Abstract
Ingestion of Bacillus anthracis spores causes gastrointestinal (GI) anthrax. Humoral immune responses, particularly immunoglobulin A (IgA)–secreting B-1 cells, play a critical role in the clearance of GI pathogens. Here, we investigated whether B. anthracis impacts the function of colonic B-1 cells to establish active infection. GI anthrax led to significant inhibition of immunoglobulins (eg, IgA) and increased expression of program death 1 on B-1 cells. Furthermore, infection also diminished type 2 innate lymphoid cells (ILC2) and their ability to enhance differentiation and immunoglobulin production by secreting interleukin 5 (IL-5). Such B-1–cell and ILC2 dysfunction is potentially due to cleavage of p38 and Erk1/2 mitogen-activated protein kinases in these cells. Conversely, mice that survived infection generated neutralizing antibodies via the formation of robust germinal center B cells in Peyer's patches and had restored B-1–cell and ILC2 function. These data may provide additional insight for designing efficacious vaccines and therapeutics against this deadly pathogen.
Keywords: anthrax toxin, Bacillus anthracis, B-1 cells, type 2 innate lymphoid cells
Anthrax is a zoonotic disease caused by the introduction of Bacillus anthracis endospores either by respiratory, oral, or cutaneous routes. With the gastrointestinal (GI) form of the disease, symptoms begin as nausea, vomiting, mild diarrhea, fever, and headaches, which soon progress into hemorrhagic diarrhea, hematemesis, ascites, and, eventually, septic shock and death. Virulent B. anthracis contains 2 plasmids (pXO1 and pXO2), for toxin production and capsule formation, respectively [1]. The pXO1 encodes protective antigen (PA), lethal factor (LF), and edema factor (EF); lethal factor cleaves mitogen-activated protein kinases (MAPKs) to subvert immune cells, while EF increases cellular levels of cyclic adenosine monophosphate (cAMP), causing edema [2]. PA binds to its receptors expressed on host cells and facilitates cellular entry of LF and EF. The nonphagocytic capsule that protects the bacteria from innate cells is encoded by pXO2 [3].
Both the respiratory and GI tracts are lined by mucosae; however, the presence of digestive enzymes and a greater microbial load differentiates these 2 locations. Commensal gut microbes and the immune system have coevolved over several million years [4]. One of the most common and effective responses of the mammalian host against bacteria is secretion of immunoglobulin A (IgA) at mucosal surfaces [5]. Gut mucosae secrete massive amounts of IgA, the lack of which causes dysbiosis [6]. Currently, B cells are grouped into 2 major classes, B-1 cells and B-2 cells. B-1 cells, which include CD5+ B-1a and CD5− B-1b subsets, differ from conventional B-2 cells in that they develop from fetal liver progenitors [7], represent the major B-cell subpopulation in the peritoneal and pleural cavities [8], and in steady state, produce germ line–encoded immunoglobulin M (IgM) and IgA to maintain commensals and resist common pathogens [9, 10]. B-1 cells are instrumental in producing antibodies without T-cell help, allowing for rapid antibody responses against microbial gut residents, including Bacillus species [11]. Several bacilli compose the gut microbiota [11]; therefore, IgA may play a critical role in controlling microbial infection, including GI B. anthracis infection. Hence, we hypothesized that B. anthracis interferes with B-1–cell function to establish active infection.
Herein, we report that toxins secreted by B. anthracis impair immunoglobulin secretion and surface receptor expression on B-1 cells. Additionally, type 2 innate lymphoid cells (ILC2) that support the local expansion of B-1 cells [12] are compromised. These data strongly indicate that survival from infection necessitates neutralizing antibodies by expansion of germinal center B cells in the Peyer's patches and uncompromised function of B-1 cells and ILC2 in the gut.
MATERIALS AND METHODS
Mice and Ethics Statement
A/J mice were obtained from Jackson Laboratory and bred in-house at the animal facility at the College of Veterinary Medicine, University of Florida. Mice were provided food and water ad libitum. Mice were used at 6–8 weeks of age in accordance with the Animal Welfare Act and the Public Health Policy on Humane Care. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida under protocol number 201 107 129.
B. anthracis Spore Preparation and Mouse Infections
Spores were prepared from a toxigenic nonencapsulated strain of B. anthracis (Sterne), as described previously [13]. To calculate final concentrations, serial dilutions (1:10) were grown in triplicate on lysogeny broth agar plates (Sterne), and colonies were counted. Mice were orally infected with Sterne spores (109 spores/100 µL of phosphate-buffered saline [PBS] per mouse).
Real-Time Polymerase Chain Reaction (PCR) Analysis
RNA isolated from colons was subjected to quantitative real-time PCR analysis, as described earlier [14]. A list of primers used and their sequences can be found in Supplementary Table 1.
Flow Cytometry and Antibodies
Colonic lamina propria (LP) cells were isolated as previously described [14], with minor modifications. Digestion buffer consisted of Dulbecco's modified Eagle's medium (DMEM; (Gibco, Life Technologies) containing 0.25 mg/mL collagenase type VII (Sigma-Aldrich), 0.125 U/mL Liberase TM Research Grade (Roche Applied Science, Indianapolis, IN), 10 mM HEPES, 0.1 M CaCl2 (Sigma-Aldrich), and 5% fetal bovine serum (FBS; 3 × 10-minute digestions). Flow cytometric analyses were performed using a BD LSRFortessa (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, Ashland, OR). A list of antibodies used can be found in Supplementary Table 2.
Ex Vivo Evaluation of MAPKs
Colonic cells were isolated from A/J mice as mentioned above, and live cells were evaluated. Cells were equilibrated in DMEM with 10% FBS for 30 minutes at 37°C and 5% CO2. The cells were incubated with 1 multiplicity of infection of B. anthracis Sterne spores or left untreated for 1, 3, and 6 hours. Treated and untreated cells were stained for B-1 and ILC2 cell markers along with MAPK phospho-specific fluorescently tagged antibodies and analyzed.
Sera Analyses
Immunoglobulins in the sera of Sterne-infected and uninfected A/J mice were measured, as described previously [14].
Lethal Toxin Neutralization Assay
Sera of Sterne-infected A/J mice were analyzed for the presence of anti-LT antibodies, as previously described [15].
Statistical Analyses
Unless stated otherwise, representative data indicate mean ± standard error of the mean. Significance was determined by 2-tailed unpaired t tests for 2-group comparisons (GraphPad Prism 5 for Mac OS X, La Jolla, CA).
RESULTS
Dysfunctional Innate B-Cell Responses in GI Anthrax
B. anthracis is a category A select agent and can only be handled in biosafety level 3 (BSL3) facilities. To avoid working in the BSL3 laboratory, we used the extensively studied A/J murine model, which is highly susceptible to infection with B. anthracis Sterne, a strain that has lost pXO2 [16]. Data showed that 80% of the mice (n = 10) infected with 109 colony-forming units died from oral infection (data not shown) 5 days after infection and exhibited symptoms seen with human GI anthrax.
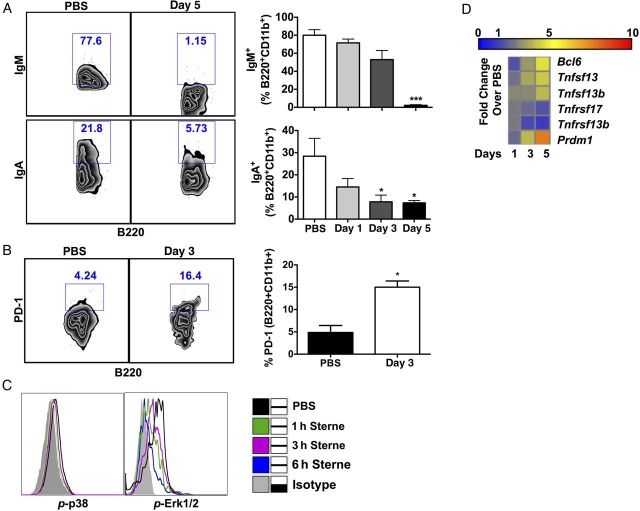
Gut-associated B-1 cells were defined by surface expression of CD45, B220, and CD11b (Supplementary Figure 1A). We observed a time-dependent decrease in IgM and IgA levels on B-1 cells (Figure 1A). Furthermore, we also found an increase of PD-1 on B-1 cells (Figure 1B), a molecule that is tightly regulated by MAPKs [17, 18] and inhibits B-cell receptor (BCR) signaling [19] to suppress immunoglobulin production, especially in B-1 cells [20]. Consequently, we investigated whether Sterne infection hampered the phosphorylation status of p38 and Erk1/2 in these cells. Data from in vivo and ex vivo experiments showed decreased phosphorylation of these MAPKs (Figure 1C and data not shown), suggesting a direct inhibition of B-1–cell function by LT secreted during infection.
Figure 1.
Gastrointestinal anthrax limits the function of B-1 cells. A/J mice were orally gavaged with 109 spores of the Sterne strain of Bacillus anthracis, and adaptive immune responses in the colon were analyzed at various time points. A, Frequency of immunoglobulin M (IgM)+ and immunoglobulin A (IgA)+ B cells in the colon. B, PD-1 expression on colonic B-1 cells. Left panel shows representative plots and compiled data from 3 independent experiments. C, Activity of mitogen-activated protein kinases in colonic B-1 cells was analyzed by flow cytometry. Gray tinted line, isotype control; black line, phosphate-buffered saline (PBS) group; green line, 1 hour; magenta line, 3 hours; blue line, 6 hours Sterne-infected colonic cells isolated from A/J mice. D, Gene expression profile of the distal colons from Sterne-infected A/J mice. Data represent observations from 4 independent experiments and are shown as mean ± standard error of the mean. *P < .05 and ***P < .001, compared with the PBS group.
Transcription of genes involved in B-cell activation and survival in the colon was analyzed; the data revealed conflicting outcomes in transcriptional activity (Figure 1D). For example, recognition of bacteria or bacterial products by epithelial cells and dendritic cells (DCs) typically leads to transcription and secretion of B-cell–activating factors, including a proliferation-inducing ligand (APRIL; also known as TNFSF13) and B cell–activating factor of the tumor necrosis factor family (BAFF; also known as TNFSF13b). These molecules then bind to their cognate receptors, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI; also known as TNFRSF13b) and B-cell maturation antigen (BCMA; also known as TNFRSF17), respectively, for B-cell activation, maturation, and survival [21]. While we observed increased April/Tnfsf13 and Baff/Tnfsf13b transcripts, Taci/Tnfrsf13b and Bcma/Tnfrsf17 expression were suppressed in the colons of infected mice (Figure 1D), suggesting that, despite potential survival signals provided by epithelial cells or DCs, B cells may not be capable of appropriately responding to their growth factors. Similarly, transcription of 2 opposing B-cell transcription factors, Bcl6 and B lymphocyte-induced maturation protein-1 (Blimp-1; also known as Prdm1), were enhanced during infection. The presence of Bcl6 leads to inhibition of Blimp1 transcription by its association with MAPK-dependent AP-1 activation [22]. The simultaneous presence of these 2 transcription factors may be due to the lack of active AP-1 in the infected cells via decreased MAPK activation.
Impact of GI B. anthracis Infection on ILC2 Function
ILC are an emerging family of cell types that play key roles in inflammation and tissue remodeling, particularly at barrier surfaces [23]. ILC2 are defined by the absence of lineage markers and the expression of the transcription factor GATA3 and of CD90 (Supplementary Figure 1B) [23], known to assist in B-1–cell survival and proliferation [24]. ILC2 also express higher levels of CD25, ICOS, IL-7Ra, IL33Ra, and c-kit [25], which were used to further characterize these cells (Supplementary Figure 1C). Accordingly, we found a sharp decrease in ILC2 within the LP of mice with GI anthrax 3 days after infection (Figure 2A). This steep drop in ILC2 in colonic tissue could adversely impact the maintenance of B-1 cells. ILC2 survival is maintained by several secreted factors such as thymic stromal lymphopoietin (TSLP), interleukin 7 (IL-7), IL-33, and IL-25 [26]. To examine whether the absence of these secreted factors causes depletion of ILC2, we evaluated the transcription of the aforementioned gene products in the colon. Surprisingly, with the exception of Tslp (Figure 2B), expression levels of all other growth factors were unchanged in the intestinal stroma. However, all of these growth factors except IL-7 require MAPKs to exert proliferative effects on target cells [27]. Thus, we evaluated phosphorylation of p38 and Erk1/2 MAPK in colonic cells treated with Sterne spores ex vivo. The phosphorylation status followed a similar pattern to that seen for B-1 cells; activation of these kinases was not maintained during infection and provides a potential cause for the absence of ILC2 (Figure 2C). These data suggest that reduced colonic ILC2 in infected mice may result from 2 important factors: the lack of TSLP and the inability of growth factors to signal in the absence of functional MAPKs. We also evaluated IL-5 and IL-13 production by the surviving ILC2 in the LP and found a significant decrease 3 days after infection (Figure 2D and 2E). These data indicate that diminished ILC2 during anthrax may be due to lack of survival signals; however, their impaired function is likely due to insufficient MAPK activation.
Figure 2.
Sterne infection reduces the gut-associated population of type 2 innate lymphoid cells (ILC2) and their secretion of interleukin 5 (IL-5). A/J mice were orally gavaged with 109 spores of the Sterne strain of Bacillus anthracis, and the ILC2 population was analyzed at indicated time points. A, Frequency of ILC2 in the colon. B, Transcriptional expression of Tslp in colonic tissues. C, Activity of mitogen-activated protein kinases in colonic ILC2 was analyzed by flow cytometry. Gray tinted line, isotype control; black line, phosphate-buffered saline (PBS) group; green line, 1 hour; magenta line, 3 hours; blue line, 6 hours Sterne-infected colonic cells isolated from A/J mice. D, Frequency of IL-5+ ILC2 in the colon. Left panel shows representative plots and compiled data from three independent experiments. E, Frequency of interleukin 13 (IL-13)+ ILC2 in the colon. Left panel shows representative plots and compiled data from 3 independent experiments. Data represent observations from 4 independent experiments and are shown as mean ± standard error of the mean. *P < .05 and **P < .01, compared with the PBS group.
Influence of B. anthracis Infection on B-1–Cell Recruitment
To investigate whether the presence of B-1 cells was also affected in the colonic LP, we evaluated total B-1–cell number, which showed no significant difference with infection (data not shown). However, an increase in the ratio of B-1a (B220+CD11b+CD5+) over B-1b (B220+CD11b+CD5−) cells was observed (Figure 3A). This change in proportion of B-1 cells was attributed to an increase in B-1a cells and a mild decrease in B-1b cells within the colon (Figure 3B). B-1 cells have the capacity to multiply at mucosal sites in the presence of certain growth factors (eg, IL-5, APRIL, and BAFF) and signals (eg, Toll-like receptor [TLR] ligands and BCR cross-linking) [28]. We saw an increase in certain growth factors (APRIL and BAFF) with a concurrent decrease in their receptors (BCMA and TACI, respectively; Figure 1D). However, the gut microbiota potentially provides constant stimuli to B-1 cells via various TLR and BCR ligands. To determine whether the increase in B-1a cells in the infected gut is due to increased proliferation, we compared levels of the proliferation marker Ki-67 in B-1a cells from infected mice and uninfected controls and found no difference (data not shown). This suggested an influx of B-1a cells into the LP; thus, we analyzed the transcriptional activation of important chemokines, such as CCL3, CXCL10, CXCL12, CXCL13, and CXCL14. A small to moderate increase in the transcription of potent chemoattractants for myeloid cells [29], CCL3, CXCL12, and CXCL14, was seen. Similarly, a small increase in the transcription of CXCL10, which is implicated in T-cell, natural killer (NK)–cell, and monocyte recruitment, was also noted. Importantly, transcription of CXCL13, a critical chemokine involved in B-1a–cell recruitment [30], increased >10-fold as compared to the uninfected controls, suggesting a greater influx of B-1a cells into the gut (Figure 3C). We also evaluated the expression of different gut-homing receptors along with the receptor for CXCL13 on the B-1a cells (α4β7, CCR6, CCR9, and CXCR5). Not surprisingly, the only difference in expression on the B-1a cells was with CXCR5, which is the receptor for CXCL13 (Figure 3D). These data suggest that GI anthrax results in increased CXCL13, which can recruit B-1a cells into the gut via its receptor, CXCR5.
Figure 3.
Gastrointestinal anthrax increases the recruitment of B-1a cells in the colonic tissues. A/J mice were orally gavaged with 109 spores of the Sterne strain of Bacillus anthracis, and the presence of B-1a and B-1b cells in the colon was analyzed at various time points. A, Frequency of B-1a and B-1b cells in the colon. Left panel shows representative plots and compiled data from 3 independent experiments. B, Frequency of B-1a and B-1b cells from the total live cell population in the colon. C, Gene expression profile of the distal colon from Sterne-infected A/J mice. D, Expression of indicated gut homing/chemokine receptors were evaluated on B-1a cells, using geometric mean fluorescent intensity (GMFI). Data represent observations from 4 independent experiments and are shown as mean ± standard error of the mean. *P < .05 and **P < .01, compared with the PBS group.
Germinal Center B-Cell Formation for Protective Antibody Response Against B. anthracis
Generation of protective antibodies against B. anthracis toxins and its bacterial capsule protects against bacterial challenge, suggesting a pivotal role for humoral B-cell responses [31]. Accordingly, it is well documented that neutralizing antibodies are exceptionally effective against B. anthracis; however, the exact immune mechanisms that lead to the generation of high-affinity antibodies in natural infection are unknown.
Colonic B-1 cells do not undergo somatic hypermutation required for the generation of high-affinity neutralizing antibodies against pathogenic challenge; conversely, conventional B-2 cells undergo somatic hypermutation with the help of activated T cells, which takes a relatively long time. Congruent with the acute nature of this disease, we did not observe increased germinal center (GC) B-cell formation in infected mice; however, in mice that survived infection (2 weeks after infection), GC B cells accumulated within the Peyer's patches. We evaluated various immunologic sites (mesenteric lymph nodes [MLNs], Peyer's patches, and spleen) for B-cell activation and for GC B-cell development. Of the sites tested, a significant induction of B-cell activation, as measured by downregulation of surface IgD, was observed in the Peyer's patches of survivor mice (Figure 4A). GC B cells, defined as peanut agglutinin (PNA)+ and Fas+, were also significantly enhanced in the Peyer's patches of these mice (Figure 4B). Consistent with the lack of an increase in GC B cells until 2 weeks after infection, sera from mice 5 days after infection failed to neutralize LT (Figure 4C), despite the availability of circulating antigen for the potential induction of antigen-specific responses (Figure 4C). We then addressed whether sera from mice that survived Sterne challenge for a minimum of 2 weeks would have neutralizing capacity against LT. Data clearly demonstrated that only sera from survivor mice significantly prevented cell death in the macrophage cell line J774A.1, compared with uninfected and moribund day 5–infected mice in a toxin neutralization assay (Figure 4C). We also tested the serum levels of circulating antibodies and had predicted a decrease or no change in their levels; surprisingly and inconsistent with GC B-cell formation, all infected mice showed a significant increase in IgG2b levels (Supplementary Figure 2A). These conflicting data could result from a lack of c-fos, which is a negative regulator of IgG2b production and is downstream of MAPK [32–34]. Accordingly, these data strongly indicate that intestinal B-cell activation and GC formation are essential responses against GI anthrax challenge when their function is not impaired.
Figure 4.
Germinal center B cells and protective antibody formation in surviving Sterne-infected A/J mice. A/J mice were orally gavaged with 109 spores of the Sterne strain of Bacillus anthracis, and after 2 weeks after infection indicated organs were analyzed for germinal reactions. A, Frequency of B220+ immunoglobulin D (IgD)+ cells in the mesenteric lymph nodes (MLNs), Peyer's patches (PPs), and spleen. Left panel shows representative plots and compiled data from 3 independent experiments. B, Frequency of Fas+ and peanut agglutinin (PNA)+ cells of activated B cells (B220+IgD+). C, Sera collected at days 5 and 14 after infection were analyzed for their neutralization capacity. In vitro lethal toxin neutralization assay was performed using sera derived from Sterne-infected or uninfected mice. Data shown as mean ± standard error of the mean. **P < .01 and ***P < .0001, compared with untreated J774A.1 cells. Data represent observations from 3 independent experiments and are shown as mean ± standard error of the mean. ***P < .001, compared with the phosphate-buffered saline (PBS) group. Abbreviations: LT, lethal toxin; St, Sterne-infected sera (day 5); survivor, Sterne-infected sera (day 14).
Furthermore, 2 weeks after infection, B-1 cells from mice that recovered from GI Sterne infection were not impaired in their expression of IgA or IgM, compared with B-1 cells derived from uninfected mice (Supplementary Figure 2B and 2C). Similarly, neither the presence of ILC2 nor their capacity to secrete IL-5 was compromised (Supplementary Figure 2D and 2E), suggesting the reestablishment of protective innate and B-cell immune responses against Sterne infection.
DISCUSSION
GI anthrax in humans and other mammals results from the ingestion of B. anthracis after consumption of tainted meat or via the handling of infected animals [35, 36]. To attain active infection, A/J mice were orally infected with 109 B. anthracis Sterne spores (approximately 2 times the median lethal dose), which resulted in the death of 70%–80% of the mice within 5 days. The mice that died from infection showed the typical signs of bacterial dissemination in various visceral organs as early as 1 day after infection. Mice that survived infection showed the generation of neutralizing antibodies.
Upon microbial infection, regulated induced inflammation may elicit protective immunity to overcome pathogen challenge. However, certain successful pathogens (eg, Yersinia, Shigella, and Salmonella organisms) [37, 38] escape immune clearance by inducing immune suppression [39] of innate cells through the inhibition of NF-κB and MAPK signaling. Factors secreted by B. anthracis lead to immune suppression [40]; LF is a Zn+2-dependent matrix metalloproteinase that cleaves MAPKs, while EF is an adenylate cyclase that generates cAMP to induce devastating immune dysfunction. The entry of these toxins (LT and EF) is dependent upon the interaction between another secreted protein, PA, and its receptors [41], which are ubiquitously expressed on immune cells, including DCs and T and B cells [42]. However, the effect of these toxins on GI immune cells has not been studied in detail. MAPK signaling is a central component in the survival and function of these cells, the inhibition of which would disturb immune homeostasis. DCs and macrophages are the most studied cells in regard to anthrax toxins, which, upon infection, are compromised in cytokine production and MAPK activation. Reduced MAPK activity in myeloid cells may have also contributed to the availability of growth factors such as TSLP, BAFF, and APRIL, which aid in the survival of B cells and ILC2 in the LP. Like myeloid cells, other cells are also impacted by infection, including the function of colonic B cells and ILC2.
Studies implicate B cells and CD11b+ cells in systemic bacterial dissemination [43, 44]; B-1 cells, which reside in the GI tract, express surface CD11b, making them an important immune cell subset to study during the course of GI infection. The major function of B-1 cells in the GI tract is to produce germ line–encoded immunoglobulins against commensal and commonly invading pathogens [9, 10]. Several bacilli strains are part of the gut commensal microbiota; therefore, antibody directed against these bacteria may also impact the replication of pathogenic B. anthracis. However, unlike other bacillus species, B. anthracis releases toxins to inhibit MAPK activity, an essential component of multiple critical signaling pathways [45]. Here, we show a direct impact on MAPK activation in B-1 cells and other downstream events. MAPKs control the expression of PD-1, and upon B. anthracis infection, MAPK inhibition led to PD-1 upregulation. PD-1 disrupts BCR signaling required for the activation of B cells [19, 20]. We also observed a significant decrease in IgA and IgM levels with infection, likely due to cleavage of MAPKs and subsequent increases in PD-1 expression. Additionally, a MAPK-dependent secreted factor from epithelial cells and DCs, Tslp, was also found to be significantly downregulated upon infection, suggesting an environment that is not conducive to B-1–cell growth. Despite an adverse condition for the proliferation of B-1 cells in the infected intestine, we observed an increase in the B-1a–cell population. This increase in B-1a cells may be due to an increase in the B-1–cell chemoattractant, CXCL13.
To investigate whether the altered B-1 population resulted from dysfunctional ILC2, we tested for their presence and found it to be reduced to 10% of that of uninfected mice. This reduction of ILC2 in the LP was associated with reduced MAPK activity, which is consistent with MAPK being the central signaling molecule for ILC2 survival and function. Several receptors for growth factors required for ILC2 survival, such as IL-33Ra, c-kit, and sca-1 [46], involve MAPK signaling, and its absence could potentially cause ablation of this population. Therefore, not only are the growth factors necessary for ILC2 and B-1 cell depletion, but their signaling receptors are also incapacitated by degraded MAPKs.
B. anthracis toxins affect multiple aspects of adaptive immunity [47]. While robust memory B-cell responses were reported in patients who survived cutaneous or inhalation anthrax [48], anthrax LT was found to inhibit B-cell function in mice receiving toxin injected intraperitoneally [49]. Humoral responses are pivotal to neutralizing infectious agents and their toxins, including B. anthracis [24, 50]. Expression of Bcl-6 and Blimp-1, along with expression of essential chemokines, such as Cxcl13, that regulate B-cell trafficking were significantly elevated in response to bacterial infection; however, 80% of the mice succumbed to infection within days, potentially shortening the interval to generate sufficient neutralizing antibodies by GC B cells against Sterne. Indeed, mice that survived infection exhibited a robust increase in GC B cells in the Peyer's patches, indicating the potential induction of antigen-dependent humoral responses.
In summary, toxins secreted by B. anthracis led to diminished MAPK activity in B-1 cells and ILC2. Upon infection, B-1 cells downregulated the expression of immunoglobulins and several surface receptors required for their survival, such as TACI and BCMA. At the same time, ILC2 that support proliferation of B-1 cells were reduced to 10% of their original population, suggesting that survival signals besides BAFF and APRIL were not available to B-1 cells, making the gut environment hostile for their survival. However, with infection, we saw a gradual increase in gut B-1a cells, which could be due to a sharp increase in chemoattractants such as CXCL13. Neutralizing antibodies against LT were seen in the sera of survivors 2 weeks after infection; however, they were absent in the early stages of the disease, when they could have affected survival. It is still unclear which factor(s) determine survival versus death of the animal. Further studies are required to understand these survival factors in a low-dose infection, factors that could include the composition of the gut microbiota, natural antibodies, and/or other innate immune cells.
Supplementary Data
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01 AI093370 to M. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mikesell P, Ivins BE, Ristroph JD, Dreier TM. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983;39:371–6. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonello F, Zornetta I. Bacillus anthracis factors for phagosomal escape. Toxins (Basel) 2012;4:536–53. doi: 10.3390/toxins4070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino S, Watarai M, Cheun HI, Shirahata T, Uchida I. Effect of the lower molecular capsule released from the cell surface of Bacillus anthracis on the pathogenesis of anthrax. J Infect Dis. 2002;186:227–33. doi: 10.1086/341299. [DOI] [PubMed] [Google Scholar]
- 4.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagarasan S. Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Curr Top Microbiol Immunol. 2006;308:137–53. doi: 10.1007/3-540-30657-9_6. [DOI] [PubMed] [Google Scholar]
- 6.Shulzhenko N, Morgun A, Hsiao W, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–93. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Shin DM, Abbasi S, et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proc Natl Acad Sci U S A. 2012;109:20077–82. doi: 10.1073/pnas.1212428109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–18. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagarasan S, Honjo T. T-independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 10.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal vacteria. Science. 2000;288:2222–6. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 11.Hong HA, Khaneja R, Tam NM, et al. Bacillus subtilis isolated from the human gastrointestinal tract. Res Microbiol. 2009;160:134–43. doi: 10.1016/j.resmic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 13.Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathania M, Zadeh M, Lightfoot YL, et al. Colonic immune stimulation by targeted oral vaccine. PLoS One. 2013;8:e55143. doi: 10.1371/journal.pone.0055143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamadzadeh M, Durmaz E, Zadeh M, et al. Targeted expression of anthrax protective antigen by Lactobacillus gasseri as an anthrax vaccine. Future Microbiol. 2010;5:1289–96. doi: 10.2217/fmb.10.78. [DOI] [PubMed] [Google Scholar]
- 16.Goossens PL. Animal models of human anthrax: the Quest for the Holy Grail. Mol Aspects Med. 2009;30:467–80. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann M, Dragoi C, Poleganov MA, Pfeilschifter J, Mühl H. Interleukin-18 directly activates T-bet expression and function via p38 mitogen-activated protein kinase and nuclear factor-kB in acute myeloid leukemia-derived predendritic KG-1 cells. Mol Cancer Ther. 2007;6:723–31. doi: 10.1158/1535-7163.MCT-06-0505. [DOI] [PubMed] [Google Scholar]
- 18.Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–71. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–95. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169:1922–9. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- 23.Spencer SP, Wilhelm C, Yang Q, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–7. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenneman KE, Doganay M, Akmal A, et al. The early humoral immune response to Bacillus anthracis toxins in patients infected with cutaneous anthrax. FEMS Immunol Med Microbiol. 2011;62:164–72. doi: 10.1111/j.1574-695X.2011.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 26.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 27.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–68. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 28.Sahay B, Owen JL, Yang T, et al. Activation of B cells by a dendritic cell-targeted oral vaccine. Curr Pharmaceut Biotechnol. 2014;14:867–77. doi: 10.2174/1389201014666131226120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comerford I, McColl SR. Mini-review series: focus on chemokines. Immunol Cell Biol. 2011;89:183–4. doi: 10.1038/icb.2010.164. [DOI] [PubMed] [Google Scholar]
- 30.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 31.Oscherwitz J, Yu F, Jacobs JL, Cease KB. Recombinant vaccine displaying the loop-neutralizing determinant from protective antigen completely protects rabbits from experimental inhalation anthrax. Clin Vaccine Immunol. 2013;20:341–9. doi: 10.1128/CVI.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada M, Koizumi T, Bachiller D, Ruther U, Tokuhisa T. Deregulated c-fos modulates IgG2b production of B cells mediated by lipopolysaccharide. Immunobiology. 1993;188:233–41. doi: 10.1016/S0171-2985(11)80232-9. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene. 2000;19:1379–85. doi: 10.1038/sj.onc.1203443. [DOI] [PubMed] [Google Scholar]
- 34.Tanos T, Marinissen MJ, Leskow FC, et al. Phosphorylation of c-Fos by members of the p38 MAPK family. Role in the AP-1 response to UV light. J Biol Chem. 2005;280:18842–52. doi: 10.1074/jbc.M500620200. [DOI] [PubMed] [Google Scholar]
- 35.Xie T, Sun C, Uslu K, et al. A new murine model for gastrointestinal anthrax infection. PLoS One. 2013;8:e66943. doi: 10.1371/journal.pone.0066943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz MN. Recognition and management of anthrax--an update. N Engl J Med. 2001;345:1621–6. doi: 10.1056/NEJMra012892. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky IE, Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11:521–6. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- 38.Roy CR, Mocarski ES. Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol. 2007;8:1179–87. doi: 10.1038/ni1528. [DOI] [PubMed] [Google Scholar]
- 39.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol. 2007;7:556–67. doi: 10.1038/nri2098. [DOI] [PubMed] [Google Scholar]
- 40.Ebrahimi CM, Sheen TR, Renken CW, Gottlieb RA, Doran KS. Contribution of lethal toxin and edema toxin to the pathogenesis of anthrax meningitis. Infect Immun. 2011;79:2510–8. doi: 10.1128/IAI.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–65. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Leppla SH. Anthrax toxin uptake by primary immune cells as determined with a lethal factor-beta-lactamase fusion protein. PLoS One. 2009;4:e7946. doi: 10.1371/journal.pone.0007946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayamajhi M, Delgado C, Condon TV, Riches DW, Lenz LL. Lung B cells promote early pathogen dissemination and hasten death from inhalation anthrax. Mucosal Immunol. 2012;5:444–54. doi: 10.1038/mi.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliva CR, Swiecki MK, Griguer CE, et al. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proc Natl Acad Sci U S A. 2008;105:1261–6. doi: 10.1073/pnas.0709321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–92. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 46.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–9. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 47.Baldari CT, Tonello F, Paccani SR, Montecucco C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends Immunol. 2006;27:434–40. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Quinn CP, Dull PM, Semenova V, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–36. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 49.Fang H, Xu L, Chen TY, Cyr JM, Frucht DM. Anthrax lethal toxin has direct and potent inhibitory effects on B cell proliferation and immunoglobulin production. J Immunol. 2006;176:6155–61. doi: 10.4049/jimmunol.176.10.6155. [DOI] [PubMed] [Google Scholar]
- 50.Hadler JL. Learning from the 2001 anthrax attacks: immunological characteristics. J Infect Dis. 2007;195:163–4. doi: 10.1086/510317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.