Abstract
FTO is the strongest known genetic susceptibility locus for obesity. Experimental studies in animals suggest the potential roles of FTO in regulating food intake. The interactive relation among FTO variants, dietary intake and body mass index (BMI) is complex and results from previous often small-scale studies in humans are highly inconsistent. We performed large-scale analyses based on data from 177 330 adults (154 439 Whites, 5776 African Americans and 17 115 Asians) from 40 studies to examine: (i) the association between the FTO-rs9939609 variant (or a proxy single-nucleotide polymorphism) and total energy and macronutrient intake and (ii) the interaction between the FTO variant and dietary intake on BMI. The minor allele (A-allele) of the FTO-rs9939609 variant was associated with higher BMI in Whites (effect per allele = 0.34 [0.31, 0.37] kg/m2, P = 1.9 × 10−105), and all participants (0.30 [0.30, 0.35] kg/m2, P = 3.6 × 10−107). The BMI-increasing allele of the FTO variant showed a significant association with higher dietary protein intake (effect per allele = 0.08 [0.06, 0.10] %, P = 2.4 × 10−16), and relative weak associations with lower total energy intake (−6.4 [−10.1, −2.6] kcal/day, P = 0.001) and lower dietary carbohydrate intake (−0.07 [−0.11, −0.02] %, P = 0.004). The associations with protein (P = 7.5 × 10−9) and total energy (P = 0.002) were attenuated but remained significant after adjustment for BMI. We did not find significant interactions between the FTO variant and dietary intake of total energy, protein, carbohydrate or fat on BMI. Our findings suggest a positive association between the BMI-increasing allele of FTO variant and higher dietary protein intake and offer insight into potential link between FTO, dietary protein intake and adiposity.
INTRODUCTION
Obesity is a major health problem throughout the world. A recent large-scale analysis including 9.1 million participants from 199 countries reported that the mean body mass index (BMI) has increased substantially since 1980 worldwide (1). Obesity is the results of a complex interplay between environmental and genetic factors that have additive and interactive effects. Among the obesity-susceptibility genes recently identified through genome-wide association studies, single-nucleotide polymorphisms (SNPs) that cluster in the first intron of the FTO gene show the strongest association with BMI (effect size = ∼0.35 kg/m2 per allele) and obesity risk (2–4). FTO is highly expressed in the hypothalamus, a region involved in regulation of food intake and energy expenditure (5,6). Previous studies have reported that the BMI-increasing allele of the FTO variant is associated with higher energy intake or higher specific macronutrient intake, (7–13) whereas other studies could not confirm such associations (14–23).
There is growing evidence supporting a gene–environment (diet/lifestyle) interaction in relation to BMI and obesity risk (24–26). Our previous meta-analysis of >218 000 individuals demonstrated that physical activity may attenuate the association between the FTO locus on BMI and obesity risk (24) A recent study identified that FTO variants are associated with phenotypic variability of BMI, suggesting interactions between FTO and environment in relation to BMI (27). Fewer studies have investigated the interaction between FTO variants and dietary factors on BMI and have reported conflicting results (12,15,19,23,28,29). While some studies found that high energy intake, high dietary fat intake or low carbohydrate intake might strengthen the association between FTO genetic variants and BMI/obesity risk, (12,15,28) others failed to confirm such interactions (19,23,29).
These inconsistent observations might be due to insufficient statistical power of individual studies to identify interactions, which typically require much large sample sizes. Moreover, inevitable measurement errors in dietary factors further reduce statistical power and necessitate studies with large sample sizes (30). Therefore, we analyzed cross-sectional data from 40 individual studies with a total of 177 330 adults to examine (i) the association between the FTO-rs9939609 variant (or a proxy SNP) and dietary intakes of total energy and macronutrients (protein, carbohydrate and fat) and (ii) the interaction between the FTO variant and these dietary factors on BMI.
RESULTS
Study characteristics
Study-specific characteristics for each study are shown in Supplementary Material, Table S1. The ranges of mean age, mean BMI and median of total energy, protein, carbohydrate and fat intakes (% of total energy intake) across studies were 31–75 years, 22.1–31.6 kg/m2, 1184–2703 kcal/day, 12.5–19.3, 41.8–69.4 and 14.9–39.7%, respectively.
FTO variant and BMI
The minor allele (A-allele) of FTO-rs9939609 variant (or of its proxy) was associated with increased BMI in Whites (effect per allele [95% CI] = 0.34 [0.31, 0.37] kg/m2, P = 1.9 × 10−105), Asians (effect per allele = 0.25 [0.14, 0.35] kg/m2, P = 6.2 × 10−6) and all participants combined (effect per allele = 0.33 [0.30, 0.35] kg/m2, P = 3.6 × 10−107), but no association was observed in African Americans (effect per allele = 0.00 [−0.20, 0.20], P = 0.98) (Table 1).
Table 1.
Associations of FTO SNP rs9939609 or a proxy with BMI and intakes of total energy, protein, carbohydrate and fat in a fixed effects meta-analysis of up to 177 330 adultsa
| Model 1b |
Model 2c |
|||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | I2 (%) | β (95% CI) | P | I2 (%) | |
| BMI (kg/m2) | ||||||
| Whites | 0.34 (0.31,0.37) | 1.9 × 10−105 | 46 | – | – | – |
| African Americans | 0.00 (−0.20, 0.20) | 0.98 | 0 | – | – | – |
| Asians | 0.25 (0.14, 0.35) | 6.2 × 10−6 | 48 | – | – | – |
| All | 0.33 (0.30, 0.35) | 3.6 × 10−107 | 47 | – | – | – |
| Total energy (kcal/day) | ||||||
| Whites | −7.8 (−11.8, −3.9) | 1.5 × 10−4 | 5 | −7.2 (−11.1, −3.3) | 3.3 × 10−4 | 0 |
| African Americans | 4.0 (−19.9, 27.9) | 0.74 | 40 | 4.6 (−19.3, 28.5) | 0.70 | 39 |
| Asians | 13.2 (−2.8, 29.2) | 0.11 | 30 | 10.5 (−5.5, 26.5) | 0.20 | 26 |
| All | −6.4 (−10.1, −2.6) | 0.001 | 18 | −5.9 (−9.7, −2.1) | 0.002 | 13 |
| Protein (% of energy) | ||||||
| Whites | 0.08 (0.06, 0.10) | 3.8 × 10−15 | 26 | 0.05 (0.03, 0.07) | 8.8 × 10−8 | 20 |
| African Americans | 0.15 (0.01, 0.29) | 0.03 | 0 | 0.14 (0.00, 0.28) | 0.05 | 5 |
| Asians | 0.06 (−0.02, 0.15) | 0.14 | 57 | 0.06 (−0.02, 0.15) | 0.15 | 57 |
| All | 0.08 (0.06, 0.10) | 2.4 × 10−16 | 32 | 0.05 (0.04, 0.07) | 7.5 × 10−9 | 29 |
| Carbohydrate (% of energy) | ||||||
| Whites | −0.07 (−0.11, −0.02) | 0.005 | 30 | −0.04 (−0.09, 0.01) | 0.10 | 22 |
| African Americans | −0.06 (−0.40, 0.27) | 0.71 | 60 | −0.05 (−0.38, 0.28) | 0.77 | 59 |
| Asians | −0.07 (−0.32, 0.18) | 0.57 | 0 | −0.08 (−0.33, 0.17) | 0.53 | 0 |
| All | −0.07 (−0.11, −0.02) | 0.004 | 29 | −0.04 (−0.09, 0.01) | 0.08 | 23 |
| Fat (% of energy) | ||||||
| Whites | 0.02 (−0.02, 0.07) | 0.30 | 1 | 0.00 (−0.04, 0.05) | 0.85 | 0 |
| African Americans | 0.03 (−0.22, 0.27) | 0.84 | 43 | 0.01 (−0.23, 0.26) | 0.92 | 42 |
| Asians | 0.07 (−0.12, 0.26) | 0.47 | 0 | 0.08 (−0.11, 0.28) | 0.39 | 0 |
| All | 0.03 (−0.02, 0.07) | 0.24 | 3 | 0.01 (−0.03, 0.05) | 0.69 | 0 |
aData are β coefficients (95% CI) per minor allele of rs9939609 or a proxy (r2 > 0.8) for each trait. Analyses from individual studies were conducted separately in men and women, and then combined by meta-analysis of up to 177 330 adults (154 439 Whites, 5776 African Americans and 17 115 Asians).
bModel 1, adjusted for age, physical activity (if available), region (if available) and eigenvectors (GWAS data only).
cModel 2, further adjusted for BMI.
FTO variant and dietary intake
The minor allele (BMI-increasing allele) of the FTO variant was associated with lower total energy intake (effect per allele = −6.4 [95% CI −10.1, −2.6] kcal/day, P = 0.001) (Table 1; Supplementary Material, Fig. S1), which was only modestly attenuated after further adjustment for BMI (P = 0.002). The heterogeneity among studies was low (I2 = 18%), and there was no significant factor accounting for the heterogeneity in the meta-regression (all P > 0.05) (Supplementary Material, Table S2). The results in stratified meta-analyses for association between FTO variant and total energy intake are shown in Supplementary Material, Figure S2.
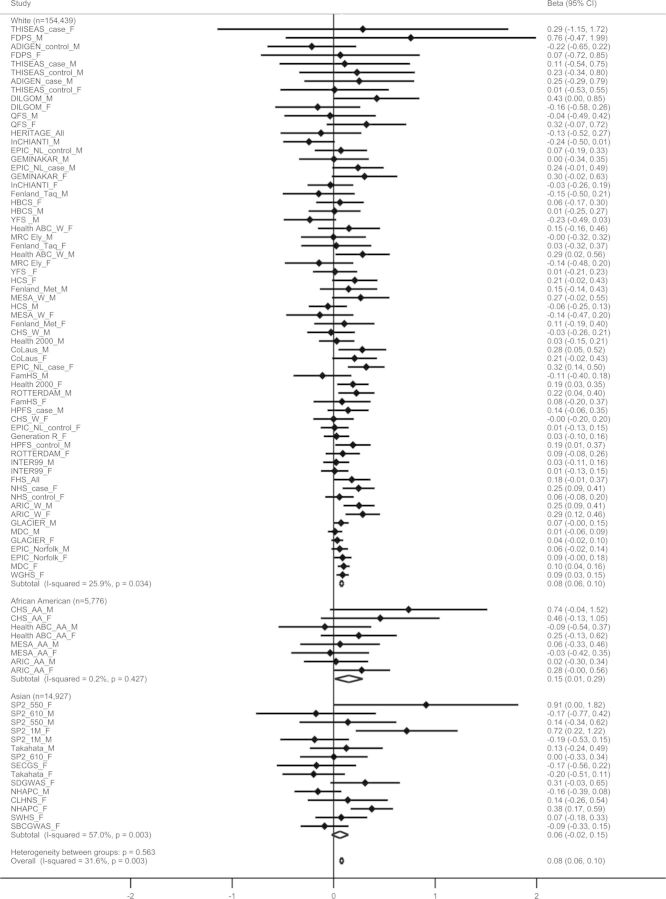
The minor allele of the FTO variant was significantly associated with higher dietary protein intake (% of energy) (effect per allele = 0.08 [0.06, 0.10] %, P = 2.4 × 10−16), and results were directionally consistent across ethnicities, with moderate heterogeneity (Table 1 and Fig. 1). The association was attenuated but remained significant after further adjustment for BMI (effect per allele = 0.05 [0.04, 0.07] %, P = 7.5 × 10−9). The meta-regression identified geographic region (North America versus Europe, P = 0.006; North America versus Asia, P = 0.03) and ethnicity groups (White versus Asian, P = 0.03) to be contributing to the observed moderate heterogeneity (Supplementary Material, Table S2). In stratified meta-analyses (Supplementary Material, Fig. S3), the directions of the association between the FTO variant and protein intake were consistent across the subgroups, though P-values for heterogeneities were nominally significant for geographic region (P = 0.02) and median of protein intake (P = 0.03).
Figure 1.
Forest plot of the association between FTO-rs9939609 SNP or a proxy and protein intake in a fixed effects meta-analysis of 175 142 adults. The studies are shown in men (_M), women (_F) or mixed (_All), cases (_case) and controls (_control) for case–control studies, and Whites (_W) and African Americans (_AA) for studies with multiple ethnicities separately, sorted by sample size (smallest to largest). The β represents the difference in protein intake (% of energy intake) per minor allele of SNP rs9939609 or a proxy (r2 > 0.8), adjusted for age, physical activity (if available), region (if available) and eigenvectors (GWAS data only).
The minor allele of the FTO variant was associated with lower intake of dietary carbohydrate (effect per allele = −0.07 [−0.11, −0.02] %, P = 0.004) (Table 1; Supplementary Material, Fig. S4) and was attenuated after further adjustment for BMI (P = 0.08). The heterogeneity among studies was moderate (I2 = 29%). The meta-regression indicated heterogeneity by geographic region (North America versus Europe, P = 0.03) (Supplementary Material, Table S2). We observed no significant association between the FTO variant and dietary fat intake (effect per allele = 0.03 [−0.02, 0.07] %, P = 0.24) (Table 1; Supplementary Material, Fig. S5), and heterogeneity among studies was low (I2 = 3%). The results in stratified meta-analyses for the association between FTO SNP and carbohydrate and fat intakes are shown in Supplementary Material, Figure S6 and S7.
In addition, the minor allele of the FTO variant was associated with higher absolute intake (g/day) of protein (P = 7.9 × 10−12) and lower absolute intake (g/day) of carbohydrate (P = 0.003) (Supplementary Material, Table S3). We also performed meta-analyses for the FTO SNP and dietary intakes using random effects methods, resulting in similar findings (Supplementary Material, Table S4).
Comparison of associations of FTO and MC4R variants with dietary intake
To show some specificity of the FTO variant, we compared the FTO with the MC4R, the second strongest obesity gene identified so far, in associations with dietary intakes. As expected, the MC4R genetic variant (SNP rs17782313 or a proxy) was significantly associated with BMI (P = 5.4 × 10−76); however, there is no significant association between the MC4R variant and total energy, protein, carbohydrate or fat intake (Supplementary Material, Table S5). As the effect size of the FTO variant on BMI is larger than that of the MC4R variant on BMI (0.33 versus 0.23 kg/m2), we calculated the adjusted effect sizes of the FTO variant on dietary intake after accounting for the strength of the effect of the MC4R variant on BMI. There is a significant difference in effect sizes of the FTO variant (adjusted) and the MC4R variant on dietary protein intake (P = 0.008) (Supplementary Material, Table S5).
Dietary intake and BMI
There was no significant difference in BMI between participants in the high- and low-energy intake groups (β = −0.07 [−0.17, 0.04] kg/m2, P = 0.21) (Supplementary Material, Table S6). Participants in the high-protein (0.66 kg/m2, P = 2.6 × 10−29) and high-fat intake groups (0.20 kg/m2, P = 0.002) had a higher mean BMI compared with those in the low intake groups, whereas the mean BMI was lower (0.31 kg/m2, 4.3 × 10−8) among participants in the high carbohydrate intake group compared with those in the low intake group (Supplementary Material, Table S6). Most of these differences seem to be driven by those observed in Whites, whereas they are less apparent or absent in African Americans and Asians. We observed moderate-to-high heterogeneity for these comparisons (I2 = 40–90%).
Interaction between FTO variant and dietary intake on BMI
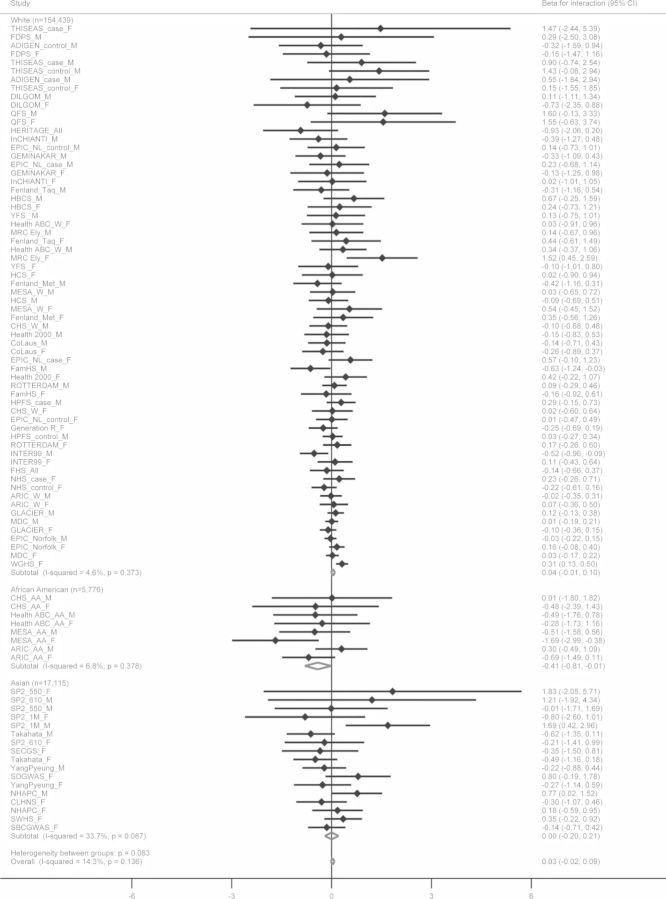
There was no significant interaction between the FTO variant and total energy intake on BMI (effect for interaction = 0.03 [−0.02, 0.09], Pinteraction = 0.25), and heterogeneity among studies was low (I2 = 14%) (Table 2 and Fig. 2). The meta-regression identified ethnicity (White versus African Americans, P = 0.01) and sample size (P = 0.02) as sources of heterogeneity (Supplementary Material, Table S7). When we stratified our meta-analysis by ethnicity, we observed a nominally significant result that showed interaction in opposite direction in African Americans (effect for interaction = −0.41 [−0.81, −0.01], Pinteraction = 0.04) (Table 2). We did not observe significant interactions between the FTO genetic variant and total energy intake on BMI in the stratified meta-analyses by other characteristics (Supplementary Material, Fig. S8).
Table 2.
Interaction between FTO-rs9939609 SNP or a proxy and dietary intakes on BMI in a fixed effects meta-analysis of up to 177 330 adultsa
| Main effect of SNP in high intake groupb |
Main effect of SNP in low intake groupb |
Interaction effect |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | I2 (%) | β (95% CI) | P | I2 (%) | β (95% CI) | P | I2 (%) | |
| Total energy (kcal/day) | |||||||||
| Whites | 0.36 (0.32, 0.40) | 1.4 × 10−61 | 32 | 0.31 (0.26, 0.35) | 3.9 × 10−43 | 28 | 0.04 (−0.01, 0.10) | 0.13 | 5 |
| African Americans | −0.23 (−0.52, 0.06) | 0.12 | 36 | 0.20 (−0.08, 0.48) | 0.16 | 0 | −0.41 (−0.81, −0.01) | 0.04 | 7 |
| Asians | 0.26 (0.11, 0.41) | 0.001 | 48 | 0.20 (0.04, 0.35) | 0.01 | 37 | 0.00 (−0.21, 0.21) | 0.97 | 34 |
| All | 0.34 (0.30, 0.38) | 7.1 × 10−61 | 42 | 0.30 (0.26, 0.34) | 1.6 × 10−44 | 26 | 0.03 (−0.02, 0.09) | 0.25 | 14 |
| Protein (% of energy) | |||||||||
| Whites | 0.32 (0.28, 0.37) | 5.8 × 10−48 | 32 | 0.33 (0.29, 0.37) | 4.8 × 10−52 | 33 | 0.00 (−0.06, 0.06) | 0.90 | 0 |
| African Americans | −0.07 (−0.36, 0.21) | 0.62 | 0 | 0.07 (−0.21, 0.35) | 0.61 | 23 | −0.11 (−0.51, 0.29) | 0.58 | 0 |
| Asians | 0.37 (0.21, 0.53) | 8.6 × 10−6 | 26 | 0.15 (−0.02, 0.31) | 0.08 | 23 | 0.22 (−0.01, 0.44) | 0.06 | 0 |
| All | 0.32 (0.28, 0.36) | 1.2 × 10−50 | 32 | 0.31 (0.27, 0.35) | 3.0 × 10−51 | 33 | 0.01 (−0.05, 0.06) | 0.80 | 0 |
| Carbohydrate (% of energy) | |||||||||
| Whites | 0.34 (0.29, 0.38) | 2.2 × 10−54 | 37 | 0.33 (0.28, 0.37) | 2.0 × 10−49 | 21 | 0.00 (−0.05, 0.06) | 0.94 | 0 |
| African Americans | 0.10 (−0.18, 0.38) | 0.48 | 22 | −0.11 (−0.40, 0.18) | 0.45 | 0 | 0.24 (−0.16, 0.64) | 0.25 | 1 |
| Asians | 0.19 (0.03, 0.35) | 0.02 | 40 | 0.36 (0.20, 0.51) | 7.5 × 10−6 | 33 | −0.19 (−0.42, 0.04) | 0.10 | 0 |
| All | 0.32 (0.28, 0.36) | 1.4 × 10−54 | 38 | 0.32 (0.28, 0.36) | 5.6 × 10−52 | 26 | 0.00 (−0.06, 0.05) | 0.87 | 0 |
| Fat (% of energy) | |||||||||
| Whites | 0.34 (0.30, 0.38) | 6.6 × 10−53 | 26 | 0.32 (0.28, 0.36) | 7.0 × 10−50 | 38 | 0.03 (−0.03, 0.09) | 0.37 | 0 |
| African Americans | 0.02 (−0.27, 0.31) | 0.90 | 2 | 0.00 (−0.28, 0.27) | 0.98 | 46 | 0.02 (−0.37, 0.42) | 0.91 | 49 |
| Asians | 0.43 (0.27, 0.58) | 7.4 × 10−8 | 13 | 0.11 (−0.05, 0.28) | 0.18 | 31 | 0.30 (0.07, 0.52) | 0.01 | 0 |
| All | 0.34 (0.30, 0.38) | 5.7 × 10−58 | 25 | 0.30 (0.26, 0.34) | 3.8 × 10−48 | 41 | 0.04 (−0.01, 0.10) | 0.13 | 7 |
aData are β (95% CI) per minor allele of rs9939609 or a proxy (r2 > 0.8), adjusted for age, physical activity (if available), region (if available) and eigenvectors (GWAS data only). Analyses from individual studies were conducted separately in men and women, and then combined by meta-analysis of up to 177 330 adults (154 439 Whites, 5776 African Americans and 17 115 Asians).
bHigh and low intake groups were defined by medians for each dietary intake.
Figure 2.
Forest plot of the interaction between FTO-rs9939609 SNP or a proxy and total energy intake on BMI in a fixed effects meta-analysis of 177 330 adults. The studies are shown in men (_M), women (_F) or mixed (_All), cases (_case) and controls (_control) for case–control studies, and Whites (_W) and African Americans (_AA) for studies with multiple ethnicities separately, sorted by sample size (smallest to largest). The β represents the difference in BMI per minor allele of SNP rs9939609 or a proxy (r2 > 0.8) comparing participants in the high total energy intake to those in the low total energy intake group, adjusted for age, physical activity (if available), region (if available) and eigenvectors (GWAS data only).
Overall, we did not find a significant interaction between the FTO variant and dietary protein intake (effect for interaction = 0.01 [−0.05, 0.06], Pinteraction = 0.80), carbohydrate intake (effect for interaction = 0.00 [−0.06, 0.05], Pinteraction = 0.87) or dietary fat intake (effect for interaction = 0.04 [−0.01, 0.10], Pinteraction = 0.13) on BMI (Table 2; Supplementary Material, Figs S9, S10 and S11). The heterogeneity among studies was low (I2 = 0, 0 and 7%, respectively).
We observed a significant interaction between the FTO genetic variant and fat intake on BMI in Asians (effect for interaction = 0.30 [0.07, 0.52], Pinteraction = 0.01), but not in Whites (Pinteraction = 0.37) or African Americans (Pinteraction = 0.91) (Table 2). When stratified by other characteristics, we observed a significant interaction between the FTO variant and fat intake on BMI in studies from Asia (identical result in Asians since participants of these studies are all Asians) and in studies with low fat intake (effect for interaction = 0.10 [0.01, 0.18], P for interaction = 0.03) (Supplementary Material, Fig. S12). We also conducted the stratified analyses by excluding Asian studies (Supplementary Material, Fig. S13). Most results were similar except that interaction between the FTO variant and fat intake on BMI in studies with low fat intake did not remain significant (effect for interaction = 0.05 [−0.04, 0.14], P for interaction = 0.25). No significant interactions between the FTO genetic variant and dietary protein or fat intake on BMI were observed in the stratified meta-analyses (Supplementary Material, Fig. S14 and S15).
Since there was little or no heterogeneity in interactions between FTO genetic variants and dietary intake on BMI among studies, the results were similar when we performed meta-analyses using random effects method (Supplementary Material, Table S8).
DISCUSSION
By combining data from 40 studies including up to177 330 individuals, we confirmed the association between the minor allele (A-allele) of the FTO-rs9939609 variant (or its proxy) and higher BMI in Whites and Asians and all participants combined, but not in African Americans. This is consistent with the previous results that this index SNP is not associated with adiposity in African-ancestry populations (31–34), which can likely be explained by different LD patterns of FTO between European and African populations (35). We also found significant association with dietary protein intake where the BMI-increasing allele of FTO variant was associated with higher protein intake. This association was only slightly attenuated after adjustment for BMI. Dietary intakes of total energy, protein, carbohydrate and fat did not influence the association between the FTO variant and BMI.
We observed that the BMI-increasing allele of FTO was associated with lower total energy intake in our study. Moreover, the observed inverse association was slightly stronger among studies with a higher mean BMI compared with those with a lower mean BMI. This might be partly explained by the underreporting of total energy intake, as individuals with higher BMI are more likely to be underreporters. In line with our results, Sonestedt et al. (12) found an inverse association between FTO and total energy intake, and interestingly, the frequency of individuals who underreported their dietary energy intake was higher in the BMI-increasing allele carriers compared with non-carriers. The difference in energy intake between FTO genotypes was not significant when misreporters were excluded (12). Misreporting is a common and inevitable type of measurement error found in any dietary assessment that relies on self-reports, such as the food frequency questionnaire (FFQ) and dietary records. Thus, the observed associations between BMI-associated genetic variants (such as genetic variants in the FTO and MC4R) and total energy intake could be biased among studies using self-reported data on dietary intakes if this measurement error (underreporting in obese participants) is not taken into account. Unfortunately, we were unable to do further analysis by excluding underreporters in the current study because detailed data on energy expenditure to evaluate the magnitude of misreporting in each study were not available. For macronutrient intakes, we primarily focused on relative intakes (% of energy) rather than absolute intakes, since the results might be less influenced by misreporting (36). Moreover, it has been suggested that underreporting was particularly frequent with fat- and/or carbohydrate-rich foods (37). We observed a strong association between the FTO genetic variant and dietary protein intake, and the association was consistent across subgroups stratified by study characteristics. Moreover, we found that another obesity-related genetic variant, MC4R-rs17782313, was not associated with dietary intake and there was a significant difference in effect sizes of the FTO variant and the MC4R variant on dietary protein intake, after adjusting for the strengths of the effects of these two genetic variants on BMI. These results clearly suggest the specificity of the FTO genetic variant in association with dietary protein intake, and this observed association is less likely due to measurement errors in reporting of dietary intakes which are related to obesity.
Previous animal studies have supported a role of the FTO in energy homeostasis, but it remains unclear whether the FTO primarily affects energy expenditure or food intake (38,39). Emerging evidence from animal and in vitro studies suggests a role of the FTO in protein metabolism and cellular amino acids sensing (40–42). The sensing of amino acid levels in the brain has critical impacts on hypothalamic mTOR pathways regulating food intake (43). Moreover, a very recent study suggested a link between the FTO, ghrelin (a key mediator of ingestive behavior), and neural responses to food cues (45). Taken together, it is possible that the FTO might be involved in the central sensing of dietary macronutrients composition (41) and our data from current large population studies suggest a preference for protein-rich diets by the FTO BMI-increasing allele carriers.
We found no evidence for dietary total energy or macronutrient intake influencing the association between the FTO variant and BMI. Although sample size, age, gender, BMI, dietary intake, ethnicity, geographic region, study design and measurement of dietary intake varied among the participating studies, our meta-regression indicated that these study-specific characteristics were less likely to have affected the interaction effects. There was very little or no between-study heterogeneity in the meta-analyses of interaction effects. It is unlikely that the lack of significant interaction was due to low statistical power because we have adequate sample size to detect interactions of small magnitude (>80% power to detect gene–diet interaction effect size of 0.08 kg/m2). Indeed, the observed interaction effects were close to null, and therefore, it is unlikely that even larger sample sizes would reveal significant interactions. Nevertheless, it is possible that the estimate of the interaction effect may be an underestimation of the true effect because of measurement error in dietary intake and heterogeneous measurement methods across studies. A previous methodology paper suggested that gene–environment interaction studies would benefit more from better measurement of environmental factors than from increasing sample size (30). Studies with repeated and more precise measurement of dietary intake are needed in future gene–diet interaction analyses. In addition, our previously reported approach using a genetic risk score based on multiple genetic variants rather than analysis of individual variants might be preferable in gene–environment interaction studies (25,26,44).
Interestingly, we observed a nominally significant interaction between dietary fat intake and FTO genetic variant on BMI in Asians. Besides genetic differences, we may speculate that the observed interaction specific to Asians might be related to the lower dietary fat intake in Asian populations compared with others from North America or Europe, as we also found a similar but weaker interaction in studies with a low dietary fat intake. As protein intake is relatively stable across different populations, lower fat as percentage of energy intake likely reflects higher carbohydrate intake in Asian populations. Thus, either dietary fat or carbohydrate may be involved. However, it was also difficult to tease out whether genetic differences, geographic differences in dietary intakes or both are responsible for the observations. Asians included in our analyses were all from Asia, and we did not have data for Asians living in North America or Europe. Nevertheless, considering the compelling interaction effect size of 0.30 kg/m2 observed in Asians (approximately equivalents to the main effect of the FTO variant on BMI), it would be worth confirming this result in future studies with larger samples.
Major strengths of our study include the designed meta-analysis based on de novo analysis of both published and unpublished data, analytical consistency across studies, and a large sample size of >177 000 individuals. Together with our previous interaction analysis between physical activity and FTO genetic variants, (24) we have demonstrated that large-scale international collaborations are feasible and useful for confirming or refuting moderate interactions between genes and diet/lifestyle.
Several limitations need to be acknowledged. The present meta-analysis was based on cross-sectional data, which limits the interpretation of observations. Although genetic variants do not change throughout the life course, dietary intake may change and obesity status and other environmental factors may influence individuals' dietary intake. As measurement errors in self-reported dietary intake data are inevitable and accurate dietary assessment remains a major challenge in the gene–diet interaction analyses, repeated measures using longitudinally collected data might be helpful in reducing measurement errors and improving the study power. Furthermore, randomized dietary intervention trials may provide reliable evidence because the study conditions, especially the intakes of specific foods and nutrients, are prescribed, and the confounding effects are maximally reduced. Therefore, a meta-analysis of longitudinal studies or intervention trials would be required, but few studies have investigated the long-term interactions between FTO variants and dietary intake (46–48). In addition, we did not include data on other adiposity proxies or different types of fatty acid intake, while several studies reported interactions of FTO genetic variants with total fat intake on body fat mass, (49) and with saturated fat intake on BMI and risk of obesity (15,50,51). Participants included in our analysis were mostly from studies of Caucasian populations (87%), and more studies are needed in other ethnic groups such as African Americans in which different effective FTO variants may be involved. Finally, our current analysis did not include data from children and adolescents in which a positive association between FTO genetic variants and food intake has been reported previously (7–9,11,13).
In summary, our findings, based on a large-scale meta-analysis, suggest a strong association between FTO genetic variants and dietary protein intake, independent of its association with BMI; this is consistent with emerging insights from functional studies (41,45). The current study did not provide evidence supporting an interaction between total energy or macronutrient intake and the FTO genetic variant in relation to adiposity. Due to the inevitable measurement errors in self-reported data on dietary intakes, our results should be interpreted with caution. Future studies with repeated and precise measurement of dietary intakes are warranted to investigate interrelationships between the FTO genetic variants, dietary intakes and adiposity.
MATERIALS AND METHODS
Study design
Considering the limitations of a literature-based meta-analysis, such as publication bias and inconsistency in statistical methods, exposures and outcomes across individual studies, (52) we designed a meta-analysis based on de novo analyses of data conducted according to a standardized analytical plan (see details in Statistical analysis). We identified 45 eligible studies with data on FTO genotype and dietary intake through PubMed and through the network of collaborators who have joined our previous meta-analysis on the interaction between the FTO variant and physical activity in relation to BMI and obesity (24). A standardized analytical plan was sent to 40 studies that agreed to participate in the meta-analysis. Analyses according to our standardized plan were performed by each study locally, and detailed summary statistics were subsequently collected using our standardized data collection form.
Study participants
Our meta-analysis included cross-sectional data on 177 330 adults (62 275 men and 115 055 women; 154 439 Whites, 5776 African Americans and 17 115 Asians) from 40 studies (21 from Europe, 10 from North America and 9 from Asia) (Supplementary Material, Table S9).
Measurement of BMI and dietary intake
BMI was calculated as body weight (kg)/height2 (m2). Body weight and height were measured in 37 studies, and three studies used validated, self-reported data (Supplementary Material, Table S10). Dietary intakes (total energy, protein, carbohydrate and fat) were assessed using an FFQ (33 studies), dietary record (thee studies), both FFQ and dietary record (two studies) or diet recall over a 1-month period (two studies) (Supplementary Material, Table S10). All the studies used data on height and weight measured within 1 year of dietary intake assessment.
Genotyping
The FTO-rs9939609 SNP or a proxy (linkage disequilibrium [LD] r2 > 0.8 in the corresponding ethnic group) and the MC4R-rs17782313 SNP or a proxy were genotyped using direct genotyping methods or Affymetrix and Illumina genome-wide genotyping arrays or imputed using MACH (http://www.sph.umich.edu/csg/abecasis/MACH/) or IMPUTE software (https://mathgen.stats.ox.ac.uk/impute/impute.html) with a high imputation quality (r2 > 0.99) (Supplementary Material, Table S11 and S12). The studies provided summary statistics based on data that met their quality control criteria for genotyping call rate, concordance in duplicate samples and Hardy–Weinberg equilibrium P-value.
Statistical analysis
Each participating study analyzed the data according to our standardized plan described below. We used linear regression model to test (i) the difference in BMI between the low and high dietary intake groups (dichotomized at median of respective dietary intake variable) and (ii) the associations of the FTO variant with BMI, total energy intake and absolute intakes (g/day) and relative intakes (expressed as the percentage of total energy) of fat, protein and carbohydrate, adjusted for age, geographic regions (if available), physical activity (if available) and eigenvectors (GWAS data only). We additionally adjusted for BMI when evaluating the association between the FTO variant and dietary intake. The association between the FTO variant and BMI was also tested stratified by low and high dietary intake groups. Interactions between the FTO variant and dietary intake on BMI were tested by including the respective interaction terms in the models (e.g. interaction term = rs9939609 SNP × total energy intake [dichotomized at the medians]). All the analyses were conducted in men and women separately, except for two family studies that combined the data from men and women. In studies with multiple ethnicities, each ethnicity was analyzed separately. In case–control studies, cases and controls were analyzed separately.
We pooled β-coefficients and standard errors from individual studies using Mantel and Haenszel fixed effects as well as the DerSimonian and Laird random effects meta-analysis methods in Stata, version 12 (StataCorp LP, College Station, TX, USA). Between-study heterogeneity was tested by Cochran's Q statistic and quantified by the I2 value. Low heterogeneity was defined as an I2 value of 0–25%, moderate heterogeneity as an I2 of 25–75% and high heterogeneity as an I2 of 75%–100%. P for heterogeneity was derived from a χ2 test. We also performed meta-regression analyses to explore sources of heterogeneity in our meta-analyses. Meta-regression included the following study-specific variables as covariates: study sample size, mean age (or age group <60 versus ≥60 years), mean BMI, median of dietary intake, gender, ethnicity (White, African American and Asian), geographic region (North America, Europe and Asia), study design (population- or family-based versus. case–control), dietary intake measurement method (FFQ versus. dietary record or other) and adjustment for physical activity (yes versus no). Stratified meta-analyses were performed in subgroups according to these covariates.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
Funding sources for the individual studies included in this work are listed in Supplementary Material.
REFERENCES
- 1.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. The Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U., Allen H.L., Lindgren C.M., Luan J.a., Magi R., et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scuteri A., Sanna S., Chen W.-M., Uda M., Albai G., Strait J., Najjar S., Nagaraja R., Orrú M., Usala G., et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R.B., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthoud H.R., Morrison C. The brain, appetite, and obesity. Ann. Rev. Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 6.Hofker M., Wijmenga C. A supersized list of obesity genes. Nat. Genet. 2009;41:139–140. doi: 10.1038/ng0209-139. [DOI] [PubMed] [Google Scholar]
- 7.Cecil J.E., Tavendale R., Watt P., Hetherington M.M., Palmer C.N.A. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 8.Timpson N.J., Emmett P.M., Frayling T.M., Rogers I., Hattersley A.T., McCarthy M.I., Davey Smith G. The fat mass- and obesity-associated locus and dietary intake in children. Am. J. Clin. Nutr. 2008;88:971–978. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardle J., Llewellyn C., Sanderson S., Plomin R. The FTO gene and measured food intake in children. Int. J. Obes. 2008;33:42–45. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 10.Haupt A., Thamer C., Staiger H., Tschritter O., Kirchhoff K., Machicao F., Häring H.U., Stefan N., Fritsche A. Variation in the FTO gene influences food intake but not energy expenditure. Exp. Clin. Endocrinol. Diabetes. 2009;117:194–197. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.J., Kim I.K., Kang J.H., Ahn Y., Han B.G., Lee J.Y., Song J. Effects of common FTO gene variants associated with BMI on dietary intake and physical activity in Koreans. Clin. Chim. Acta. 2010;411:1716–1722. doi: 10.1016/j.cca.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Sonestedt E., Roos C., Gullberg B., Ericson U., Wirfält E., Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 2009;90:1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 13.Tanofsky-Kraff M., Han J.C., Anandalingam K., Shomaker L.B., Columbo K.M., Wolkoff L.E., Kozlosky M., Elliott C., Ranzenhofer L.M., Roza C.A., et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am. J. Clin. Nutr. 2009;90:1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer F., Elbers C.C., Adan R.A.H., Loos R.J.F., Onland-Moret N.C., Grobbee D.E., van Vliet-Ostaptchouk J.V., Wijmenga C., van der Schouw Y.T. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am. J. Clin. Nutr. 2009;90:951–959. doi: 10.3945/ajcn.2009.27781. [DOI] [PubMed] [Google Scholar]
- 15.Corella D., Arnett D.K., Tucker K.L., Kabagambe E.K., Tsai M., Parnell L.D., Lai C.Q., Lee Y.C., Warodomwichit D., Hopkins P.N., et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J. Nutr. 2011;141:2219–2225. doi: 10.3945/jn.111.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do R., Bailey S.D., Desbiens K., Belisle A., Montpetit A., Bouchard C., Pérusse L., Vohl M.C., Engert J.C. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57:1147–1150. doi: 10.2337/db07-1267. [DOI] [PubMed] [Google Scholar]
- 17.Hakanen M., Raitakari O.T., Lehtimaki T., Peltonen N., Pahkala K., Sillanmaki L., Lagstrom H., Viikari J., Simell O., Ronnemaa T. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J. Clin. Endocrinol. Metab. 2009;94:1281–1287. doi: 10.1210/jc.2008-1199. [DOI] [PubMed] [Google Scholar]
- 18.Hasselbalch A.L., Angquist L., Christiansen L., Heitmann B.L., Kyvik K.O., Sorensen T.I. A variant in the fat mass and obesity-associated gene (FTO) and variants near the melanocortin-4 receptor gene (MC4R) do not influence dietary intake. J. Nutr. 2010;140:831–834. doi: 10.3945/jn.109.114439. [DOI] [PubMed] [Google Scholar]
- 19.Holzapfel C., Grallert H., Huth C., Wahl S., Fischer B., Doring A., Ruckert I.M., Hinney A., Hebebrand J., Wichmann H.E., et al. Genes and lifestyle factors in obesity: results from 12,462 subjects from MONICA/KORA. Int. J. Obes. 2010;34:1538–1545. doi: 10.1038/ijo.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubacek J.A., Pikhart H., Peasey A., Kubinova R., Bobak M. FTO variant, energy intake, physical activity and basal metabolic rate in Caucasians. The HAPIEE study. Physiol. Res. 2011;60:175–183. doi: 10.33549/physiolres.932066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonassaint C.R., Szatkiewicz J.P., Bulik C.M., Thornton L.M., Bloss C., Berrettini W.H., Kaye W.H., Bergen A.W., Magistretti P., Strober M., et al. Absence of association between specific common variants of the obesity-related FTO gene and psychological and behavioral eating disorder phenotypes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:454–461. doi: 10.1002/ajmg.b.31182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karasawa S., Daimon M., Sasaki S., Toriyama S., Oizumi T., Susa S., Kameda W., Wada K., Muramatsu M., Fukao A., et al. Association of the common fat mass and obesity associated (FTO) gene polymorphism with obesity in a Japanese population. Endocr. J. 2010;57:293–301. doi: 10.1507/endocrj.k09e-305. [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Zhu H., Lagou V., Gutin B., Stallmann-Jorgensen I., Treiber F., Dong Y., Snieder H. FTO variant rs9939609 is associated with body mass index and waist circumference, but not with energy intake or physical activity in European- and African-American youth. BMC. Med. Genet. 2010;11:57. doi: 10.1186/1471-2350-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpeläinen T.O., Qi L., Brage S., Sharp S.J., Sonestedt E., Demerath E., Ahmad T., Mora S., Kaakinen M., Sandholt C.H., et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8:e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Q., Chu A.Y., Kang J.H., Jensen M.K., Curhan G.C., Pasquale L.R., Ridker P.M., Hunter D.J., Willett W.C., Rimm E.B., et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Q., Li Y., Chomistek A.K., Kang J.H., Curhan G.C., Pasquale L.R., Willett W.C., Rimm E.B., Hu F.B., Qi L. Television watching, leisure time physical activity, and the genetic predisposition in relation to body mass index in women and men. Circulation. 2012;126:1821–1827. doi: 10.1161/CIRCULATIONAHA.112.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Loos R.J.F., Powell J.E., Medland S.E., Speliotes E.K., Chasman D.I., Rose L.M., Thorleifsson G., Steinthorsdottir V., Magi R., et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad T., Lee I.M., Paré G., Chasman D.I., Rose L., Ridker P.M., Mora S. Lifestyle Interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women . Diabetes Care. 2011;34:675–680. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor A.E., Sandeep M.N., Janipalli C.S., Giambartolomei C., Evans D.M., Kranthi Kumar M.V., Vinay D.G., Smitha P., Gupta V., Aruna M., et al. Associations of FTO and MC4R variants with obesity traits in Indians and the role of rural/urban environment as a possible effect modifier. J. Obes. 2011;2011:307542. doi: 10.1155/2011/307542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong M.Y., Day N.E., Luan J.A., Chan K.P., Wareham N.J. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int. J. Epidemiol. 2003;32:51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- 31.Wing M.R., Ziegler J.M., Langefeld C.D., Roh B.H., Palmer N.D., Mayer-Davis E.J., Rewers M.J., Haffner S.M., Wagenknecht L.E., Bowden D.W. Analysis of FTO gene variants with obesity and glucose homeostasis measures in the multiethnic Insulin Resistance Atherosclerosis Study cohort. Int. J. Obes. 2011;35:1173–1182. doi: 10.1038/ijo.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassanein M.T., Lyon H.N., Nguyen T.T., Akylbekova E.L., Waters K., Lettre G., Tayo B., Forrester T., Sarpong D.F., Stram D.O., et al. Fine mapping of the association with obesity at the FTO locus in African-derived populations. Hum. Mol. Genet. 2010;19:2907–2916. doi: 10.1093/hmg/ddq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant S.F.A., Li M., Bradfield J.P., Kim C.E., Annaiah K., Santa E., Glessner J.T., Casalunovo T., Frackelton E.C., Otieno F.G., et al. Association analysis of the FTO gene with obesity in children of Caucasian and African ancestry reveals a common tagging SNP. PLoS ONE. 2008;3:e1746. doi: 10.1371/journal.pone.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollepalli S., Dolan L.M., Deka R., Martin L.J. Association of FTO gene variants with adiposity in African-American adolescents. Obesity (Silver Spring) 2010;18:1959–1963. doi: 10.1038/oby.2010.82. [DOI] [PubMed] [Google Scholar]
- 35.Peters U., North K.E., Sethupathy P., Buyske S., Haessler J., Jiao S., Fesinmeyer M.D., Jackson R.D., Kuller L.H., Rajkovic A., et al. A Systematic mapping approach of 16q12.2/FTO and BMI in more than 20,000 African Americans narrows in on the underlying functional variation: results from the Population Architecture using Genomics and Epidemiology (PAGE) Study. PLoS Genet. 2013;9:e1003171. doi: 10.1371/journal.pgen.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poslusna K., Ruprich J., de Vries J.H.M., Jakubikova M., van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24-hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009;101:S73–S85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 37.Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5:889–892. doi: 10.1079/phn2002388. [DOI] [PubMed] [Google Scholar]
- 38.Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Bruning J.C., Ruther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 39.Church C., Moir L., McMurray F., Girard C., Banks G.T., Teboul L., Wells S., Bruning J.C., Nolan P.M., Ashcroft F.M., et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung M.K., Gulati P., O'Rahilly S., Yeo G.S.H. FTO expression is regulated by availability of essential amino acids. Int. J. Obes. 2013;37:744–777. doi: 10.1038/ijo.2012.77. [DOI] [PubMed] [Google Scholar]
- 41.Gulati P., Cheung M.K., Antrobus R., Church C.D., Harding H.P., Tung Y.C.L., Rimmington D., Ma M., Ron D., Lehner P.J., et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl. Acad. Sci. USA. 2013;110:2257–2262. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMurray F., Church C.D., Larder R., Nicholson G., Wells S., Teboul L., Tung Y.C.L., Rimmington D., Bosch F., Jimenez V., et al. Adult onset global loss of the FtoGene alters body composition and metabolism in the mouse. PLoS Genet. 2013;9:e1003166. doi: 10.1371/journal.pgen.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cota D., Proulx K., Smith K.A.B., Kozma S.C., Thomas G., Woods S.C., Seeley R.J. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 44.Li S., Zhao J.H., Luan J.a., Ekelund U., Luben R.N., Khaw K.T., Wareham N.J., Loos R.J.F. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk Prospective Population Study. PLoS Med. 2010;7:e1000332. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karra E., O'Daly O.G., Choudhury A.I., Yousseif A., Millership S., Neary M.T., Scott W.R., Chandarana K., Manning S., Hess M.E., et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 2013;123:3539–3551. doi: 10.1172/JCI44403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grau K., Hansen T., Holst C., Astrup A., Saris W.H.M., Arner P., Rossner S., Macdonald I., Polak J., Oppert J.M., et al. Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. Int. J. Obes. 2009;33:1227–1234. doi: 10.1038/ijo.2009.159. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Qi Q., Zhang C., Hu F.B., Sacks F.M., Qi L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: the POUNDS LOST Trial. Diabetes. 2012;61:3005–3011. doi: 10.2337/db11-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vimaleswaran K.S., Ängquist L., Hansen R.D., Van der A D.L., Bouatia-Naji N., Holst C., Tjønneland A., Overvad K., Jakobsen M.U., Boeing H., et al. Association between fto variant and change in body weight and its interaction with dietary factors: the DiOGenes Study. Obesity. 2012;20:1669–1674. doi: 10.1038/oby.2012.49. [DOI] [PubMed] [Google Scholar]
- 49.Sonestedt E., Gullberg B., Ericson U., Wirfält E., Hedblad B., Orho-Melander M. Association between fat intake, physical activity and mortality depending on genetic variation in FTO. Int. J. Obes. 2011;35:1041–1049. doi: 10.1038/ijo.2010.263. [DOI] [PubMed] [Google Scholar]
- 50.Phillips C.M., Kesse-Guyot E., McManus R., Hercberg S., Lairon D., Planells R., Roche H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012;142:824–831. doi: 10.3945/jn.111.153460. [DOI] [PubMed] [Google Scholar]
- 51.Moleres A., Ochoa M.C., Rendo-Urteaga T., Martínez-González M.A, Azcona San Julián M.C., Martínez J.A., Marti A. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br. J. Nutr. 2012;107:533–538. doi: 10.1017/S0007114511003424. [DOI] [PubMed] [Google Scholar]
- 52.Palla L., Higgins J.P.T., Wareham N.J., Sharp S.J. Challenges in the use of literature-based meta-analysis to examine gene-environment interactions. Am. J. Epidemiol. 2010;171:1225–1232. doi: 10.1093/aje/kwq051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.