Abstract
Background. Killing of bacterial pathogens by granulocytes is a saturable process, as previously demonstrated. There is virtually no quantitative information about how granulocytes interact with antimicrobial chemotherapy to kill bacterial cells.
Methods. We performed a dose-ranging study with the aminoglycoside plazomicin against Pseudomonas aeruginosa ATCC27853 in a granulocyte-replete murine pneumonia model. Plazomicin was administered in a humanized fashion (ie, administration of decrementing doses 5 times over 24 hours, mimicking a human daily administration profile). Pharmacokinetic profiling was performed in plasma and epithelial lining fluid. All samples were simultaneously analyzed with a population model. Mouse cohorts were treated for 24 hours; other cohorts treated with the same therapy were observed for another 24 hours after therapy cessation, allowing delineation of the therapeutic effect necessary to reduce the bacterial burden to a level below the half-saturation point.
Results. The mean bacterial burden (±SD) at which granulocyte-mediated kill was half saturable was 2.45 × 106 ± 6.84 × 105 colony-forming units of bacteria per gram of tissue (CFU/g). Higher levels of plazomicin exposure reduced the bacterial burden to <5 log10 CFU/g, allowing granulocytes to kill an additional 1.0–1.5 log CFU/g over the subsequent 24 hours.
Conclusions. For patients with large bacterial burdens (eg, individuals with ventilator-requiring hospital-acquired pneumonia), it is imperative to kill ≥2 log10 CFU/g early after treatment initiation, to allow the granulocytes to contribute optimally to bacterial clearance.
Keywords: mathematical modeling, P. aeruginosa pneumonia, antimicrobial therapy
Ventilator-requiring hospital-acquired bacterial pneumonia (VRHABP) caused by Pseudomonas aeruginosa remains a difficult therapeutic problem for clinicians. While antimicrobial therapy can be optimized [1], the relationship between the interaction of antibiotics and the bacterial cell killing that is mediated by granulocytes is unclear.
We recently demonstrated in both murine thigh and pneumonia models that bacterial cell killing by granulocytes is a saturable process [2, 3]. In both evaluations, antibiotics were not used. Granulocytes unaided by antibiotics were able to mediate substantial bacterial killing as long as the organism burden remained below the half-saturation point.
In this evaluation, we used the new aminoglycoside plazomicin in a granulocyte-replete murine pneumonia model [3] to examine the interaction of granulocytes with antimicrobial therapy. We initiated a pneumonia with a dense bacterial burden at therapy start and performed a humanized dose-ranging experiment [4]. Other treated cohorts of animals were observed for 24 hours after therapy cessation to examine the impact of granulocyte killing on the residual bacterial burden.
METHODS
Microorganism
P. aeruginosa strain ATCC 27853 was used. The minimum inhibitory concentration (MIC) of plazomicin was determined by the CLSI microbroth method [5].
Pharmacokinetic Studies
To correlate the doses of drug administered to mice with measures of exposure, pharmacokinetic studies were conducted in separate cohorts of mice with P. aeruginosa pneumonia. We administered plazomicin [6] on a humanized dosing scheme in which the drug was delivered 5 times over a 24-hour interval. The humanization scheme was derived from earlier single-dose pharmacokinetic studies performed with plazomicin (data not shown). The fraction of the total daily dose at each injection was 0.555, 0.238, 0.127, 0.047, and 0.033 mg/kg of body weight. These doses were administered 2, 6, 10, 16, and 22 hours, respectively, after bacterial challenge. Plazomicin total daily doses of 5.5, 10.9, 14.6, 18.2, 21.9, 43.8, 87.5, 175, and 225 mg/kg/day were evaluated.
Briefly, granulocyte-replete mice were infected with P. aeruginosa ATCC 27853 via the intranasal route. Two hours later, the humanized regimens for the total daily doses of plazomicin were administered to different cohorts of mice. Plasma and bronchoalveolar lavage (BAL) fluid specimens were collected from 3 euthanized mice per time point (and dosage) at 10 time points over the 24-hour pharmacokinetic study. Plasma and BAL fluid specimens were stored at −80°C until they were assayed for drug content by liquid chromatography with tandem mass spectrometry (LC/MS-MS). The prediluted concentration of antibiotic in the BAL fluid (which consisted of epithelial lining fluid [ELF]) was calculated by comparing the ratio of the amounts of urea measured in simultaneously collected plasma and BAL fluid specimens.
Murine Pneumonia Model
The model and detailed methods have been previously described [7]. All animal experimentation was approved by the local institutional animal care and use committee. A mouse pneumonia model previously described [7] was used. Female, 24–26-g, outbred Swiss-Webster mice (Taconic Farms, Taconic, NY) were provided water and food ad libitum. Anesthetized mice were infected via the intranasal route with a 20-µL volume, using 2 × 107 colony-forming units (CFU) of P. aeruginosa. The bacterial inoculum was confirmed by quantitative cultures. Two hours after bacterial inoculation and just prior to therapy initiation, 5 mice were euthanized for baseline quantitative cultures of lung homogenates.
Eighteen animal cohorts were administered plazomicin. There was also an untreated control group (cohorts 19 and 20). Twenty-six hours after infection, therapy was stopped, and 1 untreated control group and 9 cohorts of treated mice were humanely euthanized. The other control group and 9 treated cohorts were followed to hour 50. At this time, these groups were humanely euthanized.
The tissues were homogenized and washed with normal saline solution to prevent drug carryover. Homogenates were then quantitatively cultured onto drug-free agar to characterize the effect of each regimen on the total bacterial population.
After incubation of the plates at 35°C for 48 hours, colonies were enumerated. Means and standard deviations for the quantitative culture samples were calculated.
Assays of Plazomicin and Urea Levels in Plasma and BAL Fluid Specimens
Mouse plasma samples (0.050 mL) were deproteinated with acetonitrile (0.150 mL). The samples were centrifuged, and an aliquot of the supernatant (0.050 mL) was transferred into an appropriately labeled autosampler vial containing 1.00 mL of high-pressure liquid chromatography (HPLC)–grade water. Samples were analyzed by high-pressure LC/MS-MS for plazomicin concentrations. Mouse BAL fluid samples (0.050 mL) were diluted with 0.100 mL of HPLC-grade water and were also analyzed by high-pressure LC/MS-MS for plazomicin concentration determinations. The LC/MS-MS system was composed of a Shimadzu Prominence HPLC system and an Applied Biosystems/MDS Sciex API5000 LC/MS-MS system.
For plazomicin, the coefficient of variation over 0.1–100 mg/L (2 regression curves) was 2.15%–8.35% for plasma. For BAL fluid, these values ranged from 1.54% to 9.17%. For urea, the range of the coefficient of variation was 3.63%–10.5% for plasma and 5.85%–9.77% for BAL.
Mathematical Models
As mentioned above, we performed a separate pharmacokinetic study in infected mice. This model has been described previously [7]:
| (1) |
| (2) |
| (3) |
and
| (4) |
where CL/F is defined as the apparent plasma clearance rate; Ka is the first order absorption rate constant; Kgmax is the first order growth rate constant; K23, K32, K24, and K42 are first order intercompartmental transfer rate constants; VELF is the volume of the ELF compartment; and X1–X4 represent the amount of plazomicin in the absorption compartment (ie, the site of injection), the central compartment (rapidly exchangeable volume, closely associated with blood), the rest of the mouse, and the ELF, respectively. The plasma concentration of plazomicin is calculated as X2/[Vc/F], where Vc is defined as the volume of the central compartment. The plazomicin concentration in the ELF is defined as X4/VELF. F is absolute bioavailability which was not measured.
The relationship between drug exposure and cell killing, as well as the cell killing attributable to granulocytes, was also evaluated, as follows:
| (5) |
and
| (6) |
where Kkillmax is the maximal kill rate constant for plazomicin therapy, KkillWBC is the maximal kill rate attributable to granulocytes; PlazoKill is the sigmoid Emax function for cell kill, in which C50-Plazo-Kill is the plazomicin concentration at which the plazomicin-attributable kill is half maximal and H is Hill's constant; Popmax is the maximal size of the bacterial population; and WBCKill50 is the bacterial burden at which granulocyte-mediated kill is half saturable. In the system output, CFU/g values are log10 transformed.
The BigNPAG program described by Leary et al [8] was used for population modeling. The inverse of the estimated observation variance was used for weighting. Bayesian estimates were obtained using the population-of-one utility in BigNPAG. Model evaluation was performed by predicted-observed plots. The mean weighted error served as the measure of bias. The bias-adjusted weighted mean squared error served as the measure of precision.
Simulation was performed using the ADAPT V package of programs [9].
RESULTS
Plazomicin MIC and Mutational Frequency to Plazomicin Resistance
The plazomicin MIC for the challenge strain was 2 mg/L, as determined in triplicate. The mutational frequency to plazomicin resistance for P. aeruginosa ATCC 27853 ranged from 1/389 045 colonies (−5.59 log10 CFU/mL) to 1/426 580 colonies (−5.63 log10 CFU/mL).
Murine Pharmacokinetics of Plazomicin
The mean and median parameter vectors as well as the estimates of standard deviations of the parameter values for the population are shown in Table 1. By simulation, the fractional penetration (AUCELF/AUCPLASMA) for plazomicin into ELF was 0.876.
Table 1.
Pharmacokinetic Parameter Values
| Measure | Vc, L | CL/F, L/H | K23, H−1 | K32, H−1 | K24, H−1 | K42, H−1 | VELF, L | Ka, × 1/H |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 0.0189 ± 0.0164 | 0.0301 ± 0.0219 | 11.09 ± 2.221 | 10.69 ± 3.555 | 6.120 ± 2.297 | 13.74 ± 2.147 | 0.0045 ± 0.0025 | 28.04 ± 1.775 |
| Median | 0.0071 | 0.0109 | 9.060 | 13.92 | 4.292 | 15.45 | 0.0023 | 28.86 |
Abbreviations: CL/F, apparent plasma clearance rate; H, Hill's constant; Ka, first order absorption rate constant; K23, K24, K32, K42, first order intercompartmental transfer rate constants; Vc/F, apparent volume of the central compartment; VELF, volume of the epithelial lining fluid compartment.
The model fit the data acceptably well. For the pre-Bayesian (population) estimation, the plasma concentration predicted-observed plot had the following regression line: observed = 1.40 × predicted–1.336; r2 = 0.983; bias = 1.770; precision = 96.75.
For the ELF concentrations, the predicted-observed plot had the following regression line: observed = 1.940 × observed−1.940; r2 = 0.934; bias = −0.304; precision = 2.465.
For the Bayesian (individual) analysis, the plasma concentration predicted-observed plot the following regression line: observed = 1.315 × predicted–0.306; r2 = 0.994; bias = −0.922; precision = 9.878.
For the ELF concentrations, the predicted-observed plot the following regression line: observed = 1.083 × observed + 0.0613; r2 = 0.998; bias = −0.181; precision = 0.719.
The median estimates were used for fitting drug exposure and the impact of white blood cells on colony counts over time in the second pharmacodynamic population analysis.
Pharmacodynamics of Plazomicin and Impact of Granulocytes on Cell Killing in Murine Lungs
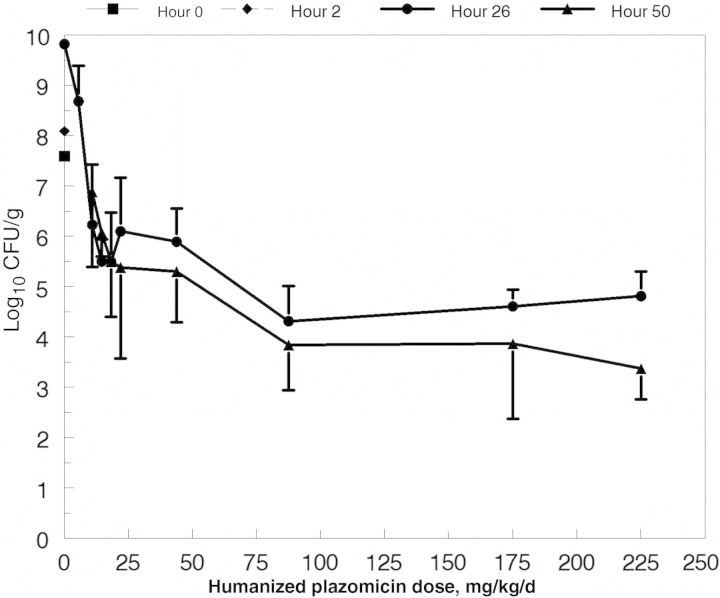
The colony counts over time are displayed for all groups in Figure 1. No animals from the untreated control group or the lowest dosing group for plazomicin survived to hour 50. Colony counts in the other lower dosing groups (10.9 and 14.6 mg/kg/day) displayed net increases between hours 26 and 50. This transitioned to stasis at intermediate doses (18.2 and 21.9 mg/kg/day) and, at higher doses (≥43.8 mg/kg/day), there was net cell kill between hours 26 and 50, even though there was no further plazomicin dosing after hour 20 and plazomicin concentrations were <0.61 mg/L in plasma and <0.56 mg/L in ELF at hour 26 (plazomicin MIC, 2.0 mg/L).
Figure 1.
Colony counts of Pseudomonas aeruginosa ATCC 27853 in the lungs of mice. Cohorts were euthanized at baseline (2 hours after challenge), at hour 26, and at hour 50. The difference between the data for 26 h and 50 h reflects the influence of granulocytes acting alone on the bacterial burden at hour 26. The untreated controls and the group treated with the lowest dose of plazomicin (5.5 mg/kg/d) were humanely euthanized at hour 26.
The point estimates and measures of dispersion of the pharmacodynamic model parameters are displayed in Table 2. Again, the fit of the model to the data was acceptable. For the pre-Bayesian regression, the predicted-observed plot had the following regression line: observed = 1.178 × predicted–1.200; r2 = 0.747; bias = 0.011; precision = 3.339.
Table 2.
Parameter Values for the Pharmacodynamic Model
| Measure | Kgmax, H−1 | Kkillmax, H−1 | C50-Plazo-Kill, mg/L | H | Popmax, CFU | IC5, CFU | KkillWBC, H−1 | WBCKill50, CFU/g |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 0.523 ± 1.370 | 2.578 ± 2.305 | 15.32 ± 5.408 | 16.77 ± 2.277 | 9.08 × 109 ± 2.23 × 109 | 8.50 × 107 ± 2.87 × 107 | 0.730 ± 1.007 | 2.45 × 106 ± 6.84 × 105 |
| Median | 0.057 | 1.256 | 16.94 | 18.49 | 9.86 × 109 | 5.60 × 107 | 0.354 | 2.68 × 106 |
In the system output, CFU/g values are log10 transformed.
Abbreviations: C50-Plazo-Kill, plazomicin concentration at which the plazomicin-attributable kill is half maximal; H, Hill's constant; IC5, initial bacterial burden at therapy initiation; Kgmax, first order growth rate constant; Kkillmax, maximal kill rate constant for plazomicin therapy; KkillWBC, maximal kill rate attributable to granulocytes; Popmax, maximal size of the bacterial population; WBCKill50, bacterial burden at which granulocyte-mediated kill is half saturable.
For the Bayesian (individual) estimation, the predicted-observed had the following regression line: observed = 1.104 × predicted - 0.724; r2 = 0.896; bias = 0.079; precision = 1.402.
For this output, the P value was << .001.
The estimate for WBCk50 was 2.68 × 106 ± 6.84 × 105 CFU/g in this experiment. In previous experiments [2, 3], the estimate for this parameter was 4.30 × 106 ± 3.75 × 106 (in a murine thigh infection model) and 2.15 × 106 ± 2.66 × 106 (in a murine pneumonia model).
DISCUSSION
How antimicrobial therapy interacts with granulocyte clearance of pathogens at an infection site has been a neglected question. There are infections in which it is known that obtaining therapy deemed “adequate” early in the therapeutic course has a major impact on the infection outcome. An example is staphylococcal bacteremia [10]. Another example is VRHABP [11, 12]. For the latter, “adequate” is merely the use of an agent to which the pathogen of interest is considered “susceptible.”
In this investigation, we have built on previous work [2, 3] in which we demonstrated that granulocytes can have a major effect on the clearance of bacterial pathogens (P. aeruginosa and Staphylococcus aureus were studied in murine models of pneumonia and thigh infection). These investigations were performed without the use of antimicrobial therapy. We demonstrated that granulocyte-mediated bacterial cellular killing is saturable and that the half-saturation point is slightly in excess of 106 CFU/g of tissue, both for S. aureus (thigh infection model) and P. aeruginosa (thigh infection and pneumonia models).
Here, we examined whether this effect is altered when antimicrobial therapy is initiated. In this study, we examined a range of plazomicin doses (an aminoglycoside antimicrobial) such that we would expect in this granulocyte-replete model, some mice would have their initial bacterial burden remain above the half-saturation point in spite of the (lower) drug exposures, while others would remain in the vicinity of the half-saturation point while the higher drug exposures would reduce the bacterial burden below this point. The last fraction of the total daily dose was administered at hour 22 (challenge was at hour 2), ensuring that both plasma and ELF drug concentrations would be far below the MIC by hour 26. Cohorts of animals were then observed for another 24 hours.
The results were clear-cut. A large mathematical model was fit to the colony count data. We were able to demonstrate that both drug exposure and granulocyte-mediated bacterial cell killing had an important effect on bacterial clearance.
The model demonstrated that, after plazomicin levels declined below an effective level, the granulocytes were able to mediate an important level of bacteria killing over the succeeding 24 hours (hours 26–50), as long as the plazomicin therapy could bring the P. aeruginosa colony counts substantially below the half-saturation point (Figure 1).
Table 2 shows that the half-saturation point for granulocyte-mediated bacterial killing (parameter WBCK50) was essentially unchanged from that seen in our previous investigations (2.68 × 106 ± 6.84 × 105 CFU/g here vs 2.15 × 106 ± 2.66 × 106 for murine pneumonia and 4.30 × 106 ± 3.75 × 106 CFU/g for murine thigh infection). This indicates that the granulocytes are likely an independent source of bacterial cell clearance. The effect of the antimicrobial, while highly important on its own, attains added importance when it brings the bacterial burden down to a level that allows the granulocytes to kill an additional 1-2 log bacterial CFU over the subsequent 24 hours. This will hasten the resolution of the infection.
Such optimization of both antimicrobial killing and granulocyte-mediated bacterial killing likely has special importance in the therapy of VRHABP. Multiple authors [11–14] have demonstrated the importance of early adequate therapy. These data may provide the explanation for these clinical findings.
VRHABP is an infection in which the bacterial burden is very high. The definition of pneumonia after BAL is the finding of ≥104 CFU/mL of BAL fluid. This is the resultant colony count after dilution by the BAL fluid. Previous investigations have shown that this dilution factor is on the order of 30–100-fold [15], as demonstrated by urea dilution between plasma and BAL fluid. Consequently, the bacterial burden after BAL in a documented case of VRHABP is >3 × 105 CFU/mL. This is at the bottom of the range specified in the definition of the condition. All other patients will have greater burdens. Given our identified half-saturation point, attainment of early, adequate chemotherapy and its subsequent salutary effect becomes understandable.
Zaccard et al [16] examined bilateral BAL specimens from patients with suspected VRHABP. There were 134 samples from patients infected with P. aeruginosa, Enterobacter species, K. pneumonia, Acinetobacter species, and Serratia marcescens. After correction for the ≥30-fold dilution (which was not done in the study by Zaccard et al), the bacterial baseline burdens were distributed as shown in Table 3. Greater than a majority of patients had a baseline bacterial burden that met or exceeds the half-saturation point seen in our studies. The burden in one quarter of patients was near the range of full saturation. We speculate that this observation is a major reason that these patients are so difficult to treat successfully. In many, the granulocytes are essentially taken out of the equation by the size of the bacterial burden. This large burden also markedly increases the likelihood of resistance emergence, because in many instances the organism number will exceed the inverse of the mutational frequency of resistance. Rapid emergence of resistance has been documented during therapy in randomized trials for this condition, particularly in P. aeruginosa [17, 18]. Finally, this also provides insight into why early adequate antimicrobial therapy has a salutary impact on patient outcome. Aggressive, adequate therapy will reduce the bacterial burden below the half-saturation point and allow granulocytes to increase bacterial clearance. Rapid clearance of the bacterial infection may result in better clinical outcomes. Alvarez-Lerma et al demonstrated that, in patients with hospital-acquired bacterial pneumonia [19], early, adequate antimicrobial therapy significantly lowered attributable mortality, the number of complications per patient, and the incidence of shock. The results of our investigation are concordant with those of the study by Alvarez-Lerma et al [19] but are also more detailed. Here, the mean half-saturation point (WBCK50) was 2.45 × 106 CFU/g (95% confidence interval [CI], 1.11 × 106–3.79 × 106 CFU/g), and the median was 2.68 × 106 CFU/g.
Table 3.
Dilution-Transformed Colony Counts for Patients With Gram-Negative Pneumonia Documented by Bronchoalveolar Lavage Analysis
| Counta | Patients, No. (%) |
|---|---|
| ≥3 × 105 | 49 (36.6) |
| ≥3 × 106 | 50 (37.3) |
| ≥3 × 107 | 35 (26.1) |
| Total | 134 (100) |
Figure 1 and Table 3 show that near-maximal granulocyte-mediated bacterial cell killing requires that the bacterial burden be reduced by chemotherapy to ≤105 CFU/g. Table 3 shows that this reduction can be achieved in a relatively high fraction of patients if 2 log10 CFU/g are killed early after therapy initiation. Consequently, when therapeutic regimens are being designed for patients with VRHABP, the minimal pharmacodynamic target (such as that seen in an animal model or from a hollow fiber system evaluation) should be to kill 2 log10 CFU/g. It would also be important to also attain resistance suppression. For the latter, particularly with P. aeruginosa infections, initiation of combination therapy may be the best way to attain these goals [20–22]. These findings set the stage for performing a clinical trial to validate this hypothesis.
Finally, duration of therapy remains an issue for these patients. Chastre et al [23] performed a randomized, double-blind trial comparing 8-day with 15-day courses of antimicrobial therapy in 401 patients with VRHABP. There were no mortality differences between the groups. The one major difference seen was in the subgroup of patients infected with nonfermenting gram-negative bacilli (eg, P. aeruginosa and Acinetobacter species). Here, there was a statistically significant difference in the recurrence rate that favored a longer therapy duration (40.6% vs 25.4%; absolute difference, 15.2% [90% CI, 3.9%–26.6%]). However, among patients who developed recurrent pulmonary infections, multidrug-resistant pathogens emerged less frequently in those who had received antibiotics for 8 days (42.1% vs 62.0%; P = .04).
We have performed this particular experiment with a single isolate of P. aeruginosa. It may be that other isolates, particularly tolerant organisms or strains that are particularly slow growing, will respond differently. Further experimentation is needed to confirm this.
By identifying a pharmacodynamic bacterial killing target of >2 log10 CFU/g for these patients, we can hopefully reduce the number of recurrences, shorten the therapy duration, and decrease the emergence of resistance. Having the help of granulocytes in bacterial clearance is key to attaining these outcomes.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01AI079578 and RO1AI079729 to the Institute for Therapeutic Innovation).
Potential conflicts of interest. R. C. is an employee of Achaogen. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scaglione F, Esposito S, Leone S, et al. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur Resp J. 2009;34:394–400. doi: 10.1183/09031936.00149508. [DOI] [PubMed] [Google Scholar]
- 2.Drusano GL, Vanscoy B, Liu W, Fikes S, Brown D, Louie A. Saturability of granulocyte kill of Pseudomonas aeruginosa in a murine model of pneumonia. Antimicrob Agents Chemother. 2011;55:2693–5. doi: 10.1128/AAC.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drusano GL, Fregeau C, Liu W, Brown DL, Louie A. Impact of burden on granulocyte clearance of bacteria in a mouse thigh infection model. Antimicrob Agents Chemother. 2010;54:4368–72. doi: 10.1128/AAC.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deziel M, Heine H, Louie A, et al. Identification of effective antimicrobial regimens for use in humans for the therapy of Bacillus anthracis infections and post-exposure prophylaxis. Antimicrob Agents Chemother. 2005;49:5099–106. doi: 10.1128/AAC.49.12.5099-5106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. Document M7-A7. [Google Scholar]
- 6.Zhanel GG, Lawson CD, Zelinitsky S, et al. Comparison of the next generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infective Ther. 2012;10:459–73. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 7.Drusano GL, Lodise TP, Melnick D, et al. Meropenem penetration into epithelial lining fluid in mice and men and delineation of exposure targets. Antimicrob Agents Chemother. 2011;55:3406–12. doi: 10.1128/AAC.01559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models. Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems; Bethesda, MD. IEEE Computer Society; 2001. pp. 389–94. [Google Scholar]
- 9.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles, CA: Biomedical Simulations Resource; 2009. [Google Scholar]
- 10.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–23. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 11.Fagon JY, Chastre J. Antimicrobial treatment of hospital-acquired pneumonia. Clin Chest Med. 2005;26:97–104. doi: 10.1016/j.ccm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Koulenti D, Rello J. Gram-negative bacterial pneumonia: aetiology and management. Curr Opin Pulm Med. 2006;12:198–204. doi: 10.1097/01.mcp.0000219269.73180.5d. [DOI] [PubMed] [Google Scholar]
- 13.Piskin N, Aydemir H, Oztoprak N, et al. Inadequate treatment of ventilator-associated and hospital-acquired pneumonia: risk factors and impact on outcomes. BMC Infect Dis. 2012;12:268. doi: 10.1186/1471-2334-12-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollef MH. Appropriate empiric antimicrobial therapy of nosocomial pneumonia: the role of the carbapenems. Respir Care. 2004;49:1530–41. [PubMed] [Google Scholar]
- 15.Lodise TP, Sörgel F, Mason B, Melnick D, Kinzig M, Drusano GL. Penetration of meropenem into epithelial lining fluid in intubated patients with nosocomial pneumonia. Antimicrob Agents Chemother. 2011;55:1606–10. doi: 10.1128/AAC.01330-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaccard CR, Schell RF, Spiegel CA. Efficacy of bilateral bronchoalveolar lavage for diagnosis of ventilator-associated pneumonia. J Clin Microbiol. 2009;47:2918–24. doi: 10.1128/JCM.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink MP, Snydman DR, Niederman MS, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother. 1994;38:547–57. doi: 10.1128/aac.38.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peloquin CA, Cumbo TJ, Nix DE, Sands MF, Schentag JJ. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med. 1989;149:2269–73. [PubMed] [Google Scholar]
- 19.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996;22:387–94. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 20.Louie A, Grasso C, Bahniuk N, et al. The combination of meropenem and levofloxacin is synergistic with respect to both Pseudomonas aeruginosa kill rate and resistance suppression. Antimicrob Agents Chemother. 2010;54:2646–54. doi: 10.1128/AAC.00065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drusano GL, Bonomo RA, Bahniuk N, et al. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:231–42. doi: 10.1128/AAC.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louie A, Liu W, Fikes S, Brown D, Drusano GL. Impact of meropenem in combination with tobramycin in a murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 2013;57:2788–92. doi: 10.1128/AAC.02624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. J Amer Med Assoc. 2003;290:2588–98. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]