar]
- 19. Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304(1):16–21. [DOI] [PubMed] [Google Scholar]
- 20. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 21. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. [DOI] [PubMed] [Google Scholar]
- 23. Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. [DOI] [PubMed] [Google Scholar]
- 24. Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. [DOI] [PubMed] [Google Scholar]
- 25. Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. [DOI] [PubMed] [Google Scholar]
- 26. Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141. [DOI] [PubMed] [Google Scholar]
- 27. Fan P, Agboke FA, McDaniel RE, et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2014;50(2):457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDaniel RE, Maximov PY, Jordan VC. Estrogen-mediated mechanisms to control the growth and apoptosis of breast cancer cells: a translational research success story. Vitam Horm. 2013;93:1–49. [DOI] [PubMed] [Google Scholar]
- 29. Brauch H, Schwab M. Prediction of tamoxifen outcome by genetic variation of CYP2D6 in post-menopausal women with early breast cancer. Br J Clin Pharmacol. 2014;77(4):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Province MA, Goetz MP, Brauch H, et al. CYP2D6 genotype and adjuvant tamoxifen: meta-analysis of heterogeneous study populations. Clin Pharmacol Ther. 2014;95(2):216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5(3):207–213. [DOI] [PubMed] [Google Scholar]
- 32. Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26(18):3073–3082. [DOI] [PubMed] [Google Scholar]
- 33. McCowan C, Wang S, Thompson AM, et al. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109(5):1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7(4):378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jordan VC, Ford LG. Paradoxical clinical effect of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res (Phila). 2011;4(5):633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. [DOI] [PubMed] [Google Scholar]
- 37. Lien EA, Ueland PM, Solheim E, et al. Determination of tamoxifen and four metabolites in serum by low-dispersion liquid chromatography. Clin Chem. 1987;33(9):1608–1614. [PubMed] [Google Scholar]
- 38. Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991;51(18):4837–4844. [PubMed] [Google Scholar]
- 39. Daniel P, Gaskell SJ, Bishop H, et al. Determination of tamoxifen and biologically active metabolites in human breast tumours and plasma. Eur J Cancer Clin Oncol. 1981;17(11):1183–1189. [PubMed] [Google Scholar]
- 40. Jordan VC, Fritz NF, Tormey DC. Endocrine effects of adjuvant chemotherapy and long-term tamoxifen administration on node-positive patients with breast cancer. Cancer Res. 1987;47(2):624–630. [PubMed] [Google Scholar]
- 41. MacCallum J, Cummings J, Dixon JM, et al. Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer. 2000;82(10):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gjerde J, Gandini S, Guerrieri-Gonzaga A, et al. Tissue distribution of 4-hydroxy-N-desmethyltamoxifen and tamoxifen-N-oxide. Breast Cancer Res Treat. 2012;134(2):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jordan VC, Collins MM, Rowsby L, et al. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75(2):305–316. [DOI] [PubMed] [Google Scholar]
- 44. Jordan VC, Dix CJ, Naylor KE, et al. Nonsteroidal antiestrogens: their biological effects and potential mechanisms of action. J Toxicol Environ Health. 1978;4(2–3):363–390. [DOI] [PubMed] [Google Scholar]
- 45. Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol. 1980;71(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981;256(2):859–868. [PubMed] [Google Scholar]
- 47. Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J Med Chem. 2003;46(6):883–908. [DOI] [PubMed] [Google Scholar]
- 48. Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J Med Chem. 2003;46(7):1081–1111. [DOI] [PubMed] [Google Scholar]
- 49. Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50(14):4177–4189. [PubMed] [Google Scholar]
- 50. Lien EA, Solheim E, Kvinnsland S, et al. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988;48(8):2304–2308. [PubMed] [Google Scholar]
- 51. Lien EA, Solheim E, Lea OA, et al. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49(8):2175–2183. [PubMed] [Google Scholar]
- 52. Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151–159. [DOI] [PubMed] [Google Scholar]
- 53. Lim YC, Desta Z, Flockhart DA, et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55(5):471–478. [DOI] [PubMed] [Google Scholar]
- 54. Lim YC, Li L, Desta Z, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318(2):503–512. [DOI] [PubMed] [Google Scholar]
- 55. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. [DOI] [PubMed] [Google Scholar]
- 56. Jordan VC, Gosden B. Inhibition of the uterotropic activity of estrogens and antiestrogens by the short acting antiestrogen LY117018. Endocrinology. 1983;113(2):463–468. [DOI] [PubMed] [Google Scholar]
- 57. Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47(15):4020–4024. [PubMed] [Google Scholar]
- 58. Snyder KR, Sparano N, Malinowski JM. Raloxifene hydrochloride. Am J Health Syst Pharm. 2000;57(18):1669–1675; quiz 1676–1678. [PubMed] [Google Scholar]
- 59. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila). 2010;3(6):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu H, Lee ES, Gajdos C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95(21):1586–1597. [DOI] [PubMed] [Google Scholar]
- 61. Balaburski GM, Dardes RC, Johnson M, et al. Raloxifene-stimulated experimental breast cancer with the paradoxical actions of estrogen to promote or prevent tumor growth: a unifying concept in anti-hormone resistance. Int J Oncol. 2010;37(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–1989. [PubMed] [Google Scholar]
- 63. Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ward HW. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J. 1973;1(5844):13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgan LR, Jr, Schein PS, Woolley PV, et al. Therapeutic use of tamoxifen in advanced breast cancer: correlation with biochemical parameters. Cancer Treat Rep. 1976;60(10):1437–1443. [PubMed] [Google Scholar]
- 66. Jordan VC. Tamoxifen: catalyst for the change to targeted therapy. Eur J Cancer. 2008;44(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23(8):1189–1196. [DOI] [PubMed] [Google Scholar]
- 68. Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48(18):5183–5187. [PubMed] [Google Scholar]
- 69. Gottardis MM, Wagner RJ, Borden EC, et al. Differential ability of antiestrogens to stimulate breast cancer cell (MCF-7) growth in vivo and in vitro. Cancer Res. 1989;49(17):4765–4769. [PubMed] [Google Scholar]
- 70. Wolf DM, Langan-Fahey SM, Parker CJ, et al. Investigation of the mechanism of tamoxifen-stimulated breast tumor growth with nonisomerizable analogues of tamoxifen and metabolites. J Natl Cancer Inst. 1993;85(10):806–812. [DOI] [PubMed] [Google Scholar]
- 71. Gottardis MM, Jiang SY, Jeng MH, et al. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49(15):4090–4093. [PubMed] [Google Scholar]
- 72. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395. [DOI] [PubMed] [Google Scholar]
- 73. Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403. [DOI] [PubMed] [Google Scholar]
- 74. Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. [DOI] [PubMed] [Google Scholar]
- 75. Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6(5):2028–2036. [PubMed] [Google Scholar]
- 76. Haddow A, Watkinson JM, Paterson E, et al. Influence of synthetic oestrogens on advanced malignant disease. Br Med J. 1944;2(4368):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haddow A., David A. Karnofsky memorial lecture. Thoughts on chemical therapy. Cancer. 1970;26(4):737–754. [DOI] [PubMed] [Google Scholar]
- 78. Stoll B. Palliation by castration or hormone ablation. In: Stoll BA, (ed). Breast Cancer Management Early and Late. London, UK: William Herman Medical Books Ltd; 1977:135–149. [Google Scholar]
- 79. Obiorah I, Jordan VC. 2012 NAMS/PFIZER- Wulf H. Utian endowed lecture. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013;20(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67(2):111–116. [DOI] [PubMed] [Google Scholar]
- 81. Lonning PE. Additive endocrine therapy for advanced breast cancer - back to the future. Acta Oncol. 2009;48(8):1092–1101. [DOI] [PubMed] [Google Scholar]
- 82. Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302(7):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wolf DM, Jordan VC. Characterization of tamoxifen stimulated MCF-7 tumor variants grown in athymic mice. Breast Cancer Res Treat. 1994;31(1):117–127. [DOI] [PubMed] [Google Scholar]
- 84. Wolf DM, Jordan VC. The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res Treat. 1994;31(1):129–138. [DOI] [PubMed] [Google Scholar]
- 85. Liu H, Lee ES, Deb Los Reyes A, et al. Silencing and reactivation of the selective estrogen receptor modulator-estrogen receptor alpha complex. Cancer Res. 2001;61(9):3632–3639. [PubMed] [Google Scholar]
- 86. Li S, Shen D, Shao J, et al. Endocrine-Therapy-Resistant ESR1 Variants Revealed by Genomic Characterization of Breast-Cancer-Derived Xenografts. Cell Rep. 2013;4(6):1116–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, et al. D538G Mutation in Estrogen Receptor-alpha: A Novel Mechanism for Acquired Endocrine Resistance in Breast Cancer. Cancer Res. 2013;73(23):6856–6864. [DOI] [PubMed] [Google Scholar]
- 88. Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of Constitutively Active Estrogen Receptor-alpha Mutations in Pretreated Advanced Estrogen Receptor-Positive Breast Cancer. Clin Cancer Res. 2014;20(7):1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 92. LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83(8):2496–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57(15):3071–3078. [PubMed] [Google Scholar]
- 96. Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69(4):1243–1254. [DOI] [PubMed] [Google Scholar]
- 97. Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93(22):1714–1723. [DOI] [PubMed] [Google Scholar]
- 98. Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97(23):1746–1759. [DOI] [PubMed] [Google Scholar]
- 99. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. [DOI] [PubMed] [Google Scholar]
- 100. Beral V, Reeves G, Bull D, et al. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ariazi E, Cunliffe H, Lewis-Wambi JS, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A. 2011;108(47):18879–18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fan P, Griffith OL, Agboke FA, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73(14):4510–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sengupta S, Obiorah I, Maximov PY, et al. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169(1):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Obiorah I, Sengupta S, Curpan R, et al. Defining the conformation of the estrogen receptor complex that controls estrogen induced apoptosis in breast cancer. Molecular Pharmacology. 2014;85:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Obiorah IE, Jordan VC. Differences in the Rate of Oestrogen-induced Apoptosis in Breast Cancer by Estradiol and the Triphenylethylene Bisphenol. Br J Pharmacol. 2014;171(17):4062–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lundgren K, Brown M, Pineda S, et al. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res. 2012;14(2):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14(11):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31(22):2783–2790. [DOI] [PubMed] [Google Scholar]
- 109. Jordan VC. A(nother) scientific strategy to prevent breast cancer in postmenopausal women by enhancing estrogen-induced apoptosis? Menopause. 2014;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jordan VC. Timing is key to avoid the bad and enhance the good of soy supplements. J Natl Cancer Inst. 2014:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Murdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–717. [DOI] [PubMed] [Google Scholar]
- 112. Maximov PY, McDaniel RE, Fernandes DJ, et al. Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. British Journal of Pharmacology. 2014;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]