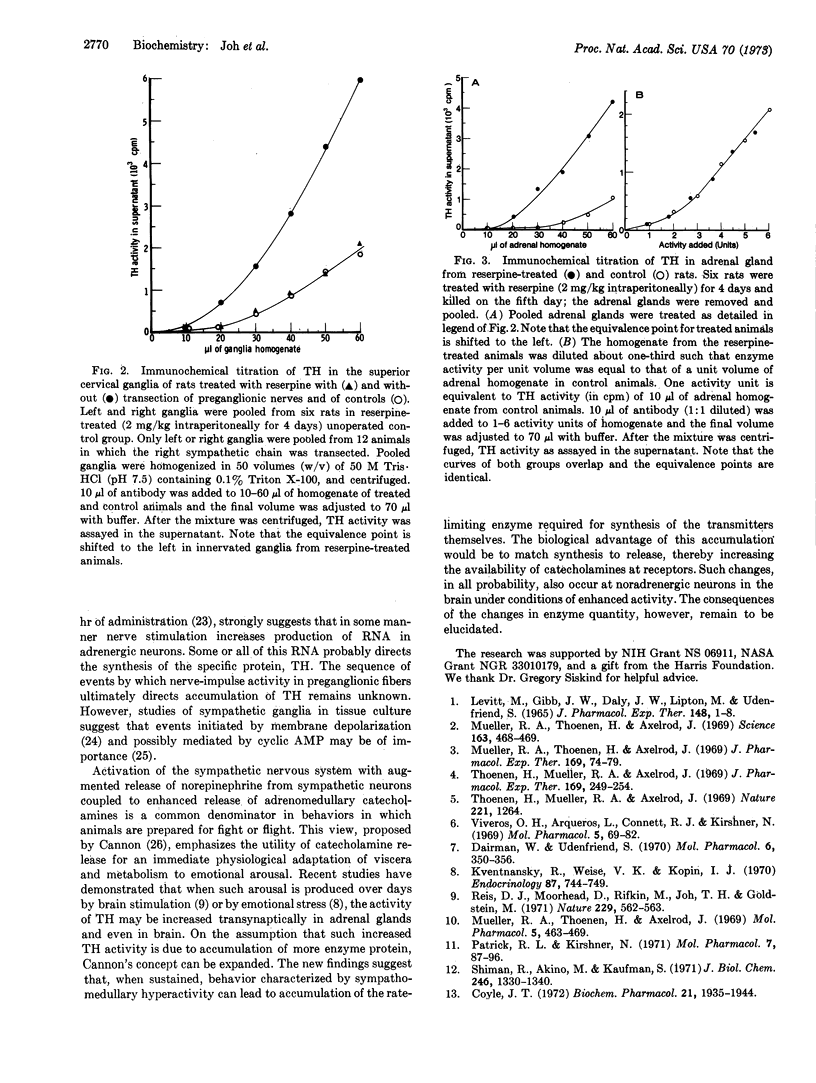
Abstract
Chronic administration of reserpine to rats increases, in sympathetic ganglia and adrenal medulla, the activity of tyrosine hydroxylase (EC 1.14.3.x), the enzyme catalyzing the rate-limiting step in the biosynthesis of catecholamines. Immunochemical titration of the enzyme in both adrenal gland and innervated superior cervical ganglia demonstrates that enhanced enzyme activity is entirely attributable to accumulation of more specific enzyme protein and not activation of preexistent enzyme molecules.
Keywords: rats, superior cervical ganglion
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dairman W., Udenfriend S. Increased conversion of tyrosine to catecholamines in the intact rat following elevation of tissue tyrosine hydroxylase levels by administered phenoxybenzamine. Mol Pharmacol. 1970 Jul;6(4):350–356. [PubMed] [Google Scholar]
- DeQuattro V., Nagatsu T., Maronde R., Alexander N. Catecholamine synthesis in rabbits with neurogenic hypertension. Circ Res. 1969 Apr;24(4):545–555. doi: 10.1161/01.res.24.4.545. [DOI] [PubMed] [Google Scholar]
- Jarlstedt J., Dahlström A. Changes in RNA-content of sympathetic ganglion cells of reserpine-pretreated rats. Neuropharmacology. 1972 May;11(3):447–450. doi: 10.1016/0028-3908(72)90030-5. [DOI] [PubMed] [Google Scholar]
- KRIEGER E. M. NEUROGENIC HYPERTENSION IN THE RAT. Circ Res. 1964 Dec;15:511–521. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R., Weise V. K., Kopin I. J. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology. 1970 Oct;87(4):744–749. doi: 10.1210/endo-87-4-744. [DOI] [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackay A. V., Iversen L. L. Increased tyrosine hydroxylase activity of sympathetic ganglia cultured in the presence of dibutyryl cyclic AMP. Brain Res. 1972 Dec 24;48:424–426. doi: 10.1016/0006-8993(72)90204-1. [DOI] [PubMed] [Google Scholar]
- Mackay A. V., Iversen L. L. Trans-synaptic regulation of tyrosine hydroxylase activity in adrenergic neurones: effect of potassium concentration on cultured sympathetic ganglia. Naunyn Schmiedebergs Arch Pharmacol. 1972;272(2):225–229. doi: 10.1007/BF00508770. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Adrenal tyrosine hydroxylase: compensatory increase in activity after chemical sympathectomy. Science. 1969 Jan 31;163(3866):468–469. doi: 10.1126/science.163.3866.468. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Inhibition of trans-synaptically increased tyrosine hydroxylase activity by cycloheximide and actinomycin D. Mol Pharmacol. 1969 Sep;5(5):463–469. [PubMed] [Google Scholar]
- Musacchio J. M., Wurzburger R. J., D'Angelo G. L. Different molecular forms of bovine adrenal tyrosine hydroxylase. Mol Pharmacol. 1971 Mar;7(2):136–146. [PubMed] [Google Scholar]
- Patrick R. L., Kirshner N. Effect of stimulation on the levels of tyrosine hydroxylase, dopamine beta-hydroxylase, and catecholamines in intact and denervated rat adrenal glands. Mol Pharmacol. 1971 Jan;7(1):87–96. [PubMed] [Google Scholar]
- Petrack B., Sheppy F., Fetzer V. Studies on tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1968 Feb 25;243(4):743–748. [PubMed] [Google Scholar]
- Reis D. J., Moorhead D. T., Rifkin M., Joh T. H., Goldstein M. Changes in adrenal enzymes synthesizing catecholamines in attack behaviour evoked by hypothalamic stimulation in the cat. Nature. 1971 Feb 19;229(5286):562–563. doi: 10.1038/229562b0. [DOI] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Increased tyrosine hydroxylase activity after drug-induced alteration of sympathetic transmission. Nature. 1969 Mar 29;221(5187):1264–1264. doi: 10.1038/2211264a0. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Connett R. J., Kirshner N. Mechanism of secretion from the adrenal medulla. IV. The fate of the storage vesicles following insulin and reserpine administration. Mol Pharmacol. 1969 Jan;5(1):69–82. [PubMed] [Google Scholar]



