Among individuals with HIV, 6.9% are colonized with methicillin-resistant Staphylococcus aureus (MRSA). Exposure to healthcare settings and correctional facilities significantly increases MRSA colonization risk. Screening of extranasal sites increases the number of identified MRSA carriers by at least 31%.
Keywords: MRSA, HIV, colonization, methicillin-resistant Staphylococcus aureus, meta-analysis
Abstract
Background. Human immunodeficiency virus (HIV)–infected individuals who are colonized with methicillin-resistant Staphylococcus aureus (MRSA) have increased risk for MRSA infection. We conducted a meta-analysis of published studies to estimate the prevalence of MRSA colonization in this population.
Methods. We performed a systematic literature review and meta-analysis. The PubMed and Embase databases were searched and studies reporting prevalence of MRSA colonization among HIV-infected individuals were included.
Results. Among 7940 citations, 32 studies reporting data on 6558 HIV-infected individuals were considered eligible for our meta-analysis. We found that 6.9% (95% confidence interval [CI], 4.8–9.3) of individuals with HIV infection are MRSA carriers, with the corresponding figure across North American studies being 8.8% (95% CI, 6.0–12.2). History of hospitalization during the previous 12 months was associated with a 3.1 times higher risk of MRSA colonization (risk ratio [RR], 3.11 [95% CI, 1.62–5.98]). Previous or current incarceration was also associated with a higher risk for carriage (RR, 1.77 [95% CI, 1.26–2.48]). Current antiretroviral therapy or use of trimethoprim-sulfamethoxazole did not impact the risk of MRSA carriage (RR, 1.02 [95% CI, .64–1.63] and 1.45 [95% CI, .69–3.03], respectively). Extranasal screening increased the detection of MRSA colonization by at least 31.6% (95% CI, 15.8–50.0). The added yield from groin screening was 19.3% (95% CI, 11.5–28.5), from perirectal screening 18.5% (95% CI, 7.4–33.2), and from throat cultures 17.5% (95% CI, 12.0–24).
Conclusions. Individuals with HIV infection constitute a highly vulnerable population for MRSA colonization, and prior exposure to hospital or incarceration are significant factors. Nasal screening alone will underestimate the rate of colonization by at least one-third.
Methicillin-resistant Staphylococcus aureus (MRSA) infections are a major public health problem, both in healthcare settings and in the community. MRSA colonization has been linked to infection in many high-risk populations [1–4], and different strategies of surveillance for colonization and/or decolonization have been proven effective in decreasing the rate of infections [5]. Individuals infected with the human immunodeficiency virus (HIV) have a 6- to 18-fold higher risk of MRSA infections compared with the general population [6, 7]. These infections include skin and soft tissue infections (SSTIs), pneumonia, endocarditis, and bacteremia and are associated with significant morbidity and mortality [8, 9]. Although the relative contribution of colonization in the risk of developing MRSA infection in HIV-infected individuals is unknown, there is evidence that infections are associated with previous carriage [10, 11], as in other populations [1–4]. Moreover, HIV-infected individuals seem to be particularly vulnerable to MRSA colonization; whether this is due to immune mechanisms or previous exposures to settings with high prevalence of infection is debatable [12].
To study the prevalence of MRSA carriage among HIV-infected individuals and determine the underlying basis of the vulnerability of this population to colonization, we performed a meta-analysis of published studies.
METHODS
Study Selection
A literature search of the PubMed and Embase databases [13] was conducted up to 3 April 2014. Two authors (F. N. Z., I. M. Z.) performed the literature search independently, using as keywords the following terms: (HIV OR immunodeficiency OR [human immunodeficiency virus] OR AIDS) AND (MRSA OR [methicillin-resistant Staphylococcus aureus] OR Staphylococcus OR [methicillin AND resistant]). Among the citations extracted, titles and abstracts were reviewed in an attempt to retrieve the clinical studies that reported the prevalence of MRSA colonization among HIV-infected individuals. Articles that were relevant by title and abstract were accessed in full text to determine those that fulfilled the inclusion criteria. In our analysis, we included both published articles and abstracts from conference proceedings published in Embase. Finally, reviewing of the reference lists of eligible studies complemented the search. We performed our review and meta-analysis in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) checklist (Supplementary Table 1) [14].
Inclusion/Exclusion Criteria
Studies were considered eligible if they reported the prevalence of MRSA colonization in nares and/or extranasal sites among individuals with HIV infection. Studies reporting data exclusively on pediatric populations were excluded due to the difference in the behavioral characteristics of adult and pediatric HIV-infected populations, which could impact the estimated prevalence. A restriction to English-language articles was imposed.
Outcomes of Interest
The main outcome of interest was the prevalence of MRSA colonization among HIV-infected individuals. This was calculated by dividing the number of colonized individuals by the total number of individuals at risk, that is, HIV-infected individuals who were screened for MRSA carriage. Also, we were interested in studying the impact of prior hospitalization, current antiretroviral therapy, current use of trimethoprim-sulfamethoxazole (TMP-SMX), illicit drug use, and history of incarceration. Moreover, we studied the added yield of different sites of extranasal screening on the estimated prevalence of MRSA colonization.
Data Extraction
Two reviewers (F. N. Z., I. M. Z.) independently extracted data from eligible studies, and discrepancies between reviewers were resolved by consensus. Among studies that reported the prevalence of MRSA colonization among HIV-infected individuals, the extracted data included the study period, the country, the number of screenings, the setting from which individuals were recruited, the number of CD4 cells/µL (mean, median, range) and the viral load, the body sites that were screened, the method of MRSA isolation, the number of individuals screened, the total number of colonized individuals, the number of colonized individuals by site screened, and the number of MRSA infections among colonized and noncolonized individuals. We also extracted information on current use of TMP-SMX, use of antiretroviral therapy, illicit drug use, and history of recent hospitalization and incarceration among colonized and noncolonized individuals.
Quality Assessment
We rated the methodological quality of included studies using the Newcastle-Ottawa Quality Assessment Scale (NOS) [15], which is a “star-based” rating system. Each study included in the meta-analysis was assessed and scored based on representativeness of the exposed cohort, ascertainment of exposure, assessment of outcome, adequacy of follow-up time for outcomes to occur, and adequacy of follow-up of cohorts. Two reviewers (F. N. Z., I. M. Z.) independently assessed the quality of eligible studies. Each study could get up to 5 stars as some fields of the NOS—namely, “selection of the non-exposed cohort,” “demonstration that the outcome of interest was not present at the start of the study,” and “comparability between cohorts”—were not applicable for our study. Studies that were awarded >4 stars were deemed of adequate quality to extract relevant data. Details of the quality assessment of all eligible studies are provided in Supplementary Table 2).
Data Analysis
A random effects (RE) analysis was used to calculate the combined prevalence and the 95% confidence intervals (CIs) [16]. To avoid an undue large weight for studies with low or high prevalence (prevalence close to 0 or 1), we used the double arcsine methodology [17], which corrects variance instability and large study weights related to the use of inverse-variance method [18]. Statistical heterogeneity was assessed using the τ2 statistic [19], and subgroup and sensitivity analyses were used to account for potential sources of heterogeneity. The effect of different factors on colonization and the effect of colonization on ensuing infections were reported as risk ratio (RR) estimates and RE CIs, with heterogeneity measured by Cochran Q. Small-study effect was assessed by Egger test [20]. We used the Stata version 13 software package (StataCorp, College Station, Texas) for data analysis.
RESULTS
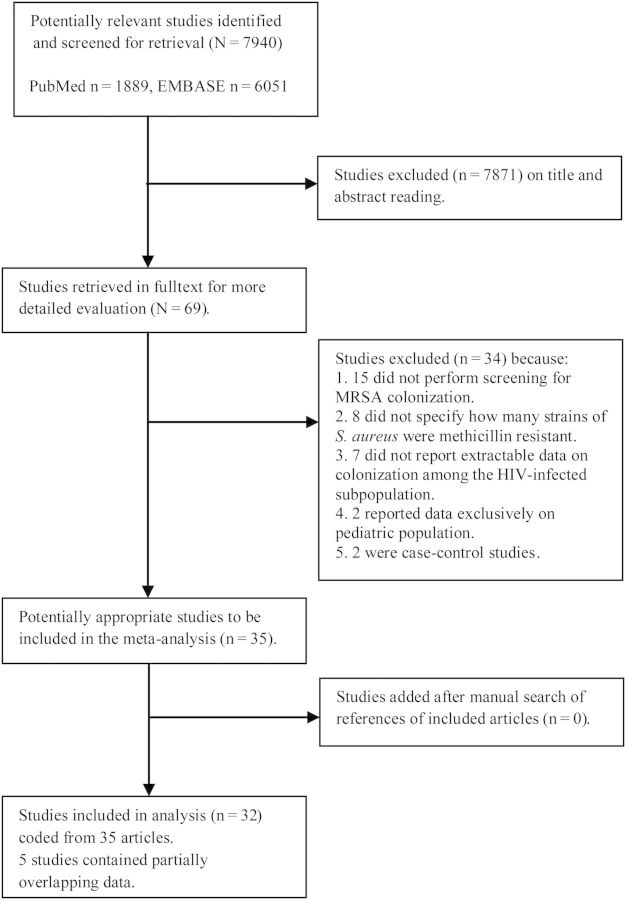
The initial database search identified 1889 publications in PubMed (1958–2014) and 6051 in Embase (1950–2014). The date of our last access to the databases was 3 April 2014. Sixty-nine studies were assessed in full text, and 35 were considered suitable for our analysis. Fifteen studies were excluded because they did not perform screening for MRSA colonization, 8 because they did not specify how many strains of S. aureus isolated in colonized individuals were methicillin resistant, 7 because they did not report extractable data on colonization among the HIV-infected subpopulation, 2 because they reported data exclusively on pediatric populations, and 2 because they were case-control studies. The review of the reference lists of full-text articles did not add any additional studies. Five studies contained partially overlapping data and they were included only once, with an effort to include the maximum amount of relevant information, leaving 32 studies for the final analysis (Figure 1). The references of eligible studies are provided in the Supplementary Appendix.
Figure 1.
Flow diagram of meta-analysis. Abbreviations: EMBASE, excerpta medica database; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus.
The characteristics of eligible studies are summarized in Table 1. All studies were prospective or cross-sectional except for one [21], which reported both cross-sectional and retrospective data. The total study population was 6558 individuals, of whom 4436 individuals were attending outpatient clinics and 1941 individuals were screened within 72 hours of hospitalization or in the emergency department. There was 1 study reporting data on 15 HIV-infected individuals attending a dialysis center, 1 reporting data on 30 newly arrested individuals (within 24 hours), and 1 reporting nonstratified data on hospitalized persons and individuals attending an outpatient clinic (n = 136). The included studies were published from 1990 to 2013 and reported data on 6558 individuals screened from 1988 to 2012. Fifteen of 32 studies reporting data on 4517 individuals were published after 2010. Seventeen studies (53%) were conducted in North America, 6 in Asia, 6 in Europe, and 3 in South America. All studies used culture methods to isolate MRSA. All studies were deemed of adequate quality regarding the outcome of interest and were included in the meta-analysis (Supplementary Table 2).
Table 1.
Characteristics of Eligible Studies
| Study, First Author | Publication Year | Location | Setting | Screening | Method | No.a | no. (%)b | Dominant Strain | Quality Score |
|---|---|---|---|---|---|---|---|---|---|
| Europe | |||||||||
| Oliva [S1] | 2013 | Italy | Outpatient ID clinic | N | C | 68 | 2 (2.9) | NR | 5 |
| Outpatient ID clinic | N | C | 63 | 0 (0.0) | NA | ||||
| Joore [S2] | 2013 | Netherlands | Outpatient MSM in STI clinic | N, T, P | C | 42 | 1 (2.38) | Spa-type t064 | 4 |
| Giuliani [S3] | 2010 | Italy | Outpatient MSM in HIV clinic | N | C | 104 | 0 (0.0) | NA | 4 |
| Seybold [S4] | 2009 | Germany | Outpatient ID clinic | N | C | 100 | 2 (2.0) | NR | 5 |
| Sissolak [S5] | 2002 | Austria | Within 48 h of admission | N | C | 47 | 0 (0.0) | NA | 4 |
| Weinke [S6] | 1992 | Germany | Outpatient and hospitalized | N, T | C | 136 | 0 (0.0) | NA | 5 |
| North America (US, Canada) | |||||||||
| Popovich [S7] | 2013 | Indiana, US | Within 72 h of hospitalization | N, A, G, P, T, W | C | 374 | 76 (20.3) | USA300 | 5 |
| Farley [S8] | 2013 | Maryland, US | Within 72 h of hospitalization | N, A, W | C | 68 | 10 (14.7) | USA300 | 4 |
| Peters [S9] | 2013 | Georgia, US | Outpatient HIV clinic | N, G | C | 600 | 79 (13.2) | USA300 | 5 |
| Popovich [S10] | 2012 | Indiana, US | Outpatient HIV clinic | N | C | 458 | 50 (10.9) | USA300 | 4 |
| Schechter-Perkins [S11] | 2011 | Massachusetts, US | ED | N, G, P, T, W, S | C | 11 | 4 (36.4) | USA300 | 5 |
| Crum-Cianflone [S12] | 2011 | California, US | Outpatient military HIV clinic | N, A, G, T, P | NR | 312 | 7 (2.2) | USA300 (in total) | 5 |
| Washington, D.C., US | Outpatient military HIV clinic | N, A, G, T, P | NR | 169 | 10 (5.9) | ||||
| Texas, US | Outpatient military HIV clinic | N, A, G, T, P | NR | 44 | 4 (9.1) | ||||
| Virginia, US | Outpatient military HIV clinic | N, A, G, T, P | NR | 25 | 1 (4.0) | ||||
| Alexander [S13] | 2011 | New York, US | Dialysis center | N | C | 15 | 1 (6.67) | NR | |
| Mermel [S14] | 2010 | Ohio, US | Inpatient | N | C | 161 | 5 (3.1) | USA300 (in total) | 5 |
| Maryland, US | Outpatient | N | C | 494 | 78 (15.8) | ||||
| Madariaga [S15] | 2009 | Nebraska, US | Outpatient HIV clinic | N, P | C | 100 | 2 (2) | NR | 4 |
| Antoniou [S16] | 2009 | Toronto, Canada | Outpatient clinic MSM | N, P | C | 298 | 4 (1.3) | USA300 | 4 |
| Shet [S17] | 2009 | New York, US | Outpatient HIV clinic | N, A | C | 107 | 18 (16.8) | USA300 | 5 |
| Farley [S18] | 2008 | Maryland, US | Within 24 h from arrest | N, W | C | 30 | 4 (13.3) | NR | 4 |
| Cenizal [S19] | 2008 | Texas, US | Outpatient HIV clinic | N, A | C | 146 | 15 (10.3) | USA300 | 5 |
| Hidron [S20] | 2005 | Georgia, US | Within 48 h of hospitalization | N | C | 81 | 14 (17.3) | NR | 5 |
| Miller [S21] | 2003 | New York, US | Community former and current drug users | N | C | 193 | 8 (4.2) | NR | 4 |
| Klein [S22] | 1997 | New York, US | Outpatient dermatology | N, A, W | C | 33 | 0 (0) | NA | 4 |
| Raviglione [S23] | 1990 | New York, US | Within 24 h of hospitalization | N | C | 64 | 3 (4.7) | NR | 5 |
| South America | |||||||||
| Reinato [S24] | 2013 | Brazil | Within 24 h of hospitalization | N | C | 169 | 10 (5.9) | NR | 5 |
| Padoveze [S25] | 2008 | Brazil | Outpatient HIV center | N | C | 111 | 0 (0.0) | NA | 5 |
| Padoveze [S26] | 2001 | Brazil | Outpatient HIV center | N | C | 126 | 48 (38.1) | NR | 5 |
| Inpatient | N | C | 52 | 14 (26.9) | |||||
| Asia | |||||||||
| Chow [S27] | 2012 | Singapore | On hospital admission | N, A, G, P, T, W | C | 914 | 96 (10.5) | NR | 5 |
| Kyaw [S28] | 2012 | Singapore | Outpatient HIV center | N, A, G, P, T | C | 296 | 15 (5.1) | NR | 5 |
| MohdNawi [S29] | 2012 | Malaysia | Outpatient | N, A, T | C | 130 | 1 (0.8) | NR | 4 |
| Chacko [S30] | 2009 | India | Outpatient dermatology center | N | C | 60 | 8 (13.3) | NR | 4 |
| Villacian [S31] | 2004 | Singapore | Outpatient HIV clinic | N | C | 195 | 6 (3.1) | NR | 5 |
| McDonald [S32] | 2003 | Taiwan | Outpatient ID clinic | N | C | 162 | 9 (5.6) | NR | 5 |
The references of eligible studies are provided in the Supplementary Appendix.
Abbreviations: A, axilla; C, culture; ED, emergency department; G, groin; HIV, human immunodeficiency virus; ID, infectious disease; MSM, men who have sex with men; N, nares; NA, not applicable; NR, not reported; P, perirectal; S, skin (palms of both hands); STI, sexually transmitted infection; T, throat; W, wound.
a Number of patients who were screened for methicillinresistant Staphylococcus aureus (MRSA) colonization.
b Number of patients who were found to be MRSA colonized.
The combined prevalence of MRSA colonization among HIV-positive individuals was estimated to be 6.9% (95% CI, 4.8–9.3; τ2 = 0.067). The Egger test yielded no evidence of small-study effects (bias −1.39; P = .17). When data from studies conducted exclusively in North America were separately analyzed, the pooled prevalence was estimated to be 8.8% (95% CI, 6.0–12.2; τ2 = 0.051), and again there was no evidence of publication bias (−0.60; P = .56). Ten of 17 studies conducted in North America also reported the strain of the isolated MRSA. All of them identified USA300 as the most prevalent strain. Among individuals attending outpatient clinics in North American studies, the pooled prevalence of MRSA colonization was lower (7.0% [95% CI, 4.1–10.6]) than among individuals screened upon their admission to the hospital (13.5% [95% CI, 6.2–23.0]), but the difference did not reach statistical significance (P = .12). Across European studies (560 individuals), the prevalence was found to be 1.0% (95% CI, .3–2.2), whereas across Asian studies (n = 1757), the corresponding figure was 5.8% (95% CI, 2.8–9.8) (Table 2).
Table 2.
Summary Estimates of Included Studies
| MRSA Colonization Prevalence | Studies (Arms) | No.a | % Combined Effect (95% CI) | τ2 | P Value |
|---|---|---|---|---|---|
| All studies | 32 (38) | 6558 | 6.9 (4.8–9.3) | 0.067 | |
| Geographic region | |||||
| North America | 17 (21) | 3783 | 8.8 (6.0–12.2) | 0.051 | Ref |
| Europe | 7 (7) | 560 | 1.0 (0.3–2.2) | 0.005 | .01 |
| Asia | 6 (6) | 1757 | 5.8 (2.8–9.8) | 0.028 | .30 |
| Setting (among North American studies) | |||||
| Outpatient | 10 (13) | 2979 | 7.0 (4.1–10.6) | 0.046 | Ref |
| On hospital admission | 6 (6) | 759 | 13.5 (6.2–23.0) | 0.079 | .12 |
Abbreviations: CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; Ref, reference.
a Number of patients who were screened for MRSA colonization.
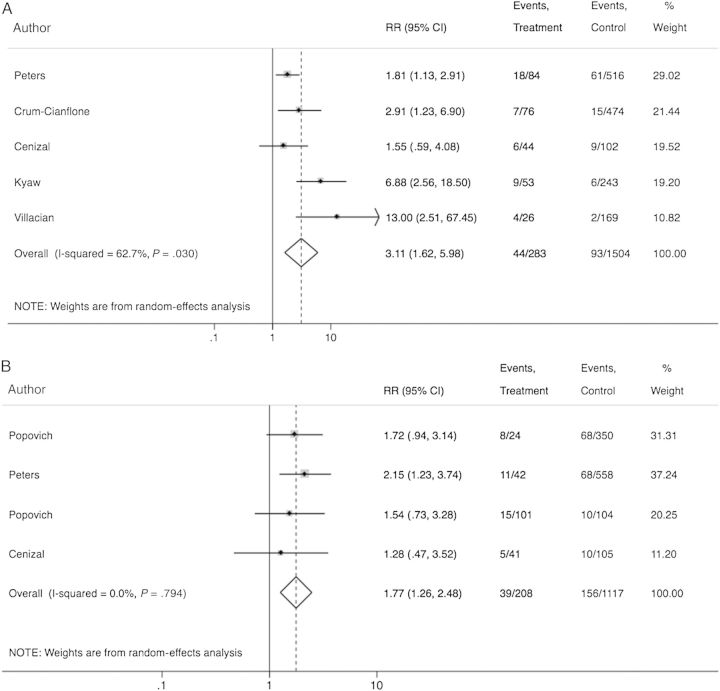
Based on 5 studies that reported stratified data on hospitalization during the previous 12 months among 1787 colonized and noncolonized individuals with HIV infection, we estimated that individuals hospitalized in the preceding year have a 3.1 times higher risk of being colonized compared with nonhospitalized individuals (RR, 3.11 [95% CI, 1.62–5.98]; Figure 2). Moreover, based on 4 studies that reported stratified data for colonized and noncolonized individuals with history of incarceration (1325 individuals), we found that individuals with history of incarceration are 77% more likely to be MRSA colonized compared with patients without (RR, 1.77 [95% CI, 1.26–2.48]; Figure 2). Current antiretroviral therapy (RR, 1.02 [95% CI, .64–1.63]), current use of TMP-SMX (RR, 1.45 [95% CI, .69–3.03]), or illicit drug use (RR, 1.26 [95% CI, .73–2.19]) was not observed to be associated with increased risk of MRSA colonization. Of note, only 1 of the studies included in the subanalysis of the impact of prior TMP-SMX use on the risk of MRSA colonization reported data on the resistance patterns of isolated MRSA strains from patients who had previously received this antibiotic. In this study, all MRSA isolates among recipients of TMP-SMX (6 patients) were resistant to this antibiotic. Finally, there was a possible increased risk for colonization among patients with a history of syphilis (RR, 2.98 [95% CI, .95–9.34]), but this did not reach statistical significance (Supplementary Figure 1). Across the included studies, we were not able to estimate the impact of HIV stage (CD4 count, viral load) on the risk of MRSA colonization because studies reported stage of disease instead of absolute CD4 or viral load counts and used different cutoffs or provided the relative effects or mean values of CD4 and viral load without providing raw data. Individual study data regarding this association are described in Supplementary Table 3. More specifically, 1 study conducted in Europe, 6 studies conducted in North America, and 4 studies conducted in Asia examined the risk of MRSA colonization based on the disease stage of the study population. Among them, 5 studies found that MRSA carriage is independent of CD4 count, whereas 6 concluded that lower CD4 counts increase the risk of MRSA colonization.
Figure 2.
Forest plot of included studies. Relative risk estimates of methicillin-resistant Staphylococcus aureus colonization among previously hospitalized patients (A) and previously incarcerated patients (B). Abbreviations: CI, confidence interval; RR, risk ratio.
Across the included studies, 7 reported concurrent data on screening nares and extranasal body sites in the same study population. Four of these were conducted in the United States, 2 in Singapore, and 1 in the Netherlands. Studies were conducted between 2007 and 2012 and included data on 2876 HIV-infected individuals. Two of the studies screened individuals on admission to the hospital; the other 5 screened subjects during outpatient visits. Testing for MRSA carriage at extranasal body sites increased detection of MRSA carriage in all but 1 study [22]. The number of MRSA carriers by extranasal testing increased by 31.6% (95% CI, 15.8–50.0). The studies also provided data on individuals colonized at individual extranasal body sites with negative nasal screening. Five studies reported on individuals with positive perirectal screening, 4 each on individuals with positive groin and throat screening, 2 on individuals with positive axilla screening, and 1 on individuals with positive wound screening. Screening of the groin identified 19.3% (95% CI, 11.5–28.5) more colonized individuals than screening nares alone and perirectal screening identified 18.5% (95% CI, 7.4–33.2), whereas the corresponding figure for throat cultures was 17.5% (95% CI, 12.0–24.0). Of note is that 1 study could not be included in the analysis as no patient was colonized in the nares, and exclusively extranasal colonization was studied [23] (Table 3).
Table 3.
Individual Study Data for Extranasal Colonization
| Author | Population | Prevalence (Any Body Site) | Prevalence (Nasal) | Prevalence (Exclusive Extranasal) | Throat | Rectum | Groin | Axilla | Wound |
|---|---|---|---|---|---|---|---|---|---|
| Popovich [S7] | On admission to hospital (within 72 h) | 76/374 | 45/374 | 31/374 | 9/374 | 17/374 | 12/374 | 5/374 | 2/374 |
| Crum-Cianflone [S12] | Outpatient clinic | 22/550 | 18/550 | 4/550 | 2/550 | 1/550 | 1/550 | 0/550 | … |
| Madariaga [S15] | Outpatient clinic | 2/100 | 2/100 | 0/100 | … | … | 0/100 | … | … |
| Peters [S9] | Outpatient clinic | 79/600 | 66/600 | 13/600 | … | … | 13/600 | … | … |
| Chowa [S27] | On admission to the hospital | 96/914 | 74/96 | 22/96 | 12/96 | 12/96 | NA | NA | … |
| Kyawa [S28] | Outpatient clinic | 15/296 | 12/296 | 3/296 | 2/296 | 1/296 | NA | NA | … |
| Joore [S2] | Outpatient STD clinic-MSM | 1/42 | 0/42 | 1/42 | … | 1/42 | … | … | … |
The references of eligible studies are provided in the Supplementary Appendix.
Abbreviations: MSM, men who have sex with men; NA, not applicable; STD, sexually transmitted disease.
a Studies performed nares, axilla, and groin swabbing.
DISCUSSION
Our meta-analysis showed that 6.9% of individuals infected with HIV are MRSA carriers, an estimation that is as high as that reported among high-risk populations, such as patients on chronic dialysis (6.2%) [1] and patients admitted to the intensive care unit (7.0%) [3]. Prior hospitalization and prior or current incarceration are significantly associated with MRSA colonization, and nasal screening alone underestimates the rate of colonization by at least one-third. This estimated prevalence of MRSA carriage is worrisome given the high rate [24–27] and morbidity [8] of MRSA infections in this population. Importantly, individuals hospitalized during the previous 12 months have a 3.1 times higher risk of MRSA carriage compared with nonhospitalized individuals. This association highlights the importance of the contact with the healthcare system in this population. Among our studies, the most commonly isolated strain was USA300, which is a community-acquired strain. However, this strain is nowadays increasingly prevalent also in the healthcare setting [28].
Previous or current incarceration was also found to increase the risk of MRSA colonization among HIV-infected individuals. Crowding; rationed access of inmates to soap, water, and clean laundry; delays in diagnosis of skin and soft tissue infections; and sharing linen, soap, or clothing are some of the factors that have been suggested to account for high MRSA colonization of inmates [29]. Jails and prisons have been characterized as settings with increased prevalence of MRSA and other drug-resistant organisms that are spread primarily by person-to-person contact even among non-HIV-infected individuals [30, 31]. This led the Federal Bureau of Prisons, as well as different states' departments of justice and public health, to develop guidelines specifically for the control of MRSA transmission in correctional facilities [32]. The implementation of these policies had inconclusive results in different facilities [33, 34]. Because our study focused on HIV-infected individuals, we cannot provide any comparisons on the prevalence of MRSA among prisoners with and without HIV. However, HIV infection has been previously shown to be independently associated with an increased risk of MRSA carriage in correctional facilities [35]. Of note, the variation in the time frame of incarceration reported in the individual studies did not allow an estimation of how long the increased risk for MRSA colonization persists.
Antiretroviral therapy is proven to reverse some of the immunologic abnormalities that may render HIV-infected individuals vulnerable to MRSA colonization and infection, and several studies have examined its association with the rate of MRSA carriage with inconclusive results [36]. Also, trimethoprim-sulfamethoxazole (TMP-SMX) has antistaphylococcal activity. However, our meta-analysis could not find an association between either current antiretroviral therapy or TMP-SMX with the estimated MRSA carriage. It should be noted here that individuals who receive antiretroviral therapy and TMP-SMX prophylaxis might have a lower CD4 count than those who do not receive therapy, and this may increase their risk of MRSA colonization. Because our RR estimates are unadjusted for the CD4 count, the possibly protective effect of antiretroviral therapy and TMP-SMX prophylaxis on MRSA carriage might be lost due to the difference in the CD4 count.
Interestingly, extranasal testing identified additional MRSA-colonized individuals in all but 1 study (which had identified only 2 colonized individuals out of 100 screened) among those that provided stratified data [22]. More specifically, extranasal screening increased the number of individuals identified as carriers by 31.6%, which means that for almost every 3 carriers, 1 will be missed if extranasal sites are not screened. This estimation might even underestimate the actual prevalence of extranasal colonization as not all studies included in this subanalysis screened all the available extranasal sites. Due to limited data, it was not possible to estimate the benefit of combining multiple extranasal body-site screenings. This high prevalence of extranasal colonization probably indicates that nasal mupirocin ointment alone might not be able to effectively decolonize the carriers, highlighting the importance of the chlorhexidine bathing in this population. The fact that groin and perirectal screening provided significant additional yield for MRSA detection over nares screening (19.3% and 18.5%, respectively) is particularly interesting given that 26%–40% of SSTIs among HIV-infected individuals are reported to be located in the buttocks/scrotum/anogenital region [6, 37]. Additional clinical studies should examine the association of the site of colonization with the site of SSTI.
Of note is that only 16 of the 32 studies (50%) included in our meta-analysis included screening of extranasal sites in their protocol, and this might have underestimated the burden of MRSA colonization in this population. Also, only culture methods were used among the included studies, which are reported to have lower sensitivity compared with polymerase chain reaction [38]. Finally, our estimates combine data from different settings, countries, and years, and our results may not apply to the individual center where local epidemiology has the most significant role. Indeed, we found that there was limited or absent MRSA colonization among HIV-infected individuals across the 7 studies with 560 individuals that were conducted in Europe, but further studies are needed to confirm this low estimated prevalence.
Among the studies included in our meta-analysis, 2 reported stratified data on MRSA infections among colonized and noncolonized individuals [10, 11]. In these studies, 68% and 100% of reported MRSA infections were among previously identified carriers and the colonizing isolates always matched clinical isolates. The possible association of colonization with infection and the identified increased burden of MRSA carriage among HIV-infected individuals raise concerns about whether screening and/or decolonization policies should be applied in clinical practice, as in high-risk hospitalized populations. In the outpatient setting, there is no straightforward answer to the efficacy of active surveillance for colonization and decolonization in reducing the rate of infections, as well as to the best policy regarding frequency of screening [39]. Approaches should be tested in clinical studies taking into account the high rate of extranasal colonization that was found in our study. Subpopulations with high-risk characteristics for MRSA infection in addition to HIV infection (eg, intravenous drug use), as well as patients with increased risk for colonization (eg, previously hospitalized or incarcerated patients), might benefit more from screening and/or decolonization and should be the first targets in such clinical outpatient studies. In the meantime, current community-associated MRSA prevention strategies seem to be of high importance [39]. In the hospital setting, it might be prudent to consider including HIV-infected individuals in the populations routinely screened on hospital admission. Decolonization for some individuals, as well as strict compliance with infection control policies, might be of significant help given the vulnerability of this population to both colonization and infection.
CONCLUSIONS
In this study, HIV-infected individuals were found to have high prevalence of MRSA colonization, with a significant proportion of carriage being detected exclusively in extranasal sites. We also found strong evidence that MRSA colonization is mainly related to previous exposure to settings with high prevalence of MRSA, such as hospitals, jails, and prisons and weaker evidence regarding the association with high-risk sexual behaviors. Given that HIV-infected individuals are at increased risk for MRSA infection and that colonization has been associated with infection, these findings emphasize the need for evaluation and implementation of MRSA prevention strategies that focus on this highly vulnerable population. In this effort, specific attention should be drawn to the important burden of colonization in extranasal sites.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse (NIDA) or the National Institutes of Health (NIH).
Financial support. J. D. R. was supported for this project by award number K24DA022112 from NIDA and in part by the infrastructure and resources provided by the Lifespan/Tufts/Brown Center for AIDS Research, an NIH-funded program (grant number P30-AI-42853), from the NIH Center for AIDS Research.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E. Meta-analysis of methicillin-resistant Staphylococcus aureus colonization and risk of infection in dialysis patients. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013091028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zervou FN, Zacharioudakis IM, Ziakas PD, Mylonakis E. MRSA colonization and risk of infection in the neonatal and pediatric ICU: a meta-analysis. Pediatrics. 2014;133:e1015–23. doi: 10.1542/peds.2013-3413. [DOI] [PubMed] [Google Scholar]
- 3.Ziakas PD, Anagnostou T, Mylonakis E. The prevalence and significance of methicillin-resistant Staphylococcus aureus colonization at admission in the general ICU setting: a meta-analysis of published studies. Crit Care Med. 2014;42:433–44. doi: 10.1097/CCM.0b013e3182a66bb8. [DOI] [PubMed] [Google Scholar]
- 4.Ziakas PD, Pliakos EE, Zervou FN, Knoll BM, Rice LB, Mylonakis E. MRSA and VRE colonization in solid organ transplantation: a meta-analysis of published studies. Am J Transplant. 2014 doi: 10.1111/ajt.12784. doi:10.1111/ajt.12784. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–65. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crum-Cianflone NF, Burgi AA, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. Int J STD AIDS. 2007;18:521–6. doi: 10.1258/095646207781439702. [DOI] [PubMed] [Google Scholar]
- 7.Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clin Infect Dis. 2010;50:979–87. doi: 10.1086/651076. [DOI] [PubMed] [Google Scholar]
- 8.Hidron AI, Kempker R, Moanna A, Rimland D. Methicillin-resistant Staphylococcus aureus in HIV-infected patients. Infect Drug Resist. 2010;3:73–86. doi: 10.2147/idr.s7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen MH, Kauffman CA, Goodman RP, et al. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Ann Intern Med. 1999;130:221–5. doi: 10.7326/0003-4819-130-3-199902020-00026. [DOI] [PubMed] [Google Scholar]
- 10.Peters PJ, Brooks JT, McAllister SK, et al. Methicillin-resistant Staphylococcus aureus colonization of the groin and risk for clinical infection among HIV-infected adults. Emerg Infect Dis. 2013;19:623–9. doi: 10.3201/eid1904.121353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shet A, Mathema B, Mediavilla JR, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. J Infect Dis. 2009;200:88–93. doi: 10.1086/599315. [DOI] [PubMed] [Google Scholar]
- 12.Cole J, Popovich K. Impact of community-associated methicillin resistant Staphylococcus aureus on HIV-infected patients. Curr HIV/AIDS Rep. 2013;10:244–53. doi: 10.1007/s11904-013-0161-0. [DOI] [PubMed] [Google Scholar]
- 13.Sampson M, Barrowman NJ, Moher D, et al. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol. 2003;56:943–55. doi: 10.1016/s0895-4356(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of norandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 18 March 2014.
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in Western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5:e225. doi: 10.1371/journal.pmed.0050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–8. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 19.Rucker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliva A, Lichtner M, Mascellino MT, et al. Study of methicillin-resistant Staphylococcus aureus (MRSA) carriage in a population of HIV-negative migrants and HIV-infected patients attending an outpatient clinic in Rome. Ann Ig. 2013;25:99–107. doi: 10.7416/ai.2013.1911. [DOI] [PubMed] [Google Scholar]
- 22.Madariaga MG, Ullrich F, Swindells S. Low prevalence of community-acquired methicillin-resistant Staphylococcus aureus colonization and apparent lack of correlation with sexual behavior among HIV-infected patients in Nebraska. Clin Infect Dis. 2009;48:1485–7. doi: 10.1086/598511. [DOI] [PubMed] [Google Scholar]
- 23.Joore IK, van Rooijen MS, Schim van der Loeff MF, de Neeling AJ, van Dam A, de Vries HJ. Low prevalence of methicillin-resistant Staphylococcus aureus among men who have sex with men attending an STI clinic in Amsterdam: a cross-sectional study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002505. doi:10.1136/bmjopen-2012-002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hidron AI, Moanna A, Rimland D. The rise and fall of methicillin-resistant Staphylococcus aureus infections in HIV patients. AIDS. 2011;25:1001–3. doi: 10.1097/QAD.0b013e328343c595. [DOI] [PubMed] [Google Scholar]
- 25.Delorenze GN, Horberg MA, Silverberg MJ, Tsai A, Quesenberry CP, Baxter R. Trends in annual incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection in HIV-infected and HIV-uninfected patients. Epidemiol Infect. 2013;141:2392–402. doi: 10.1017/S0950268813000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkey MD, Wilson LE, Moore RD, Lucas GM, Francis J, Gebo KA. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med. 2008;9:858–62. doi: 10.1111/j.1468-1293.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews WC, Caperna JC, Barber RE, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J Acquir Immune Defic Syndr. 2005;40:155–60. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- 28.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787–94. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 29.Bick JA. Infection control in jails and prisons. Clin Infect Dis. 2007;45:1047–55. doi: 10.1086/521910. [DOI] [PubMed] [Google Scholar]
- 30.Aiello AE, Lowy FD, Wright LN, Larson EL. Meticillin-resistant Staphylococcus aureus among US prisoners and military personnel: review and recommendations for future studies. Lancet Infect Dis. 2006;6:335–41. doi: 10.1016/S1473-3099(06)70491-1. [DOI] [PubMed] [Google Scholar]
- 31.Malcolm B. The rise of methicillin-resistant Staphylococcus aureus in U.S. correctional populations. J Correct Health Care. 2011;17:254–65. doi: 10.1177/1078345811401363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Federal Bureau of Prisons. Management of methicillin-resistant Staphylococcus aureus (MRSA) infections. Available at: http://www.bop.gov/resources/pdfs/mrsa.pdf. Accessed 24 April 2014.
- 33.Wootton SH, Arnold K, Hill HA, et al. Intervention to reduce the incidence of methicillin-resistant Staphylococcus aureus skin infections in a correctional facility in Georgia. Infect Control Hosp Epidemiol. 2004;25:402–7. doi: 10.1086/502413. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities—Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–6. [PubMed] [Google Scholar]
- 35.Baillargeon J, Kelley MF, Leach CT, Baillargeon G, Pollock BH. Methicillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin Infect Dis. 2004;38:e92–95. doi: 10.1086/383146. [DOI] [PubMed] [Google Scholar]
- 36.Imaz A, Pujol M, Barragan P, Dominguez MA, Tiraboschi JM, Podzamczer D. Community associated methicillin-resistant Staphylococcus aureus in HIV-infected patients. AIDS Rev. 2010;12:153–63. [PubMed] [Google Scholar]
- 37.Lee NE, Taylor MM, Bancroft E, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus skin infections among HIV-positive men who have sex with men. Clin Infect Dis. 2005;40:1529–34. doi: 10.1086/429827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luteijn JM, Hubben GA, Pechlivanoglou P, Bonten MJ, Postma MJ. Diagnostic accuracy of culture-based and PCR-based detection tests for methicillin-resistant Staphylococcus aureus: a meta-analysis. Clin Microbiol Infect. 2011;17:146–54. doi: 10.1111/j.1469-0691.2010.03202.x. [DOI] [PubMed] [Google Scholar]
- 39.Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA participants in the CDC Convened Experts’ Meeting on Management of MRSA in the Community. Strategies for clinical management of MRSA in the community: summary of an experts’ meeting convened by the Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/mrsa/pdf/MRSA-Strategies-ExpMtgSummary-2006.pdf. Accessed 30 June 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.