Abstract
Microsomal enzymes generate H2O2 in the presence of NADPH. In this reaction, referred to as “oxidase” activity, H2O2 is generated directly or indirectly via the formation of superoxide anion. In the presence of redox active transition metals, H2O2 can form highly toxic hydroxyl radicals and, depending on the “oxidase” activity of individual cytochrome P450 isoenzymes, this can compromise cellular functioning and contribute to tissue injury. In the present studies, we compared the initial rates of H2O2 generating activity of microsomal preparations containing various human recombinant cytochromes P450s. In the absence of cytochrome P450s the human recombinant NADPH cytochrome P450 reductase (CPR) generated low, but detectable amounts of H2O2 (∼0.04 nmol H2O2/min/100 units of reductase). Significantly greater activity was detected in preparations containing individual cytochrome P450s coexpressed with CPR (from 6.0 nmol H2O2/min/nmol P450 to 0.2 nmol/min/nmol P450); CYP1A1 was the most active, followed by CYP2D6, CYP3A4, CYP2E1, CYP4A11, CYP1A2, and CYP2C subfamily enzymes. H2O2 generating activity of the cytochrome P450s was independent of the ratio of CYP/CPR. Thus, similar H2O2 generating activity was noted with the same cytochrome P450s (CYP3A4, CYP2E1, and CYP2C9) expressed at or near the ratio of CYP/CPR in human liver microsomes (5–7), and when CPR was present in excess (CYP/CPR = 0.2–0.3). Because CYP3A4/5/7 represent up to 40% of total cytochrome P450 in the liver, these data indicate that these enzymes are the major source of H2O2 in human liver microsomes.
Keywords: reactive oxygen species, cytochrome P450, microsomes, cytochrome P450 reductase, “oxidase” reaction
ABBREVIATIONS
- AR
Amplex Red
- CYP
cytochrome P450
- CPR
NADPH-cytochrome P450 reductase (EC 1.6.2.4)
- DMSO
dimethyl sulfoxide
- DPI
diphenyliodonium chloride
- DETAPAC
diethylenetriaminepentaacetic acid
- HRP
horseradish peroxidase
- RFU
relative fluorescence units
- SOD
superoxide dismutase
Microsomal drug metabolizing enzyme complexes carry out the transformation of xenobiotics in several successive steps which include electron transport and oxygen activation (Cooper and Groves, 2011; Gorsky et al., 1984; Guengerich and Johnson, 1997; Gutierrez et al., 2003). Initially, nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) cytochrome P450 reductase (CPR), a flavin adenine dinucleotide (FAD) and a flavin mononucleotide (FMN) containing enzyme, accepts electrons from NADPH. An interflavin electron transfer in the enzyme forms stable semiquinone FAD/FMN intermediates which then sequentially donate two electrons to cytochrome P450 enzymes. Cytochrome P450s use these electrons and two protons to form “active” oxygen intermediates which react with substrates in the final stages of the monooxygenase reaction. The supply of reducing equivalents to the cytochrome P450 enzymes is a key step for efficient metabolism of xenobiotics. During the monooxygenase reaction, microsomal enzymes can simultaneously generate hydrogen peroxide (H2O2) in a process referred to as “uncoupling” of the microsomal electron transport chain or in absence of metabolizing substrate, referred as “NADPH oxidase” (Gillette et al., 1957). Several intracellular mechanisms have been identified that eliminate H2O2, including catalase and glutathione peroxidase mediated reactions (Bhabak and Mugesh, 2010; Kirkman and Gaetani, 2007). Excessive accumulation of H2O2 can overwhelm these pathways resulting in the formation of cytotoxic reactive hydroxyl radicals in the presence of transition metals (Boveris et al., 1972; Goswami et al., 2002; Jomova and Valko, 2011).
The precise chemistry underlying the generation of H2O2 in microsomes is not known. It can be produced either indirectly via the formation of superoxide anion which then dismutates rapidly into H2O2 or directly as the result of the decay of a hydroperoxo intermediate formed during oxygen activation in the cytochrome P450 active center (Denisov et al., 2005; Hamdane et al., 2008; Kuthan and Ullrich, 1982; Makris et al., 2002). Cytochrome P450 enzymes are thought to be the major generators of H2O2 in microsomes (Bernhardt, 1996; Bondy and Naderi, 1994; Denisov et al., 2005; Estabrook et al., 1979; Hamdane et al., 2008; Makris et al., 2002; Perret and Pompon, 1998; Zangar et al., 2004). However, the relative activities of different P450 enzymes in generating H2O2 are not well defined. Earlier studies have reported that several recombinant human cytochrome P450 enzymes can generate reactive oxygen species, but this has not been characterized in detail (Patten and Koch, 1995; Puntarulo and Cederbaum, 1998; Schlezinger et al., 1999). In the present studies, we quantitatively measured absolute rates of H2O2 generation using microsomal preparations containing a panel of individual human recombinant cytochrome P450 enzymes. For these studies, we applied a sensitive Amplex Red/horse radish peroxidase (HRP) assay. We found that individual human cytochrome P450s varied over 10-fold in their ability to generate H2O2. These data suggest that the specific composition of cytochrome P450s determines relative rates of H2O2 formation in microsomes and elevated expression of cytochrome P450 enzymes with high “oxidase” activities may contribute directly towards generating oxidative stress.
MATERIALS AND METHODS
Chemicals and reagents
Microsomal fractions from insect cells infected with a baculovirus containing no human enzymes (negative control), human CPR, human CPR and either human CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C8, CYP2C9(Arg144), CYP2C18, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, CYP3A7, CYP2J2, CYP4A11, or CYP4F2 were from BD Gentest (Woburn, MA). Mixtures of human CPR and human cytochrome P450s (CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4), referred as SUPERMIX, were also obtained from BD Gentest (Woburn, MA). Purified human cytochrome b5, expressed as histidine-tagged recombinant protein in Escherichia coli, and Vivid Cytochrome P450 Fluorogenic Probe Substrates were from Invitrogen (Carlsbad, CA). Purified CPR from rabbit liver (cat. no. C4839, 38,000 units/mg protein, where one unit of CPR activity is defined as the reduction of 1.0 nmol of cytochrome c/min at pH 7.7 at 30°C, purity ∼90%), HRP, type 1 (cat. no. P-8125), catalase (from Aspergillus niger as an ammonium sulfate suspension, cat. no. C3515), superoxide dismutase (SOD, cat. no. S2515), NADPH, sodium azide, dimethyl sulfoxide (DMSO), diethylammonium salt of diethylenetriaminepentaacetic acid (DETAPAC), diphenyliodonium chloride (DPI), and 30% H2O2, were from Sigma-Aldrich (St Louis, MO). Amplex Red (10-acetyl-3,7-dihydroxyphenoxazine, AR) was from Molecular Probes (Eugene, OR), and was prepared in oxygen-free DMSO as a 10mM stock solution. All chemicals and water were of the highest available purity and used without additional treatments.
Metabolic activities of recombinant cytochrome P450s
The activities of recombinant cytochrome P450s were evaluated using a set of Vivid fluorogenic substrates as described (Crespi et al., 2002). 7-Ethyloxymethyloxy-3- cyanocoumarin (EOMCC) was used for CYP1A2, 2C19, CYP2D6, and CYP2E1, 7-benzyloxymethyloxy-3-cyanocoumarin (BOMCC) for CYP2C8/9, and dibenzyloxy methoxyfluorescein (DBOMF) for CYP3A4. In these assays, the formation of fluorogenic products were recorded continuously using a microplate reader (SpectraMax M5, Molecular Device, Sunnyvale, CA) in fluorescence mode. Standard reaction mixtures (final volume, 200 μl) contained 50mM potassium phosphate buffer, pH 7.4, 0.5mM DETAPAC, appropriate amounts of recombinant cytochrome P450s (typically, 1–10 pmol), and the selected substrates. The reactions were initiated by the addition of a mixture containing NADPH and an NADPH regenerating system consisting of NADPH, glucose-6-phosphate, and glucose-6 phosphate dehydrogenase (final concentrations, 0.1mM, 10.0mM, and 0.5 U/ml, respectively). Under these conditions, reactions rates were directly proportional to enzyme concentrations and time of incubation.
Enrichment of recombinant microsomal enzymes with cytochrome b5
Enrichment of the enzyme preparations with purified human cytochrome b5 was accomplished as previously described (Patten and Koch, 1995). Briefly, microsomal preparations were preincubated at room temperature with intermittent gentle mixing with purified cytochrome b5 in a small volume (20–30 μl) containing 50mM potassium phosphate buffer, pH 7.7, and 0.5mM DETAPAC. After 15 min, the microsomal preparations were placed on ice and analyzed within 1 h.
Optimization and validation of the AR/HRP assay for use with recombinant microsomal enzymes
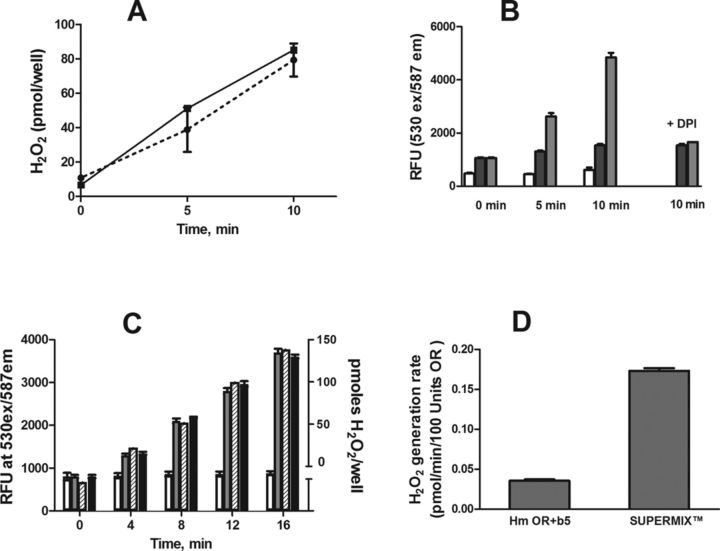
We have previously shown that the AR/HRP assay can be used to quantify H2O2 generated by microsomal enzymes (Mishin et al., 2010). In this assay, HRP-H2O2 complexes catalyze the oxidation of nonfluorescent AR to highly fluorescent resorufin (Mohanty et al., 1997; Towne et al., 2004). In our earlier studies (Mishin et al., 2010), resorufin redox cycling was found to interfere with the analysis when AR/HRP was present continuously in the assay of recombinant microsomal enzymes. Therefore, to accurately quantify H2O2 formed by microsomal enzymes, AR/HRP was only added at selected times after the enzyme reactions were terminated by the addition of ice-cold acetonitrile (25%, final concentration). Under these conditions, NADPH-dependent electron transport and cytochrome P450-dependent enzyme metabolic activities were stopped without affecting the activity of the AR/HRP assay (Fig. 1, upper and center panels).
FIG. 1.
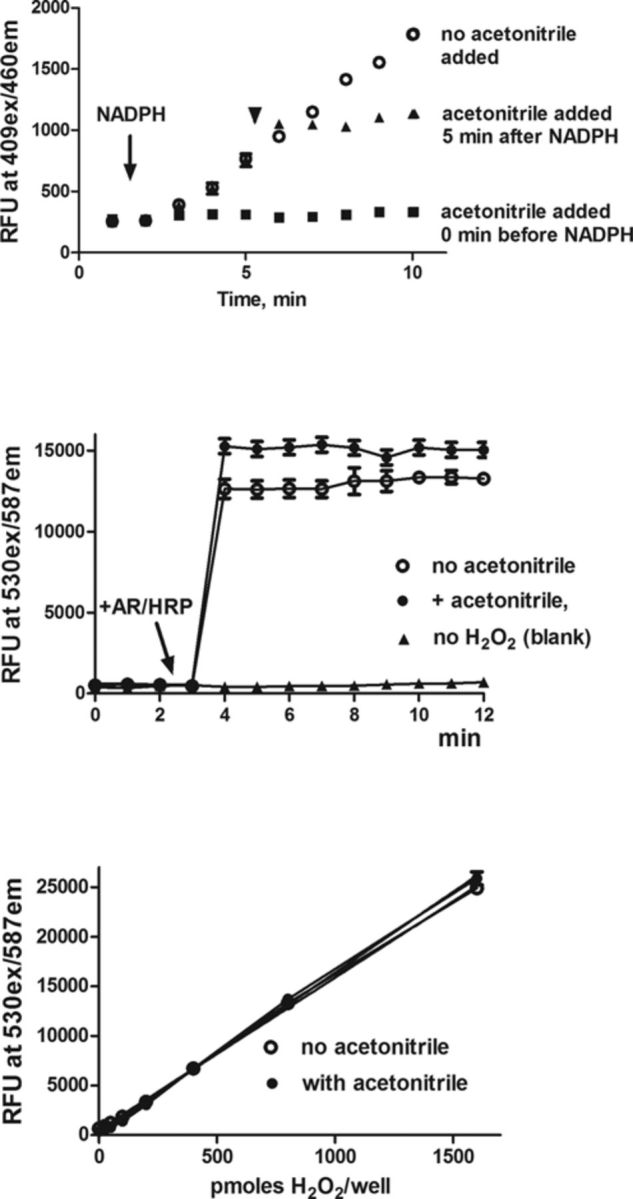
Effects of acetonitrile on cytochrome P450 activity and the measurements of H2O2. Upper panel: The activity of CYP2C9 was measured using BOMCC as a substrate. The reaction was initiated by the addition of a mixture of NADPH and an NADPH-regenerating system (indicated by an arrow). Fluorescence of the reaction product, 3-hydroxy-7-hydroxycoumarin (409 nm exitation/460 nm emission), was recorded continuously. Open circles, control CYP2C9 activity; closed squares, acetonitrile (25% final concentration) was added 1 min before the addition of NADPH to the reaction; closed triangles, acetonitrile was added 5 min after the start of the reaction (shown by the arrowhead). Center panel: Effects of acetonitrile on the measurement of H2O2. AR/HRP mixes were added to samples containing 800 pmol of H2O2 (indicated by the arrow). Open circles, measurements of H2O2 in the absence of acetonitrile; closed circles, measurements of H2O2 in the presence of acetonitrile (12.5% final concentration); closed triangles, blank samples (no H2O2 added). Assays were run in triplicate and presented as the mean ± SD. Lower panel: Effects of acetonitrile on the H2O2 standard calibration curve. Standard curves for H2O2 were obtained in the absence and presence of acetonitrile (12.5% final concentration). Open circles, no acetonitrile added; closed circles, acetonitrile added. Samples were analyzed in triplicate at least four times. Data are presented as the mean ± SD. Note that acetonitrile did not significantly affect the measurement of H2O2 standards.
Assays for measuring H2O2 formation were run in 96-well black flat-bottom microplates. Sixty microliters of reaction mixes containing 50mM potassium phosphate buffer, pH 7.7, 1.0mM sodium azide, and 0.5mM DETAPAC, were added to each well of the microplate. Aliquots (5–10 μl) of recombinant microsomal preparations were added to the wells such that the amounts of recombinant microsomal enzymes were within 2.0–10.0 pmol of cytochrome P450 enzyme/assay. After preincubating the plate for 5 min at 37°C, the H2O2 generating reaction was initiated by the addition of a 10 μl mixture containing NADPH and an NADPH-regenerating system. To terminate the reactions, 25 μl of acetonitrile were added into the wells with rapid mixing using a pipette. After 10 min, 100 μl of an AR/HRP mix (in potassium phosphate buffer, 50mM, pH 7.7) was added to the wells. The final reaction mixes (200 μl, total) contained 25μM AR, 1.0 units/ml HRP, and 12.5% acetonitrile. Fluorescence of the product, resorufin (530 nm exitation/587 nm emission), was quantified using a microplate reader. In initial experiments, the reactions were run with varying amounts of microsomal enzymes and terminated at different time points (see figure legends for details). Various alkoxyresorufins which are structurally related to AR, are known to be good substrates for selective cytochrome P450 enzymes forming resorufin during the O-dealkylation reactions (Burke et al., 1994; Lubet et al., 1990). In control experiments, we found that AR was not a substrate for CYP1A1 and CYP1A2 since the omission of HRP reduced the fluorescence changes to background levels. In some experiments, catalase was added to reaction mixtures to degrade H2O2, and in these experiments, sodium azide was omitted from the incubation medium. To calculate the absolute rates of H2O2 generation, only linear phases of the reactions were used. The amounts of H2O2 formed in the reactions were determined using H2O2 standards as described earlier (Mishin et al., 2010), except that calibration samples were analyzed in the presence of 12.5% acetonitrile (Fig. 1, lower panel). In separate experiments, we found that resorufin fluorescence was stable in the presence of acetonitrile for at least 60 min.
Data are presented as the means ± SD. Statistical analyses were performed using GraphPad Prism 5. Values were considered to differ significantly at the level of p < 0.05. The ratio of individual P450s to CPR was calculated based on data supplied by manufacturer. Because CPR was measured as activity in the reduction of cytochrome c, amounts were calculated based on the fact that 1 nmol of CPR reduces 3000 nmol of cytochrome c (Guengerich et al., 2009).
RESULTS
In initial experiments, we examined the H2O2 generating activity of a microsomal preparation of human recombinant CPR in the absence of any of cytochrome P450 enzymes. In the presence of NADPH, this preparation generated H2O2 in a time-dependent manner (Fig. 2, panel A). The rate of H2O2 production was 0.034 ± 0.013 nmol of H2O2/min/100 units of reductase activity. This activity was inhibited by diphenyliodonium chloride (DPI), a flavoenzyme inhibitor (Fig. 2, panel B). In contrast, although low levels of H2O2 generation were detected in control microsomal preparations, it was not inhibited by DPI, indicating that it was independent of CPR (Fig. 2, panel B). Recombinant CPR also contains cytochrome b5. To determine if cytochrome b5 contributes to H2O2 formation, we analyzed the activity of purified rabbit liver CPR, which lacks cytochrome b5. The H2O2 generating activity of this enzyme was similar (0.038 ± 0.04 nmol of H2O2/min/100 units of reductase activity) to the human recombinant enzyme coexpressed with cytochrome b5 (Fig. 2, panel C). When purified recombinant cytochrome b5 was added to the rabbit CPR, no changes in its H2O2 generating activity were noted (Fig. 2, panel C). Cytochrome b5 by itself, either in the absence or presence of NADPH, was unable to generate H2O2 (not shown). Taken together, these data indicate that cytochrome b5 does not play a role in the H2O2 generating activity of CPR.
FIG. 2.
H2O2 generation by CPRs and by SUPERMIX. H2O2 was quantified using AR/HRP in the presence of NADPH and an NADPH-regenerating system. At the indicated time points, acetonitrile (25%, final concentration) was added to terminate the reactions. To detect H2O2, AR/HRP was then added to the reactions and resorufin fluorescence quantified (530 nm exitation/585 nm emission). The amount of H2O2 formed in the reactions was calculated using a standard curve. The values are the means ± SD of at least two independent experiments assayed in triplicate. (Panel A) H2O2 generation by CPRs. Equal amounts (20 units/well) of recombinant human (dotted line) and purified rabbit enzymes (solid line) were assayed. Note that the human recombinant enzyme is coexpressed with human cytochrome b5. (Panel B) H2O2 production by P450-free microsomal preparations from genetically engineered insect cells. Open bars, no protein additions, NADPH only; hatched bars, microsomal proteins (5.0 μg protein) from negative control insect cells (note that these preparations have very low cytochrome c reductase activity, 42 and 29 nmol cytochrome c reduced/min/mg of protein, this is at least 20-fold less than the cytochrome c reductase activity in all other preparations used in these studies), gray bars, recombinant human CPR (40 units, 4.4 μg protein). H2O2 production was measured at zero time and after 5 and 10 min. The panel also shows the effects of 10μM DPI on the H2O2 generating activity of human CPR after 10 min. (Panel C) Lack of effect of cytochrome b5 on H2O2 generation by CPRs. Open bars, “negative control” preparations (5.0 μg protein) enriched with 10 pmol of purified human cytochrome b5; gray bars, 30 units of human CPR coexpressed with cytochrome b5; hatched bars, 20 units of purified rabbit CPR, black bars, 20 units of purified rabbit CPR enriched with 10 pmol of purified human cytochrome b5. (Panel D) Comparison of the H2O2 generating activity of CPR (Hm OR + b5) and SUPERMIX, a microsomal preparation containing CPR coexpressed with a numbers of cytochrome P450 enzymes. Microsomal proteins in both experiments contained 20 units CPR activity.
We next examined the H2O2 generating activity of a mixture of recombinant human cytochrome P450s coexpressed with CPR. In the presence of NADPH, SUPERMIX, a preparation containing recombinant CPR and various recombinant cytochrome P450 enzymes (CYP1A2, CYP2C8, CYP2C9, CYP2D6, and CYP3A4) was found to generate 4–5-fold greater amounts of H2O2 when compared with CPR alone (Fig. 2, panel D). The H2O2 generating activity of SUPERMIX and CPR was inhibited by DPI (10μM), and catalase (500 units/well), which inhibits accumulation of H2O2 by >90% (not shown). From these experiments we conclude that, although low levels of H2O2 are generated by CPR alone, cytochrome P450 enzymes coexpressed in combination with CPR in microsomal preparations are the main source of H2O2.
Individual recombinant cytochrome P450s coexpressed with CPR were also found to generate increased levels of H2O2 when compared with CPR alone (Table 1). For each of these enzymes, the rate of H2O2 production (expressed as nmol of H2O2/min/nmol of cytochrome P450) was significantly higher (10–140-fold) than the activity of recombinant CPR alone (in nmol of H2O2/min/100 units of CPR). The greatest activity was evident with CYP1A1, CYP2D6, CYP3A4, and CYP2E1 enzymes, whereas CYP2C subfamily enzymes had the lowest activity. An important variable in the expressed recombinant P450s was that the amounts of enzyme in each preparation relative to CPR were distinct. Thus, the molar ratio of cytochrome P450 enzymes to CPR varies from 0.1 to slightly higher than 6.0 nmol of individual CYPs/nmol of CPR. We next determined if differences in the ratio of individual cytochrome P450s to CPR can affect the rate of H2O2 production. For these studies, we analyzed preparations of CYP2E1 which have different CYP2E1/CPR ratios (0.27, 0.435, and 1.1, Table 2). In each of these preparations, CYP2E1 generates H2O2 in a time- and concentration-dependent manner (Fig. 3, panel A, and not shown). The reaction required NADPH; catalase (500 units/well) and DPI (10μM) inhibited accumulation of H2O2 in the assay (Fig. 3, panel B). The absolute rates of H2O2 production for these enzymes were found to be 5.24 ± 0.22, 5.16 ± 0.28, and 5.26 ± 0.31 nmol/min/nmol CYP2E1 for the preparations with CYP2E1/OR ratios of 0.27, 0.52, and 1.1, respectively (Table 2). Microsomal preparations containing recombinant CYP2E1 are only available coexpressed with cytochrome b5. Supplementing these preparations with additional cytochrome b5 caused small but reproducible decreases of H2O2 generating activity (Fig. 3, panel B, and not shown). These data demonstrate that the H2O2 generating activity of the different CYP2E1 preparations is similar despite varying ratios of CYP2E1/CPR.
TABLE 1. Rates of H2O2 Generation by Recombinant Human P450 Enzymes (nmol of H2O2/min/nmol P450)a.
| CYP enzyme | Lot no. | Expressed without cytochrome b5 | Expressed with cytochrome b5 | With added cytochrome b5b | P450:OR ratioc |
|---|---|---|---|---|---|
| CYP1A2 | 80,669 | 2.37 ± 0.19 | 1.89 ± 0.18 | 0.25 | |
| CYP1B1 | 26,314 | 0.49 ± 0.10 | 0.42 ± 0.08 | 3.0 | |
| CYP2A6 | 73,028 | 1.02 ± 0.14 | 0.82 ± 0.11 | 1.6 | |
| CYP2C8 | 41,730 | 0.41 ± 0.10 | 0.43 ± 0.0 | 4.42 | |
| CYP2C8 | 83,493 | 0.39 ± 0.10 | 4.5 | ||
| CYP2C9 | 19,924 | 0.22 ± 0.10 | 6.22 | ||
| CYP2C18 | 79,548 | 0.62 ± 0.11 | 0.60 ± 0.12 | 1.1 | |
| CYP2C19 | 10,141 | 0.83 ± 0.12 | 1.2 | ||
| CYP2D6 | 73,755 | 5.35 ± 0.88 | 4.36 ± 0.43 | 0.18 | |
| CYP2E1 | 29,047 | 5.26 ± 0.31 | 1.1 | ||
| CYP2J2 | 14,390 | 0.54 ± 0.07 | 2.6 | ||
| CYP3A4 | 32,017 | 5.77 ± 0.15 | 5.06 ± 0.14 | 4.14 | |
| CYP3A5 | 34,084 | 2.43 ± 0.21 | 0.15 | ||
| CYP3A7 | 71,915 | 1.46 ± 0.17 | Not done | 0.6 | |
| CYP4A11 | 69,060 | 3.50 ± 0.21 | 2.97 ± 0.19 | 0.22 | |
| CYP4F2 | 81,290 | 0.49 ± 0.14 | 2.0 |
aH2O2 generation by the recombinant P450 enzymes was determined using the AR/HRP assay as described in the Materials and Methods section.
The amounts of recombinant microsomal enzymes were within 2.0–10.0 pmol of cytochrome P450 enzyme/assay.
bFor every pmol of cytochrome P450/assay, 5 pmol of pure cytochrome b5 was added as described in the Materials and Methods section.
cRatio P450:OR was calculated as described in the Materials and Methods section.
TABLE 2. Rates of H2O2 Generation by Recombinant Human P450 Enzymes (nmol of H2O2/min/nmol P450)a.
| CYP enzyme | Lot no. | Expressed without cytochrome b5 | Expressed with cytochrome b5 | With cytochrome b5 addedb | P450:OR ratioc |
|---|---|---|---|---|---|
| CYP2C9 | 29,127 | 0.21 | 1.91 | ||
| CYP2C9 | 77,477 | 0.26 | 2.45 | ||
| CYP2C9 | 19,924 | 0.22 ± 0.10 | 6.22 | ||
| CYP2E1 | 87,639 | 5.24 ± 0.22 | 0.27 | ||
| CYP2E1 | 44,748 | 5.16 ± 0.28 | 0.52 | ||
| CYP2E1 | 29,047 | 5.26 ± 0.31 | 1.1 | ||
| CYP3A4 | 27,001 | 4.92 ± 0.21 | 0.2 | ||
| CYP3A4 | 10,738 | 5.29 ± 0.31 | 4.66 ± 0.28 | 2.53 | |
| CYP3A4 | 32,017 | 5.77 ± 0.15 | 5.06 ± 0.14 | 4.14 |
aH2O2 generation by the recombinant P450 enzymes was determined using the AR/HRP assay as described in the Materials and Methods section.
bFor every pmol of cytochrome P450/assay, 5 pmol of pure cytochrome b5 was added as described in the Materials and Methods section.
cThe ratio P450:OR was calculated as described in the Materials and Methods section.
FIG. 3.
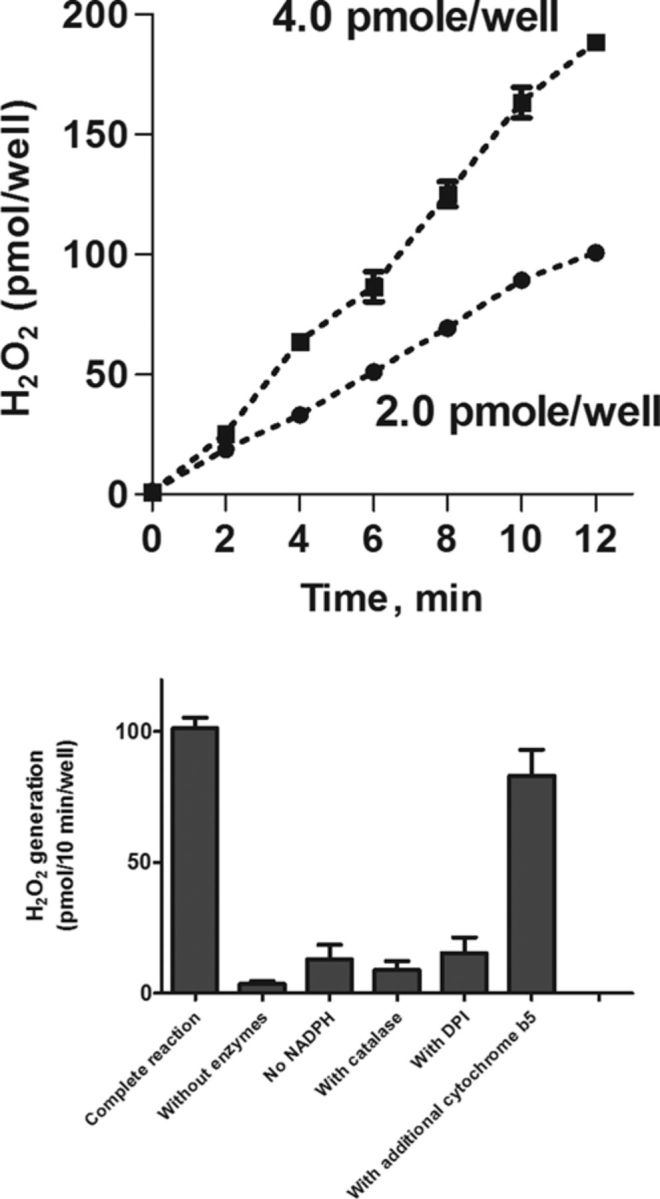
H2O2 generation by human recombinant CYP2E1 and effects of exogenous cytochrome b5. Upper panel: Protein- and time-dependent generation of H2O2 by human recombinant CYP2E1. Note that the reactions were linear for at least 10 min. Lower panel: The effects of inhibitors on the generation of H2O2 by human recombinant CYP2E1. Reactions contained 2.0 pmol of CYP2E1. All reactions were run for 6 min before analysis of H2O2 in reaction mixes. Note that the addition of purified human cytochrome b5 (at a ratio of cytochrome b5 to CYP2E1 of 5:1) had small effect on H2O2 generation activity. In all cases, the values are the mean ± SD of three independent experiments with samples analyzed in duplicate.
Similar results were found with CYP3A4. Thus, microsomal preparations with recombinant CYP3A4 have ratios of CYP3A4 to CPR of 4.14 and 2.53 and 0.21; the absolute rates of H2O2 generating activity (expressed as nmol of H2O2/min/nm of CYP3A4) for these preparations were similar (Table 2). It is important to note that preparations with high CYP3A4/OR ratios are not coexpressed with cytochrome b5. Enrichment of these CYP3A4 preparations with cytochrome b5 also caused a small decrease in their H2O2 generating activity (Table 1).
Recombinant CYP2C9 containing preparations also have different ratios of CYP2C9/CPR which ranged from 1.91 to 6.22; H2O2 generating activity in these preparations, which did not vary significantly, was much lower than preparations containing CYP2E1 and CYP3A4 (Table 2). As with CYP2C9 and CYP2E1, slightly lower H2O2 generating activity was found with CYP3A4 either coexpressed with cytochrome b5 or with added cytochrome b5 (Table 2). In general, the addition of cytochrome b5 to all other cytochrome P450 preparations also caused a small but reproducible decrease in H2O2 generating activity (Table 1). Taken together, these data indicate that the H2O2 generating activity of the cytochrome P450s is independent of the CPR content, at least at ratios where CYP is equal to or in excess (1–6) or when CPR is equal to or in excess (0.1–1). Moreover, a small decrease in the H2O2 generating activity was evident in the presence of cytochrome b5.
DISCUSSION
Earlier studies have shown that, in the absence of cytochrome P450s, purified microsomal CPR can generate superoxide anion (Grover and Piette, 1981; Kameda et al., 1979; Mishin et al., 1976; Morehouse et al., 1984) which can dismutate into H2O2 (Cho et al., 1982; Kuthan et al., 1978; Winston and Cederbaum, 1983). In the present studies, we report that H2O2 can be generated by recombinant human and purified rabbit CPRs; both enzymes possess low but detectable activities (∼0.04 nmol of H2O2/min/100 units of enzyme). CPR is typically found in human liver microsomes at levels ranging from 100 to 200 units/mg of microsomal protein, or ∼0.02 nmol/mg microsomal protein (Pearce et al., 1996). Therefore, hypothetically, this enzyme, in the absence of natural acceptors such as cytochrome P450 enzymes or heme oxygenase (the conditions favored for the auto-oxidation of flavoproteins), is able to generate ≤0.1 nmol of H2O2/min/mg of microsomal protein. Our studies also show that cytochrome b5, another member of the microsomal electron transport chain, does not generate H2O2, and does not alter the formation of H2O2 by either purified rabbit or recombinant human CPR. These data indicate that H2O2 generation in the absence of cytochrome P450s is potentially due to the slow auto-oxidation of CPR via the formation superoxide anion radicals.
When CPR is expressed together with individual cytochrome P450 enzymes or a mixture of these enzymes (Supermix), these latter hemoproteins predominate in oxygen activation and, in the absence of metabolizing substrates, become major generators of H2O2. Of particular interest is our finding that preparations of individual human recombinant cytochrome P450 enzymes, coexpressed with CPR, generate H2O2 at different rates. Thus, CYP1A1-containing preparations were the most active in generating H2O2 followed by CYP2D6, CYP3A4, CYP2E1, CYP4A11, CYP1A2, and the CYP2C subfamily. Among the cytochrome P450 enzymes analyzed in these studies, CYP1A1 is expressed in the liver at very low levels (Drahushuk et al., 1998), whereas CYP3A4 and CYP2C subfamily enzymes represent up to 60% of total cytochrome P450 in human liver microsomes (Donato and Castell, 2003; Pearce et al., 1996; Yamazaki et al., 1997). CYP1A2, CYP2E1, and CYP4A11 each represent ∼5–7%, and CYP2D6, 2–4% of the total liver microsomal cytochrome P450 content (Donato and Castell, 2003; Pearce et al., 1996; Yamazaki et al., 1997). Because CYP3A4/3A5/3A7 enzymes are the most abundant, it is likely that these enzymes are major generators of H2O2 in human liver microsomes. In earlier studies, it was suggested that CYP2E1 is the main source of reactive oxygen species generated by liver microsomes (Albano, 2006; Persson et al., 1990). However, this seems doubtful as this enzyme was not the most active of the recombinant enzymes tested for H2O2 production and the content of CYP2E1 in human liver represents only 5–10% of the total liver microsomal cytochrome P450 content (Donato and Castell, 2003; Pearce et al., 1996; Yamazaki et al., 1997). This is the case even when CYP2E1 is induced by ethanol or other specific xenobiotics; under these conditions, CYP2E1 does not exceed 15% of the total liver microsomal cytochrome P450 content (Albano, 2006; Mishin et al., 1998). This is further supported by earlier studies showing that isoniazide-induced microsomes, which contained increased level of CYP2E1, do not display elevated levels of H2O2 production (Dostalek et al., 2007, 2008). It should be noted that individual levels of the CYPs can vary widely in cells and tissues, and their expression depends on many factors including age, sex, and exposure to drugs and/or environmental conditions (Parkinson et al., 2004). These variables will likely affect the contribution of each cytochrome P450 enzyme to the total H2O2 production by microsomes.
The present studies measured H2O2 generation of various cytochrome P450 enzymes in the absence of metabolizing substrates. Thus, substrate binding in the active centers of the cytochrome P450s is not required for the oxygen activation cycle leading to the formation of H2O2. These data are consistent with studies by Vatsis et al. (2002) showing that a double mutant A298E/C436H CYP2B4 devoid of monooxygenase activity still retains its capacity to generate H2O2. The mechanism by which cytochrome P450s generate H2O2 is thought to be the result of the decay of an aberrantly protonated hydroperoxy-FeIII intermediate (Kumar et al., 2011; Porro et al., 2009). It has been suggested that distinct polar and/or acidic amino acids in the cytochrome P450 active center play a major role in preventing the decay of this hydroperoxy-FeIII intermediate (Kumar et al., 2011). This indicates that solvation of the heme pocket in the active center of the enzyme can control the rate of H2O2 formation by the different cytochrome P450 enzymes. Our data also shows that in preparations containing both CPR and various cytochrome P450s, cytochrome b5 caused a small decrease in H2O2 formation. We speculate that cytochrome b5 can alter the supply of the second electron to cytochrome P450 and this affects the rate of decay of the cytochrome P450 hydroperoxy-FeIII intermediate. Further studies are needed to explore this possibility as well as other mechanisms leading to reduced H2O2 production in the presence of cytochrome b5.
It should be noted that cytochrome P450s are present in large molar excess over CPR in native liver endoplasmic reticulum membranes (Donato and Castell, 2003; Pearce et al., 1996; Yamazaki et al., 1997). In native human liver microsomes, the typical ratio of total cytochrome P450 to CPR has been estimated to be between 5 and 7 (Nakajima et al., 2002; Venkatakrishnan et al., 2002). In most, but not all preparations containing recombinant microsomal enzymes, the molar ratio of CYP/CPR was shifted significantly to an excess of CPR. However, our data indicate that the various ratios of CYP/CPR have no control on the rate of H2O2 generation. Thus, relatively high rates of H2O2 production were demonstrated for CYP3A4 and CYP2E1 in preparations differing dramatically in their CYP/CPR ratio. Similar results were found for several CYP2C9-containing preparations varying in CPR content, although rates of H2O2 production were very low. Another notable example is the H2O2 production by CYP1A1- and CYP3A4-containing enzyme preparations. The H2O2 generating activity of these two enzymes was nearly identical despite marked differences in ratio of CYP/CPR. Taken together, these data indicate that the H2O2 generating activity is an inherent property of individual cytochrome P450 enzymes. Most likely, even relatively small amounts of CPR, in some recombinant preparations with high ratios of CYP/CPR and in native human liver microsomes, are sufficient to support high rates of H2O2 generation.
In the classical scheme for reduction of cytochrome P450s, substrate binding facilitates the electron flow to the hemoprotein from CPR which transfers the electrons sequentially in two distinct steps (Gutierrez et al., 2001). When no cytochrome P450 substrate is present in the active center of cytochrome P450, it is likely that the rate of decay of the hydroperoxy-FeIII intermediate of the cytochrome is mainly controlled by the supply of the second electron and, as indicated above, also depends on the unknown but specific factors typical for heme pocket structure of individual cytochrome P450 enzymes (Denisov et al., 2005; Hamdane et al., 2008; Makris et al., 2002). Further studies are needed to determine the role of the microenvironment of the heme pocket of the CYPs in the control of H2O2 production in native microsomes.
In summary, our data show that microsomal preparations containing individual human recombinant cytochrome P450 enzymes generate H2O2 in the absence of metabolizing substrates. The rate of H2O2 generation varies with individual cytochrome P450 enzymes and appears to depend on the intrinsic properties of each enzyme. Because CYP3A4/5/7 subfamily enzymes represent up to 40% of total cytochrome P450 in human liver microsomes, these enzymes likely represent a major source of H2O2 in vivo.
FUNDING
National Institutes of Health [AR055073, ES004738, CA132624, NS079249, ES005022]; National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases award [U54AR055073]. Funding for open access charge: National Institutes of Health.
Disclaimer: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
REFERENCES
- Albano E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- Bernhardt R. Cytochrome P450: Structure, function, and generation of reactive oxygen species. Rev. Physiol. Biochem. Pharmacol. 1996;127:137–221. doi: 10.1007/BFb0048267. [DOI] [PubMed] [Google Scholar]
- Bhabak K.P., Mugesh G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- Bondy S.C., Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem. Pharmacol. 1994;48:155–159. doi: 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M.D., Thompson S., Weaver R.J., Wolf C.R., Mayer R.T. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Cho A.K., Maynard M.S., Matsumoto R.M., Lindeke B., Paulsen U., Miwa G.T. The opposing effects of N-hydroxyamphetamine and N-hydroxyphentermine on the H2O2 generated by hepatic cytochrome P-450. Mol. Pharmacol. 1982;22:465–470. [PubMed] [Google Scholar]
- Cooper H.L., Groves J.T. Molecular probes of the mechanism of cytochrome P450. Oxygen traps a substrate radical intermediate. Arch. Biochem. Biophys. 2011;507:111–118. doi: 10.1016/j.abb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi C.L., Miller V.P., Stresser D.M. Design and application of fluorometric assays for human cytochrome P450 inhibition. Methods Enzymol. 2002;357:276–284. doi: 10.1016/s0076-6879(02)57685-0. [DOI] [PubMed] [Google Scholar]
- Denisov I.G., Makris T.M., Sligar S.G., Schlichting I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- Donato M.T., Castell J.V. Strategies and molecular probes to investigate the role of cytochrome P450 in drug metabolism: Focus on in vitro studies. Clin. Pharmacokinet. 2003;42:153–178. doi: 10.2165/00003088-200342020-00004. [DOI] [PubMed] [Google Scholar]
- Dostalek M., Brooks J.D., Hardy K.D., Milne G.L., Moore M.M., Sharma S., Morrow J.D., Guengerich F.P. In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol. Pharmacol. 2007;72:1419–1424. doi: 10.1124/mol.107.040238. [DOI] [PubMed] [Google Scholar]
- Dostalek M., Hardy K.D., Milne G.L., Morrow J.D., Chen C., Gonzalez F.J., Gu J., Ding X., Johnson D.A., Johnson J.A., et al. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J. Biol. Chem. 2008;283:17,147–17,157. doi: 10.1074/jbc.M802447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahushuk A.T., McGarrigle B.P., Larsen K.E., Stegeman J.J., Olson J.R. Detection of CYP1A1 protein in human liver and induction by TCDD in precision-cut liver slices incubated in dynamic organ culture. Carcinogenesis. 1998;19:1361–1368. doi: 10.1093/carcin/19.8.1361. [DOI] [PubMed] [Google Scholar]
- Estabrook R.W., Kawano S., Werringloer J., Kuthan H., Tsuji H., Graf H., Ullrich V. Oxycytochrome P-450: Its breakdown to superoxide for the formation of hydrogen peroxide. Acta Biol. Med. Ger. 1979;38:423–434. [PubMed] [Google Scholar]
- Gillette J.R., Brodie B.B., La Du B.N. The oxidation of drugs by liver microsomes: On the role of TPNH and oxygen. J. Pharmacol. Exp. Ther. 1957;119:532–540. [PubMed] [Google Scholar]
- Gorsky L.D., Koop D.R., Coon M.J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J. Biol. Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- Goswami T., Rolfs A., Hediger M.A. Iron transport: Emerging roles in health and disease. Biochem. Cell Biol. 2002;80:679–689. doi: 10.1139/o02-159. [DOI] [PubMed] [Google Scholar]
- Grover T.A., Piette L.H. Influence of flavin addition and removal on the formation of superoxide by NADPH-Cytochrome P-450 reductase: A spin-trap study. Arch. Biochem. Biophys. 1981;212:105–114. doi: 10.1016/0003-9861(81)90348-9. [DOI] [PubMed] [Google Scholar]
- Guengerich F.P., Johnson W.W. Kinetics of ferric cytochrome P450 reduction by NADPH-cytochrome P450 reductase: Rapid reduction in the absence of substrate and variations among cytochrome P450 systems. Biochemistry. 1997;36:14,741–14,750. doi: 10.1021/bi9719399. [DOI] [PubMed] [Google Scholar]
- Guengerich F.P., Martin M.V., Sohl C.D., Cheng Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 2009;4:1245–1251. doi: 10.1038/nprot.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Grunau A., Paine M., Munro A.W., Wolf C.R., Roberts G.C., Scrutton N.S. Electron transfer in human cytochrome P450 reductase. Biochem. Soc. Trans. 2003;31(Pt 3):497–501. doi: 10.1042/bst0310497. [DOI] [PubMed] [Google Scholar]
- Gutierrez A., Lian L.Y., Wolf C.R., Scrutton N.S., Roberts G.C. Stopped-flow kinetic studies of flavin reduction in human cytochrome P450 reductase and its component domains. Biochemistry. 2001;40:1964–1975. doi: 10.1021/bi001719m. [DOI] [PubMed] [Google Scholar]
- Hamdane D., Zhang H., Hollenberg P. Oxygen activation by cytochrome P450 monooxygenase. Photosynth. Res. 2008;98:657–666. doi: 10.1007/s11120-008-9322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kameda K., Ono T., Imai Y. Participation of superoxide, hydrogen peroxide and hydroxyl radicals in NADPH-cytochrome P-450 reductase-catalyzed peroxidation of methyl linolenate. Biochim. Biophys. Acta. 1979;572:77–82. [PubMed] [Google Scholar]
- Kirkman H.N., Gaetani G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Kumar D., Altun A., Shaik S., Thiel W. Water as biocatalyst in cytochrome P450. Faraday Discuss. 2011;148:373–383. doi: 10.1039/c004950f. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Tsuji H., Graf H., Ullrich V. Generation of superoxide anion as a source of hydrogen peroxide in a reconstituted monooxygenase system. FEBS Lett. 1978;91:343–345. doi: 10.1016/0014-5793(78)81206-x. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur. J. Biochem. 1982;126:583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- Lubet R.A., Syi J.L., Nelson J.O., Nims R.W. Induction of hepatic cytochrome P-450 mediated alkoxyresorufin O-dealkylase activities in different species by prototype P-450 inducers. Chem. Biol. Interact. 1990;75:325–39. doi: 10.1016/0009-2797(90)90075-x. [DOI] [PubMed] [Google Scholar]
- Makris T.M., Davydov R., Denisov I.G., Hoffman B.M., Sligar S.G. Mechanistic enzymology of oxygen activation by the cytochromes P450. Drug Metab. Rev. 2002;34:691–708. doi: 10.1081/dmr-120015691. [DOI] [PubMed] [Google Scholar]
- Mishin V., Gray J.P., Heck D.E., Laskin D.L., Laskin J.D. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic. Biol. Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin V., Pokrovsky A., Lyakhovich V.V. Interactions of some acceptors with superoxide anion radicals formed by the NADPH-specific flavoprotein in rat liver microsomal fractions. Biochem. J. 1976;154:307–310. doi: 10.1042/bj1540307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin V.M., Rosman A.S., Basu P., Kessova I., Oneta C.M., Lieber C.S. Chlorzoxazone pharmacokinetics as a marker of hepatic cytochrome P4502E1 in humans. Am. J. Gastroenterol. 1998;93:2154–2161. doi: 10.1111/j.1572-0241.1998.00612.x. [DOI] [PubMed] [Google Scholar]
- Mohanty J.G., Jaffe J.S., Schulman E.S., Raible D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- Morehouse L.A., Thomas C.E., Aust S.D. Superoxide generation by NADPH-cytochrome P-450 reductase: The effect of iron chelators and the role of superoxide in microsomal lipid peroxidation. Arch. Biochem. Biophys. 1984;232:366–377. doi: 10.1016/0003-9861(84)90552-6. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Tane K., Nakamura S., Shimada N., Yamazaki H., Yokoi T. Evaluation of approach to predict the contribution of multiple cytochrome P450s in drug metabolism using relative activity factor: effects of the differences in expression levels of NADPH-cytochrome P450 reductase and cytochrome b(5) in the expression system and the differences in the marker activities. J. Pharm. Sci. 2002;91:952–963. doi: 10.1002/jps.10091. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Mudra D.R., Johnson C., Dwyer A., Carroll K.M. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol. Appl. Pharmacol. 2004;199:193–209. doi: 10.1016/j.taap.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Patten C.J., Koch P. Baculovirus expression of human P450 2E1 and cytochrome b5: Spectral and catalytic properties and effect of b5 on the stoichiometry of P450 2E1-catalyzed reactions. Arch. Biochem. Biophys. 1995;317:504–513. doi: 10.1006/abbi.1995.1194. [DOI] [PubMed] [Google Scholar]
- Pearce R.E., McIntyre C.J., Madan A., Sanzgiri U., Draper A.J., Bullock P.L., Cook D.C., Burton L.A., Latham J., Nevins C., et al. Effects of freezing, thawing, and storing human liver microsomes on cytochrome P450 activity. Arch. Biochem. Biophys. 1996;331:145–169. doi: 10.1006/abbi.1996.0294. [DOI] [PubMed] [Google Scholar]
- Perret A., Pompon D. Electron shuttle between membrane-bound cytochrome P450 3A4 and b(5) rules uncoupling mechanisms. Biochemistry. 1998;37:11412–11424. doi: 10.1021/bi980908q. [DOI] [PubMed] [Google Scholar]
- Persson J.O., Terelius Y., Ingelman-Sundberg M. Cytochrome P-450-dependent formation of reactive oxygen radicals: Isozyme-specific inhibition of P-450-mediated reduction of oxygen and carbon tetrachloride. Xenobiotica. 1990;20:887–900. doi: 10.3109/00498259009046904. [DOI] [PubMed] [Google Scholar]
- Porro C.S., Kumar D., de Visser S.P. Electronic properties of pentacoordinated heme complexes in cytochrome P450 enzymes: Search for an Fe(I) oxidation state. Phys. Chem. Chem. Phys. 2009;11:10,219–10,226. doi: 10.1039/b911966c. [DOI] [PubMed] [Google Scholar]
- Puntarulo S., Cederbaum A.L. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450. Free Radic. Biol. Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Schlezinger J.J., White R.D., Stegeman J.J. Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by 3,3′,4,4′-tetrachlorobiphenyl: Production of reactive oxygen by vertebrate CYP1As. Mol. Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- Towne V., Will M., Oswald B., Zhao Q.J. Complexities in horseradish peroxidase-catalyzed oxidation of dihydroxyphenoxazine derivatives: Appropriate ranges for pH values and hydrogen peroxide concentrations in quantitative analysis. Anal. Biochem. 2004;334:290–296. doi: 10.1016/j.ab.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Vatsis K.P., Peng H.-M., Coon M.J. Replacement of active-site cysteine-436 by serine converts cytpochrome P450 2B4 into an NADPH oxidase with negligible monooxygenase activity. J. Inorg. Biochem. 2002;91:542–553. doi: 10.1016/s0162-0134(02)00438-5. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K., von Moltke L.L., Greenblatt D.J. Evaluation of SupermixTM as an in vitro model of human liver microsomal drug metabolism. Biopharm. Drug Dispos. 2002;23:183–190. doi: 10.1002/bdd.307. [DOI] [PubMed] [Google Scholar]
- Winston G.W., Cederbaum A.I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. II. Role in microsomal oxidation of ethanol. J. Biol. Chem. 1983;258:1514–1519. [PubMed] [Google Scholar]
- Yamazaki H., Inoue K., Turvy C.G., Guengerich F.P., Shimada T. Effects of freezing, thawing, and storage of human liver samples on the microsomal contents and activities of cytochrome P450 enzymes. Drug Metab. Dispos. 1997;25:168–174. [PubMed] [Google Scholar]
- Zangar R.C., Davydov D.R., Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]