Abstract
Background
Nonadherence to hormonal therapy is common and is associated with increased copayment amount. We investigated the change in adherence after the introduction of generic aromatase inhibitors (AIs) in 2010.
Methods
Using deidentified pharmacy and claims data from OptumInsight, we identified women older than 50 years on brand-name AIs (BAIs) and/or generic AIs (GAIs) for early breast cancer between January 1, 2007 and December 31, 2012. Clinical, demographic, and financial variables were evaluated. Adherence was defined as a medication possession ratio (MPR) 80% or greater.
Results
We identified 5511 women, 2815 (51.1%) on BAI, 1411 (25.6%) on GAI, and 1285 (23.3%) who switched from BAI to GAI. The median 30-day copayment was higher for BAI ($33.3) than for GAI ($9.04). In a multivariable Cox-proportional hazard analysis, women who took GAI were less likely to discontinue therapy (hazard ratio [HR] = 0.69, 95% confidence interval [CI] = 0.57 to 0.84) compared with BAI. Discontinuation was positively associated with a higher monthly copayment of $15 to $30 (HR = 1.21, 95% CI = 1.01 to 1.44) and more than $30 (HR = 1.49, 95% CI = 1.23 to 1.80) compared with less than $15. In a multivariable logistic regression analysis, adherence (medication possession ratio ≥ 80%) was positively associated with GAI use (odds ratio = 1.53, 95% CI = 1.22 to 1.91) compared with BAI and inversely associated with increased monthly copayment. In addition, adherence was associated with a high annual income of more than $100k/year (odds ratio = 1.58, 95% CI = 1.17 to 2.11).
Conclusions
Higher prescription copayment amount was associated with nonadherence and discontinuation of AIs. After controlling for copayment, discontinuation was higher and adherence was lower with Brand AIs. Because nonadherence is associated with worse survival, efforts should be directed towards reducing out-of-pocket costs for these life-saving medications.
Prescription drug prices have increased from an average of $38.43 in 1998 to $71.69 in 2008, with brand-name prescriptions on average costing $137.90 vs $35.22 for generic prescriptions (1,2). To counteract increasing medication costs, pharmacy benefit plans have added additional tiers of drugs, increased copayment rates, increased deductibles, excluded some drugs from coverage, and increased preauthorization requirements (1). When a pharmaceutical company first markets a drug, it is usually under a patent (12 years on average), which allows only the pharmaceutical company that developed the drug to sell it. During this time, the company is able to set the drug price. A generic drug is a product that is comparable to a brand drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use (3). Generic drugs are usually sold for lower prices than their branded equivalents, because competition increases among producers when drugs no longer are protected by patents (3,4).
One of the unintended consequences of a rise in prescription drug prices and the number of prescriptions per capita is an increasing rate of therapy discontinuation (ie, stopping the medication before completion) and nonadherence (ie, taking less than 80% of the intended dose). In one study of noncancer patients, over 25% admitted to not filling a prescription (1,5). It is well known that the uninsured face barriers for prescription medications, but in 2008 over 5% of those with private insurance and 13% of those with public insurance could not afford a prescription (6). One study recently estimated the national costs of medication nonadherence at $289 billion, 13% of total US health care expenditures (1,7).
Lack of compliance (discontinuation and/or nonadherence) with medications is a well-known problem in the medical literature (8–10). Oral hormonal therapy for the adjuvant treatment of breast cancer results in a greater than 30% reduction in breast cancer recurrence (11). Despite this, approximately 7% to 10% of patients discontinue therapy annually (12–18), with only 40% to 60% finishing their recommended five-year course. This nonadherence reduces the survival benefits associated with hormonal therapy (19–22). Our work and work by others suggests that treatment toxicity, patient race, prescribing physician specialty, the number and type of other prescriptions, and the degree to which the patient and physician believe in the drug’s efficacy are all associated with early discontinuation of hormonal therapy (12,16,18,23,24). One modifiable factor that may affect adherence is out-of-pocket costs. For example, doubling the copayment for various noncancer-related chronic medications reduced adherence rates between 8% and 45% (25). In a prior study by our group, we found that higher copayments were inversely associated with adherence to adjuvant aromatase inhibitor (AI) therapy (26). Less is known about the influence of brand-name vs generic drugs on adherence patterns, though generic drugs generally are cheaper with lower copayment amounts.
In 2010, generic versions of all the AIs were made available. This provided the opportunity for a natural experiment in which we investigated the change in adherence patterns before and after the introduction of generic aromatase inhibitors.
Methods
Data Source
OptumInsight maintains a proprietary research database containing claims, membership, provider, and ancillary data for over 36 million members. These include 25 million commercial members from UnitedHealthcare and six million Medicare managed care members. The deidentified database is updated frequently. Membership and provider records are linked to pharmacy claims (OptumRx) and medical claims, including diagnosis and procedure codes (CPT, HCPCS, as well as ICD9 procedures) with their dates of service and providers.
The OptumInsight database provided information on each prescription filled, including the drug, the prescriber and his/her specialty, the out-of-pocket payment and copayment. In addition to the data above, OptumInsight uses a major data syndicator, knowledge based marketing solutions (KBM) (27). The marketing database collects data from primary sources including public records, purchase transactions, census data and consumer surveys to determine income, net worth, and lifestyle information.
Sample Selection
We identified all women in the database who had filled a prescription for a brand-name AI (BAI) (Arimidex, Femara, Aromasin) and/or a generic AI (GAI) (anastrozole,exemestane,and letrozole) between January 1, 2007 and December 31, 2012. Subjects were classified based on their first prescription for hormonal therapy as BAI or GAI. We restricted our sample to patients with a diagnosis of early-stage breast cancer in the six months prior to first prescription who were at least 50 years of age at the time of the initial prescription. We defined early stage as having had a surgical resection (lumpectomy or mastectomy) within 12 months prior to the initiation of hormonal therapy. Age at diagnosis was categorized in five-year intervals. Race was classified as white, black, Asian, or Hispanic. In addition, subjects were categorized by geographic location. The pregeneric and postgeneric periods were defined as before or after July 1, 2010.
Comorbid Disease.
To assess the prevalence of comorbid disease in our cohort, we used the Episode Treatment Groups (ETGs) (28,29). This methodology uses an algorithm to compile clinical information, including prescriptions and claims for medical encounters, into episodes of care that can then be used to create a metric for chronic disease comorbidity. Subjects were categorized as having one to five or six or more comorbid conditions.
Clinical Characteristics.
For each patient, we determined the specialty of the provider who prescribed hormone therapy most frequently, categorizing the physician as medical oncologist or primary care physician/other specialty.
Financial Factors.
The copayment for the hormonal therapy was the amount paid by a subscriber for a 30-day prescription. Copayment amount for 60- or 90-day prescriptions were adjusted to 30-day copayment amounts. Copayment was categorized in roughly equal groups as less than $15, $15 to $30, or more than $30 based on common copayment amounts (multiples of $5), and so there was a roughly even distribution across the patient population. Insurance was categorized as commercial or Medicare. The number of patients on Medicaid was low, and therefore these patients were excluded from the analysis. The deductible was categorized as none, pharmacy only, or pharmacy/medical shared.
Outcomes.
We categorized patients as having discontinued therapy if the calculated drug supply, based on the last prescription date plus any surplus from a prior prescription, indicated a minimum 45-day supply gap with no hormone therapy on hand. Adherence was determined by the medication possession ratio (MPR) (ie, the number of pills supplied over a fixed period of time). We categorized subjects as being adherent if the MPR was 80% or greater during the initial two years on therapy (30).
Follow-up and Censoring.
All patients were followed for up to two years from the time of first prescription. Follow-up was available through December 31, 2012. We censored patients at the date at which they disenrolled from Optum if the patient switched AI category or if subsequent discontinuation data was missing.
Statistical Analysis.
We used multivariable Cox proportional hazards regression models to estimate the association between the effect of therapy type and discontinuation rates for hormone therapy use, controlling for clinical, financial, and demographic factors. We used multivariable logistic regression models to analyze the association between therapy type and two-year hormone therapy adherence, classified as a dichotomous variable (MPR ≥ 80% vs MPR < 80%). All variables were included that were thought to be clinically significant. Interactions were assessed using likelihood ratio tests in the models, and, when significant, a stratified analysis was performed. In an exploratory analysis, the two-year discontinuation data on the group that switched hormonal therapy was included in the model. We estimated adjusted Kaplan-Meier survival curves (STS GRAPH ADJUSTFOR) to show time to discontinuation stratified by each of the hormone therapy categories (GAI and BAI) and copayment categories, adjusting for the variables in the final model. The assumption of proportionality was confirmed visually. For all models, we rejected the null hypothesis at the P less than .05 level of statistical significance. All analyses were conducted using STATA version 12 (StataCorp, College Station, TX). All statistical tests were two-sided.
Results
We identified 5511 women who initiated hormone therapy, 2815 (51.1%) on BAI, 1411 (25.6%) on GAI, and 1285 (23.3%) who switched from BAI to GAI. Following the introduction of GAIs, 35.6% started BAI while 64.4% started GAI. Of patients who started with AI brand prior to generic availability, 73.2% switched to a GAI after they became available.
Table 1 gives the characteristics of the total cohort. The mean age of subjects at the time of breast cancer (BC) diagnosis in our study was 61 years. The majority of the study cohort was non-Hispanic white (59.9%) and had commercial insurance (88.5%). The median copayment was higher for the BAI ($33.3) than for the GAI group ($9.04). Of the patients in this cohort, 39% had copayments under $15, and 29% had copayments over $30 per month. The majority of hormone therapy prescriptions were written by hematologists/oncologists (74.5%). Most patients (85.1%) did not pay a deductible for their prescriptions.
Table 1.
Baseline characteristics of patients diagnosed with localized breast cancer at age 50 years or older who received adjuvant hormonal therapy
| Categories | All patients (n = 4226) | AI-brand (n = 2815) | AI-generic (n = 1411) |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Type of therapy | |||
| AI-brand | 2815 (66.6) | 2815 (100.0) | |
| AI-generic | 1411 (33.4) | 1411 (100.0) | |
| HT start year* | |||
| 2007 | 698 (16.5) | 698 (24.8) | 0 (0) |
| 2008 | 1002 (23.7) | 1002 (35.6) | 0 (0) |
| 2009 | 787 (18.6) | 787 (28.0) | 0 (0) |
| 2010 | 1007 (23.8) | 309 (11.0) | 698 (49.5) |
| 2011 | 732 (17.3) | 19 (0.7) | 713 (50.5) |
| Start year | |||
| Pregeneric | 2110 (50.0) | 2110 (75.0) | 0 (0) |
| Postgeneric | 2116 (50.0) | 705 (25.0) | 1411 (100.0) |
| Prescription coverage Characteristics | |||
| Adjusted 30-day copay* | |||
| <$15 | 1666 (39.4) | 458 (16.3) | 1208 (85.6) |
| $15-$30 | 1350 (32.0) | 1167 (41.5) | 183 (13.0) |
| >$30 | 1210 (28.6) | 1190 (42.3) | 20 (1.4) |
| Pharmacy deductible type | |||
| No deductible | 2390 (85.1) | 1641 (85.3) | 749 (84.6) |
| Pharmacy deductible only | 234 (8.3) | 161 (8.4) | 73 (8.3) |
| Shared pharmacy/medical | 185 (6.6) | 122 (6.3) | 63 (7.1) |
| Coverage type* | |||
| Commercial | 3739 (88.5) | 2541 (90.3) | 1198 (84.9) |
| Medicare | 487 (11.5) | 274 (9.7) | 213 (15.1) |
| Clinical characteristics | |||
| Provider specialty* | |||
| Primary care/other | 1076 (25.5) | 742 (26.4) | 334 (23.7) |
| Hematology/oncology | 3150 (74.5) | 2073 (73.6) | 1077 (76.3) |
| Surgery* | |||
| Lumpectomy/other | 1946 (46.1) | 1245 (44.2) | 701 (49.7) |
| Mastectomy | 2280 (53.9) | 1570 (55.8 | 710 (50.3) |
| Comorbidities | |||
| 1–5 | 3390 (80.2) | 2243 (79.7) | 1147 (81.3) |
| 6 + | 836 (19.8) | 572 (20.3) | 264 (18.7) |
| Age at diagnosis, y* | |||
| 50–55 | 719 (17.0) | 487 (17.3) | 232 (16.4) |
| 56–65 | 2379 (56.3) | 1612 (57.3) | 767 (54.4) |
| 66–75 | 728 (17.2) | 445 (15.8) | 283 (20.1) |
| 75+ | 400 (9.5) | 271 (9.6) | 129 (9.1) |
| Sociodemographic characteristics | |||
| Race/ethnicity | |||
| White | 2530 (59.9) | 1699 (60.4) | 831 (58.9) |
| Black | 312 (7.4) | 207 (7.4) | 105 (7.4) |
| Hispanic | 217 (5.1) | 145 (5.2) | 72 (5.1) |
| Asian | 75 (1.8) | 49 (1.7) | 26 (1.8) |
| Other/unknown race | 1092 (25.8) | 715 (25.4) | 377 (26.7) |
| Education* | |||
| High school or less | 922 (21.8) | 639 (22.7) | 283 (20.1) |
| More than high school | 3034 (78.2) | 2176 (77.3) | 1128 (79.9) |
| Household income* | |||
| Low (<$40000) | 701 (15.6) | 508 (18.1) | 193 (13.7) |
| Middle ($40K-$100K) | 1835 (43.4) | 1189 (42.2) | 646 (45.8) |
| High (>$100K) | 674 (17.4) | 447 (15.9) | 227 (16.1) |
| Unknown | 1016 (23.7) | 671 (23.8) | 345 (24.5) |
| Region | |||
| Northeast | 744 (17.6) | 476 (16.9) | 268 (19.0) |
| West | 891 (21.1) | 582 (20.7) | 296 (21.0) |
| Midwest | 1699 (40.2) | 595 (21.2) | 541 (38.3) |
| South | 888 (21.0) | 1158 (41.2) | 306 (21.7) |
* Two-sided X 2 for all comparisons, P < .01. AI-brand = brand name aromatase inhibitor; AI-generic = generic aromatase inhibitor.
Hormone therapy early discontinuation was identified in 1146 (27.1%) subjects, and nonadherence was identified in 1050 (24.8%) of subjects. Among the women on BAI, 32.6% discontinued therapy compared with 16.2% on GAI. In a multivariable Cox-proportional hazard analysis, discontinuation was decreased among women who took GAI (hazard ratio [HR] = 0.69, 95% confidence interval [CI] = 0.57 to 0.84) compared with BAI. Discontinuation was linearly associated with higher monthly copayments of $15 to $30 (HR = 1.21, 95% CI = 1.01 to 1.44) and more than $30 (HR = 1.49, 95% CI = 1.23 to 1.80) compared with less than $15. Women with more comorbid conditions (HR = 1.15, 95% CI = 1.01 to 1.34) and those over 75 years old (HR = 1.46, 95% CI = 1.16 to 1.75) were more likely to discontinue therapy early (Table 2).
Table 2.
Early discontinuation of patients diagnosed with localized breast cancer at age 50 or older who received adjuvant hormonal therapy*
| Categories | Unadjusted frequencies | Multivariable analysis | ||
|---|---|---|---|---|
| Continued therapy | Early discontinued | HR (95% CI) | P | |
| No. (%) | No. (%) | |||
| Total | 3080 (72.9) | 1146 (27.1) | ||
| Type of therapy | ||||
| AI-brand | 1898 (67.4) | 917 (32.6) | 1.0 (reference) | |
| AI-generic | 1182 (83.8) | 229 (16.2) | 0.69 (0.57 to 0.84) | <.001 |
| Prescription coverage characteristics | ||||
| Adjusted 30-day copay | ||||
| <$15 | 1356 (81.4) | 310 (18.6) | 1.0 (reference) | |
| $15-$30 | 970 (71.9) | 380 (28.5) | 1.21 (1.01 to 1.44) | .04 |
| >$30 | 754 (62.3) | 456 (37.7) | 1.49 (1.23 to 1.80) | <.001 |
| Pharmacy deductible type | ||||
| No deductible | 1813 (75.9) | 577 (24.1) | 1.0 (reference) | |
| Pharmacy deductible only | 168 (71.8) | 66 (28.2) | 1.12 (0.87 to 1.43) | .40 |
| Shared pharmacy/medical deductible | 140 (75.7) | 45 (24.3) | 1.18 (0.87 to 1.61) | .28 |
| Coverage type | ||||
| Commercial | 2806 (75.0) | 933 (24.9) | 1.0 (reference) | |
| Medicare | 274 (56.3) | 213 (43.7) | 1.27 (0.99 to 1.62) | .06 |
| Clinical characteristics | ||||
| Provider specialty (most common prov) | ||||
| Primary care/other | 787 (73.1) | 289 (26.9) | 1.0 (reference) | |
| Hematology/oncology | 2293 (72.8) | 857 (27.2) | 0.93 (0.82 to 1.08) | .37 |
| Surgery | ||||
| Lumpectomy/other | 1440 (74.0) | 506 (26.0) | 1.0 (reference) | |
| Mastectomy | 1640 (71.9) | 640 (28.1) | 0.99 (0.88 to 1.11) | .83 |
| Comorbidities (used ETG Score) | ||||
| 1–5 | 2482 (73.2) | 908 (26.8) | 1.0 (reference) | |
| 6+ | 598 (71.5) | 238 (28.5) | 1.15 (1.01 to 1.34) | .04 |
| Age at diagnosis, y | ||||
| 50–55 | 535 (74.4) | 184 (25.6) | 1.11 (0.94 to 1.31) | .21 |
| 56–65 | 1804 (75.8) | 575 (24.2) | 1.0 (reference) | |
| 66–75 | 523 (71.8) | 205 (28.2) | 0.96 (0.94 to 1.31) | .69 |
| 75+ | 218 (54.5) | 182 (45.5) | 1.46 (1.16 to 1.75) | <.001 |
| Sociodemographic characteristics | ||||
| Race/ethnicity | ||||
| White | 1912 (75.6) | 618 (24.4) | 1.0 (reference) | |
| Black | 221 (70.8) | 91 (29.2) | 1.09 (0.86 to 1.37) | .47 |
| Hispanic | 156 (71.9) | 61 (28.1) | 0.77 (0.52 to 1.13) | .18 |
| Asian | 59 (78.7) | 16 (21.3) | 0.57 (0.32 to 1.01) | .86 |
| Other/unknown race | 947 (68.4) | 437 (31.6) | 1.40 (1.04 to 1.89) | .02 |
| Education | ||||
| High school or less | 673 (73.0) | 249 (27.0) | 1.0 (reference) | |
| More than high school | 2407 (72.8) | 897 (27.2) | 0.96 (0.83 to 1.18) | .86 |
| Household income | ||||
| Low (<$40000) | 502 (71.6) | 199 (28.4) | 1.0 (reference) | |
| Middle ($40K-$100K) | 1377 (75.0) | 458 (25.0) | 0.95 (0.79 to 1.14) | .61 |
| High (>$100K) | 518 (76.8) | 156 (23.1) | 0.93 (0.73 to 1.18) | .54 |
| Unknown | 683 (67.2) | 333 (32.8) | 0.89 (0.63 to 1.25) | .52 |
| Region | ||||
| Northeast | 582 (78.2) | 162 (21.8) | 1.0 (reference) | |
| West | 599 (67.5) | 289 (32.5) | 1.24 (0.99 to 1.56) | .06 |
| Midwest | 689 (77.3) | 202 (22.7) | 1.07 (0.85 to 1.36) | .55 |
| South | 1209 (71.2) | 490 (28.8) | 1.27 (1.03 to 1.57) | .02 |
* AI-Brand =brand name aromatase inhibitor; AI-Generic = generic aromatase inhibitor; CI = confidence interval; ETG = episode treatment group.
In a multivariable logistic regression analysis, GAI users were more likely to be adherent (MPR ≥ 80%) with hormone therapy (OR = 1.53, 95% CI = 1.22 to 1.91) compared with BAI (Table 3). Women with a high annual household income of more than $100k/year (OR = 1.58, 95% CI = 1.17 to 2.11) were also more likely to be adherent with hormone therapy compared with those with a low annual income (<$40k/year). Adherence was inversely associated with copayment amount with patients with copayments of $15 to $30 per month (OR = 0.74, 95% CI = 0.59 to 0.92) and over $30 per month (OR = 0.51, 95% CI = 0.41 to 0.65) compared with patients with copayments of less than $15 per month. Medicare patients were also less likely to be adherent (OR = 0.52, 95% CI = 0.38 to 0.72) compared with those with commercial insurance. Patients with increased comorbid conditions (OR = 0.78, 95% CI = 0.65 to 0.93) and increased age (OR = 0.67, 95% CI = 0.51 to 0.88) had statistically significantly lower odds of adherence. Asian patients were more likely to be adherent compared with non-Hispanic white patients (OR = 1.98, 95% CI = 0.99 to 1.57). A sensitivity analysis was performed classifying both copayment and income as continuous variables, and the results were unchanged.
Table 3.
Adherence (Medication Possession Ration >80%) of patients diagnosed with localized breast cancer at age 50 years or older who received adjuvant hormonal therapy*
| Categories | Unadjusted frequencies | Multivariable analysis | ||
|---|---|---|---|---|
| Adhered | Nonadhered | OR (95% CI) | P | |
| No. (%) | No. (%) | |||
| Total | 3176 (75.1) | 1050 (24.8) | ||
| Type of therapy | ||||
| AI-brand | 1996 (70.9) | 819 (29.1) | 1.0 (reference) | |
| AI-generic | 1180 (83.6) | 231 (16.4) | 1.53 (122 to 1.91) | <.001 |
| Prescription coverage characteristics | ||||
| Adjusted 30-day copay | ||||
| <$15 | 1380 (82.8) | 286 (17.2) | 1.0 (reference) | |
| $15-$30 | 1012 (75.0) | 338 (25.0) | 0.74 (0.59 to 0.92) | .008 |
| >$30 | 784 (64.8) | 426 (35.2) | 0.51 (0.41 to 0.65) | <.001 |
| Pharmacy deductible type | ||||
| No deductible | 1858 (77.7) | 1038 (22.3) | 1.0 (reference) | |
| Pharmacy deductible only | 169 (72.2) | 123 (27.8) | 0.81 (0.59 to 1.10) | .17 |
| Shared pharmacy/medical deductible | 144 (77.8) | 94 (22.2) | 0.87 (0.60 to 1.27) | .48 |
| Coverage type | ||||
| Commercial | 2885 (77.2) | 854 (22.8) | 1.0 (reference) | |
| Medicare | 291 (59.7) | 196 (40.2) | 0.52 (0.38 to 0.72) | <.001 |
| Clinical characteristics | ||||
| Provider specialty (most common prov) | ||||
| Primary care/other | 819 (76.1) | 257 (23.9) | 1.0 (reference) | |
| Hematology/oncology | 2357 (74.8) | 793 (25.2) | 0.95 (0.80 to 1.12) | .55 |
| Surgery | ||||
| Lumpectomy/other | 1463 (75.2) | 483 (24.8) | 1.0 (reference) | |
| Mastectomy | 1713 (75.1) | 567 (24.9) | 1.10 (0.95 to 1.27) | .19 |
| Comorbidities (used ETG score) | ||||
| 1–5 | 2573 (75.9) | 817 (24.1) | 1.0 (reference) | |
| 6+ | 603 (72.1) | 233 (27.9) | 0.78 (0.65 to 0.93) | .007 |
| Age at diagnosis, y | ||||
| 50–55 | 539 (75.0) | 180 (25.0) | 0.86 (0.71 to 1.06) | .17 |
| 56–65 | 1842 (77.4) | 537 (22.6) | 1.0 (reference) | |
| 66–75 | 555 (76.2) | 173 (23.8) | 1.24 (0.99 to 1.57) | .06 |
| 75+ | 240 (60.0) | 160 (40.0) | 0.67 (0.51 to 0.88) | .004 |
| Sociodemographic characteristics | ||||
| Race/ethnicity | ||||
| White | 1965 (77.7) | 565 (22.3) | 1.0 (reference) | |
| Black | 230 (73.7) | 82 (26.3) | 0.93 (0.69 to 1.24) | .55 |
| Hispanic | 154 (71.0) | 63 (29.0) | 1.15 (0.72 to 1.85) | .55 |
| Asian | 61 (81.3) | 14 (18.7) | 1.98 (0.99 to 3.96) | .05 |
| Other/unknown race | 766 (70.0) | 326 (29.9) | 0.64 (0.44 to 0.93) | .02 |
| Education | ||||
| High school or less | 691 (75.0) | 431 (25.0) | 1.0 (reference) | |
| More than high school | 2485 (75.2) | 1542 (24.8) | 0.92 (0.75 to 1.13) | .44 |
| Household income | ||||
| Low (<$40000) | 504 (71.9) | 197 (28.1) | 1.0 (reference) | |
| Middle ($40K-$100K) | 1409 (76.8) | 426 (23.2) | 1.19 (0.95 to 1.49) | .12 |
| High (>$100K) | 546 (81.0) | 128 (19.0) | 1.58 (1.17 to 2.11) | .002 |
| Unknown | 717 (70.6) | 299 (29.4) | 1.52 (0.99 to 2.32) | .06 |
| Region | ||||
| Northeast | 600 (80.6) | 144 (19.3) | 1.0 (reference) | |
| West | 617 (69.5) | 271 (30.5) | 0.70 (0.53 to 0.93) | .01 |
| Midwest | 709 (79.6) | 182 (20.4) | 0.88 (0.67 to 1.17) | .38 |
| South | 1249 (73.5) | 450 (26.5) | 0.71 (0.55 to 0.91) | .006 |
* AI-brand = brand name aromatase inhibitor; AI-generic = generic aromatase inhibitor; CI = confidence interval; ETG = episode treatment group.
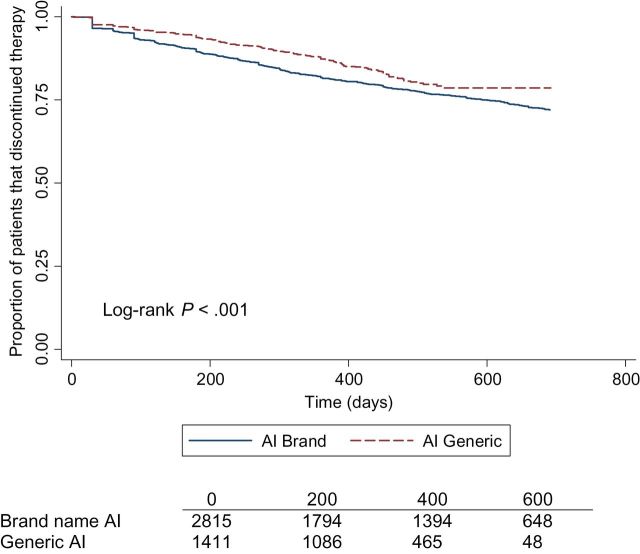
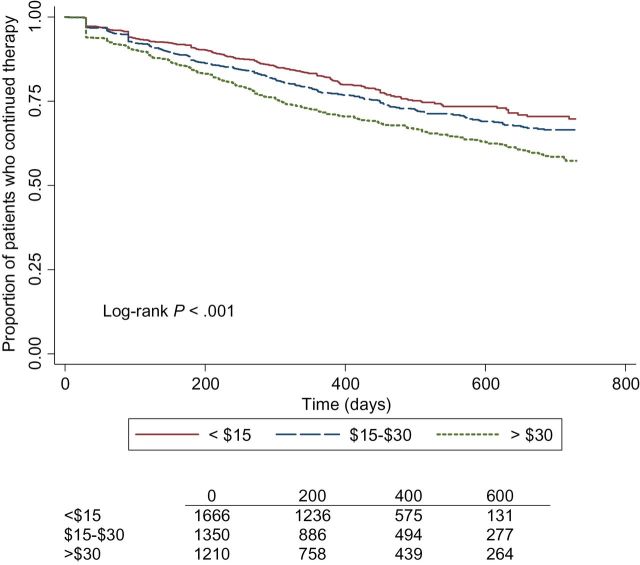
There was a statistically significant interaction between annual income and GAI/BAI and a statistically significant interaction between copayment amount and GAI/BAI (P interaction < .01). In the stratified analysis, women on BAI in the lower annual income group were more likely to discontinue therapy compared with patients on GAI. No association was found in the high annual income group. In subject with a low copayment (<$15), the BAI group was twice as likely to discontinue hormone therapy compared with GAI. No association was found in the high copayment group (Supplementary Table 1, available online). Kaplan-Meier curves were generated to show differences in hormone therapy discontinuation (Figure 1) and discontinuation by copayment amount (Figure 2) over time.
Figure 1.
Adjusted Kaplan-Meier curve for continuation of hormonal therapy by aromatase inhibitor (AI) class among women diagnosed with localized breast cancer. The log-rank P value is two-sided.
Figure 2.
Adjusted Kaplan-Meier curve for continuation of hormonal therapy by average 30-day aromatase inhibitor prescription copayment amount among women diagnosed with localized breast cancer at age 50 years or older. The log-rank P value is two-sided.
In an exploratory multivariable Cox-proportional hazard analysis including patients that switched from BAI to GAI, discontinuation was decreased among women who took GAI (HR = 0.44, 95% CI = 0.35 to 0.55) and those who switched (HR = 0.63, 95% CI = 0.52 to 0.76) compared with BAI. The association between age, comorbidity, and copayment amount and discontinuation did not change (Supplementary Table 2, available online).
Discussion
In this study of women with BC whose pharmacy benefits were administered through a large US health plan manager, we found that as monthly out-of-pocket copayment amount increased, rates of adherence to AI hormone therapy decreased and discontinuation increased. We also found that, even after controlling for monthly copayment amount, discontinuation was higher and adherence was lower in patients on brand-name AIs compared with generic AIs. Not surprisingly, the majority of women on AIs were on generic AIs after they became available in 2010.
Medication adherence is an increasingly recognized issue in oncology, particularly as the number of oral agents used for therapy increases (31). It is estimated that more than one quarter of the 400 antineoplastic agents now in the pipeline are oral drugs. As with other new cancer therapies, they are accompanied by increased costs and financial burdens for patients (32,33). While we have focused on adherence to AI hormonal therapy in this paper, there are also concerns about nonadherence with imatinib (34,35), thiopurine (36), and capecitabine (37). This issue will become increasingly important as more oral antinoeplastic drugs come into use (38). The monthly total cost of brand-name and generic AIs are, on average, $380 and $150, respectively, while the average monthly total cost of oral biologic therapies ranges from $5000 to $8000 per month. These total costs for the payer industry have translated into substantially higher out-of-pocket costs for patients.
It is increasingly recognized that the financial burden from health care costs results in patient distress. The Centers for Disease Control and Prevention estimates that one in three persons is in a family that experiences the financial burden of cancer care, and one in ten is in a family that has health care–related bills that they are not able to pay (39). As a result, increased attention has been paid to the financial toxicity of oncologic treatments. In a population-based study of treatment-related financial changes in patients with stage III colorectal cancer, a statistically significant percentage of patients (38%) reported at least one treatment-related financial hardship, defined as debt accumulation, borrowing money from family/friends, ≥20% income decline, or selling/refinancing primary home, and a minority of respondents reported discussing treatment-related expenses with their physicians (40).
Other investigators have evaluated the association between financial factors and medication adherence. In a study using the 5% Medicare random sample, Medicare beneficiaries had reduced adherence during the drug coverage gap. Patients without drug coverage had the number of prescriptions refilled reduced by 16% per month, while those with generic drug coverage reduced the number of prescriptions refilled by 10%. Patients on generic drugs had greater adherence during this timeframe than those on brand-name drugs (41). In another study that evaluated cardiac medication use following an acute myocardial infarction, Stuart and colleagues found that during the Part D coverage gap, there were statistically significant reductions in the MPR for beta blockers, statins and ACE inhibitors, despite the mortality benefit associated with these drugs (42).
The findings are consistent with prior work by our group reporting that copayment amount was associated with adherence in patients who received 90-day mail-order prescriptions (26). Ito and colleagues estimated the incremental cost effectiveness of providing Medicare beneficiaries with full coverage for AIs. They reported that full prescription coverage would result in greater quality-adjusted survival and less resource use per beneficiary, with an incremental cost-effectiveness ratio of $15128 per quality-adjusted life-year gained (43). We were surprised to see that, despite controlling for copayment, there was increased adherence and decreased discontinuation with AI-generic users compared with brand-name AIs.
Women in the highest income bracket were statistically significantly more likely to be adherent than women in the lowest income group despite controlling for copayment amount and type of hormonal therapy. Low-income groups have traditionally been found to be vulnerable with regard to quality health care, and this may be exacerbated by the overlap in the timeframe of this study with the economic downturn in the United States. However, nonfinancial interventions may be successful in improving compliance. In the California statewide survey of low-income women with breast cancer, one study found that adherence was higher in those who reported better provider-patient communication on standardized patient-reported outcome measures (44).
This study had several strengths. We utilized a large database with a nationwide sample, including patients with a wide variety of prescription benefit plans, allowing for a diversity of copayment amounts, income, and age. This study also compared changes in adherence and discontinuation rates after the introduction of generic AIs, providing support that lower out-of-pocket costs for drugs with similar effectiveness can improve compliance. Prior studies were done prior to the introduction of generic AIs. In addition, we evaluated a timeframe in which generic aromatase inhibitors were introduced to the market so we could see how this change in availability affected adherence rates. Furthermore, in addition to copayment amount and type of therapy, the dataset has information on patient income, insurance type, and deductible amount.
Some study limitations should be mentioned. All of our patients received some form of prescription coverage, and therefore our results are not generalizable to patients without prescription coverage. In addition, mail-order pharmacies such as Optum have auto-refill programs, and therefore these results may underestimate the true adherence rate. Furthermore, we did not have a full five years of follow-up following the change from brand-name to generic AI availability. In addition, we did not have detailed information on tumor stage or pathologic characteristics that may have influenced adherence. While we would not expect that stage would have a large influence on uptake of generic AIs, it is possible that patients with worse prognosis prefer brand name and may be more likely to be adherent. However, this effect would reduce the association we observed. Finally, we did not have individual information on why patients discontinued therapy, some of which may have been because of toxicity or patient preference. However, all of the AIs have similar side effects, the most common being joint discomfort and stiffness, and there are no differences in side effects between brand-name and generic AIs. We also don’t know what patient characteristics may be associated with staying on brand-name AIs or discontinuing as opposed to switching. Prior studies by our group and others have shown that attitudes and belief in efficacy contribute to initiation and adherence to hormone therapy (45,46).
In summary, copayment amount had a large impact on discontinuation and nonadherence to AI therapy. In addition, shifts in use from brand-name to generic aromatase inhibitors were associated with decreased discontinuation and increased adherence to hormone therapy that persisted after controlling for copayment amount. Since previous studies have shown that poor adherence and early discontinuation of hormonal therapy are associated with worse survival (19,21,47), public health efforts, such as the Cancer Treatment Fairness Act, should be directed towards increased drug price transparency, improving access, and reducing out-of-pocket costs for life-saving cancer treatments. This is especially important given the rapid increase of expensive oral cancer therapies.
Funding
This study was supported by a grant from the American Cancer Society (RSGT-11-012-01-CPHPS to AIN). Dr. Tsui was supported by a fellowship from the National Cancer Institute (R25 CA094061). Additional support was provided by the Witten Family Fund (to DLH).
Supplementary Material
The funders have not participated in the conduct of this study. None of the authors have any conflicts to declare with this manuscript.
References
- 1. Foundation KF. Prescription Drug Trends. In: Washington: Kaiser Family Foundation; 2010. [Google Scholar]
- 2. Stores NAoCD. Industry Facts-at-a-Glance. 2010. [Google Scholar]
- 3. Generic Drugs. Center for Drug Evaluation and Research. US Food and Drug Administration.
- 4. Food & Drug Administration, Generic Drugs: Questions and Answers. US Food and Drug Administration. January 12, 2010. Accessed February 3, 2010. [Google Scholar]
- 5. Foundation KF. Kaiser Health Tracking Poll. 2009. [Google Scholar]
- 6. Foundation KF. The Uninsured, A Primer. 2009. [Google Scholar]
- 7. Institute NEH. Thinking Outside the Pillbox: A System-wide Approach to Improving Patient Medication Adherence for Chronic Disease. 2009. [Google Scholar]
- 8. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;CD000011. [DOI] [PubMed] [Google Scholar]
- 9. Banning M. A review of interventions used to improve adherence to medication in older people. Int J Nurs Stud. 2009;46(11):1505–1515 [DOI] [PubMed] [Google Scholar]
- 10. Conn VS, Hafdahl AR, Cooper PS, et al. Interventions to Improve Medication Adherence Among Older Adults: Meta-Analysis of Adherence Outcomes Among Randomized Controlled Trials. Gerontologist. 2009;49(4):447–462 [DOI] [PubMed] [Google Scholar]
- 11. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(94724):1687–1717. [DOI] [PubMed] [Google Scholar]
- 12. Kimmick G, Anderson R, Camacho F, et al. Adjuvant Hormonal Therapy Use Among Insured, Low- Income Women With Breast Cancer. J Clin Oncol. 2009;27(21):3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431–436. [DOI] [PubMed] [Google Scholar]
- 14. Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. [DOI] [PubMed] [Google Scholar]
- 15. Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. [DOI] [PubMed] [Google Scholar]
- 16. Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(1):215–220. [DOI] [PubMed] [Google Scholar]
- 17. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. [DOI] [PubMed] [Google Scholar]
- 18. Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(21):1543–1549. [DOI] [PubMed] [Google Scholar]
- 21. Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206(1):66–75. [DOI] [PubMed] [Google Scholar]
- 22. Bryant J, Fisher B, Dignam J. Duration of adjuvant tamoxifen therapy. J Natl Cancer Inst Monogr. 2001;30:56–61. [DOI] [PubMed] [Google Scholar]
- 23. Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. [DOI] [PubMed] [Google Scholar]
- 24. Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59(1):97–102. [DOI] [PubMed] [Google Scholar]
- 25. Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291(19):2344–2350. [DOI] [PubMed] [Google Scholar]
- 26. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.http://www.kbmg.com. http://www.kbmg.com Accessed April 15, 2014.
- 28. Dang DK, Pont JM, Portnoy MA. Episode treatment groups: an illness classification and episode building system--Part I. Med Interface. 1996;9(4):118–122. [PubMed] [Google Scholar]
- 29. Dang DK, Pont JM, Portmoy MA. Episode treatment groups: an illness classification and episode building system--Part II. Med Interface. 1996;9(4):122–128. [PubMed] [Google Scholar]
- 30. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7):1280–1288. [DOI] [PubMed] [Google Scholar]
- 31. Hede K. Increase in oral cancer drugs raises thorny issues for oncology practices. J Natl Cancer Inst. 2009;101(22):1534–1536. [DOI] [PubMed] [Google Scholar]
- 32. Vanchieri C. When will the U.S. flinch at cancer drug prices? J Natl Cancer Inst. 2005;97(9):624–626. [DOI] [PubMed] [Google Scholar]
- 33. Benson R, Wilson C, Williams MV. Comments on Costs of treating advanced colorectal cancer, Ross et al., Eur J Cancer. 1996;32A:S13–S17 Eur J Cancer. 1998;34:593–594. [DOI] [PubMed] [Google Scholar]
- 34. Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–11. [DOI] [PubMed] [Google Scholar]
- 35. Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25(6):481–496. [DOI] [PubMed] [Google Scholar]
- 36. Hawwa AF, Millership JS, Collier PS, et al. The development of an objective methodology to measure medication adherence to oral thiopurines in paediatric patients with acute lymphoblastic leukaemia—an exploratory study. Eur J Clin Pharmacol. 2009;65(11):1105–1112. [DOI] [PubMed] [Google Scholar]
- 37. Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 28(14):2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. [DOI] [PubMed] [Google Scholar]
- 39. Cohen R, Gindi R, Kirzinger W. Financial Burden of Medical Care: Early Release of Estimates From the National Health Interview Survey. Available at: http://www.cdc.gov/nchs/data/nhis/earlyrelease/financial_burden_of_medical_care_032012.pdf Accessed January 15, 2014.
- 40. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–1614. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Baik SH, Lave JR. Effects of Medicare Part D coverage gap on medication adherence. Am J Manag Care. 2013;19(6):e214–e224. [PMC free article] [PubMed] [Google Scholar]
- 42. Stuart B, Davidoff A, Erten M, et al. How Medicare Part D Benefit Phases Affect Adherence with Evidence-Based Medications Following Acute Myocardial Infarction. Health Serv Res. 2013;48(6 pt 1):1960–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito K, Elkin E, Blinder V, et al. Cost-effectiveness of full coverage of aromatase inhibitors for Medicare beneficiaries with early breast cancer. Cancer. 2013;119(13):2494–2502. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Malin JL, Diamant AL, et al. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013;137(3):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. [DOI] [PubMed] [Google Scholar]
- 46. Neugut AI, Hillyer GC, Kushi LH, et al. Non-initiation of adjuvant hormonal therapy in women with hormone receptor-positive breast cancer: The Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. 2012;134(1):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hershman D, Kushi L, Kershenbaum A, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.