Abstract
Background. Sexually transmitted infections (STIs) are associated with an increased risk of human immunodeficiency virus (HIV) infection, but their biological effect on HIV susceptibility is not fully understood.
Methods. Female pig-tailed macaques inoculated with Chlamydia trachomatis and Trichomonas vaginalis (n = 9) or medium (controls; n = 7) were repeatedly challenged intravaginally with SHIVSF162p3. Virus levels were evaluated by real-time polymerase chain reaction, plasma and genital cytokine levels by Luminex assays, and STI clinical signs by colposcopy.
Results. Simian/HIV (SHIV) susceptibility was enhanced in STI-positive macaques (P = .04, by the log–rank test; relative risk, 2.5 [95% confidence interval, 1.1–5.6]). All STI-positive macaques were SHIV infected, whereas 3 controls (43%) remained uninfected. Moreover, relative to STI-negative animals, SHIV infections occurred earlier in the menstrual cycle in STI-positive macaques (P = .01, by the Wilcoxon test). Levels of inflammatory cytokines (interferon γ, interleukin 6, and granulocyte colony-stimulating factor [G-CSF]) were higher in STI-positive macaques during STI inoculation and SHIV exposure periods (P ≤ .05, by the Wilcoxon test).
Conclusions. C. trachomatis and T. vaginalis infection increase the susceptibility to SHIV, likely because of prolonged genital tract inflammation. These novel data demonstrate a biological link between these nonulcerative STIs and the risk of SHIV infection, supporting epidemiological assocations of HIV and STIs. This study establishes a macaque model for studies of high-risk HIV transmission and prevention.
Keywords: HIV risk, STI or STD, Chlamydia, Trichomonas, menstrual cycle, macaque, HIV susceptibility model
Sexually transmitted infection (STI) can increase the risk of human immunodeficiency virus (HIV) infection by 2–5-fold [1–5]. It is unclear whether this association is largely attributable to sexual or behavioral practices or to an underlying STI-associated biological mechanism. Human studies of STI coinfection and HIV susceptibility are often precluded because of study size, confounding behaviors, and ethical considerations. The use of a macaque model of STI and HIV coinfection would help to address these questions and also provide a model for preclinical testing of biomedical HIV prevention strategies. Because of the high STI prevalence among individuals at high risk for HIV infection [3, 4, 6], rigorous testing of these strategies in the context of STIs would help to ensure broad efficacy of prevention methods among varying populations.
A current focus of HIV prevention research is identifying effective preexposure prophylaxis (PrEP) strategies for high-risk groups. PrEP with Truvada was recently approved by the Food and Drug Administration for use in men who have sex with men (MSM), HIV-discordant couples, and other high-risk populations (eg, injection drug users) [7, 8]. However, some PrEP strategies in women have shown limited efficacy (as in the CAPRISA cohort) or no efficacy (as in the VOICE and FemPrEP cohorts) [9–11]. In addition to addressing adherence issues, it is also important to investigate biological factors that could lower PrEP efficacy in women, such as reproductive hormone levels, coinfections, and inflammation in the female genital tract. We and others have used female pig-tailed macaques to study topical and systemic PrEP efficacy [12, 13] and HIV susceptibility factors unique to the female host [14]. These macaques model human female reproductive tract physiology, have year-round lunar menstrual cycles, and can be vaginally infected with simian/HIV (SHIV) without progestin treatment. In these macaques, using a repeat, low-dose SHIV challenge model, we showed that susceptibility to SHIV infection is inconsistent throughout the menstrual cycle and peaks during the late-luteal and menses phases [14, 15]. With repeated low-dose exposures in pig-tailed macaques, we can carefully study factors affecting HIV susceptibility by evaluating completed menstrual cycles during virus exposures (as opposed to challenges required for infection) to account for the varying susceptibility a woman experiences throughout her menstrual cycle [13, 16–18]. We recently established a pig-tailed macaque model of concomitant STI and HIV infection [19], providing an effective system with which to assess effects of both STIs and hormonal changes on susceptibility to vaginal SHIV transmission.
Studies of macaques with concomitant STI and HIV infection are limited, with only Crostarosa et al reporting on the effect of herpes simplex virus type 2 (HSV-2) infection on SHIV susceptibility in Depo-Provera-treated rhesus macaques [20]. Yet a macaque STI model could be used to conclusively assess whether these infections do in fact increase the risk of HIV infection, to investigate associated mechanisms, and to rigorously assess HIV prevention methods. The study described here uses our previously developed pig-tailed macaque triple infection STI-SHIV model [19] to evaluate the effect of non-ulcerative STIs (ie, Chlamydia trachomatis and Trichomonas vaginalis infections) on SHIV susceptibility. Patton et al have established C. trachomatis infection models in several macaque species, investigated Chlamydia and SHIV coinfection in pig-tailed macaques, and have also studied T. vaginalis infection in pig-tailed macaques [21–24]. C. trachomatis and T. vaginalis were also selected because infections with these pathogens are epidemiologically associated with an increased risk of HIV infection and are highly prevalent among individuals at high risk for HIV [3, 6, 25, 26]. From an early report by Laga et al, women with Chlamydia or Trichomonas infections were almost 4 and 2 times as likely, respectively, to become HIV-positive [3]. These pathogens induce tissue inflammation and may also cause abrasions to the genital tract epithelium, potentially facilitating access of virus to target cells [27–30]. Cell populations involved in clearing STIs are also targets for HIV infection (and for simian immunodeficiency virus [SIV] and SHIV infections), particularly in the case of C. trachomatis and T. vaginalis [30–34]. It is hypothesized these combined factors increase susceptibility to mucosal HIV transmission. This current study incorporates methods developed in our laboratory [19] and the Patton laboratory and reports increased susceptibility to SHIV infection among macaques coinfected with C. trachomatis and T. vaginalis, introduces possible mechanisms for enhanced susceptibility in vaginal transmission, and indicates a definitive role for these coinfections in the risk of HIV infection, apart from sexual or behavioral practices alone. Moreover, this study establishes a macaque model of genital tract inflammation for studies of high-risk HIV transmission and prevention.
METHODS
Macaques and Study Design
Sixteen female pig-tailed macaques (Macaca nemestrina) were housed and studied at the Centers for Disease Control and Prevention (CDC). All procedures were approved by the CDC Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC). Enrolled macaques were evaluated during a baseline period lasting 8–12 weeks, during which blood specimens and genital secretions were collected and progesterone levels monitored to determine menstrual cycle phases (Figure 1). Plasma samples were assayed for progesterone levels at the Wisconsin National Primate Research Center. Day 1 of the menstrual cycle was designated as the first sampling time point after the steep decline in progesterone levels [14]. Daily observations of perineal tumescence [35] and detection of menstrual blood supplemented and confirmed determinations of progesterone-based menstrual cycle phase.
Figure 1.
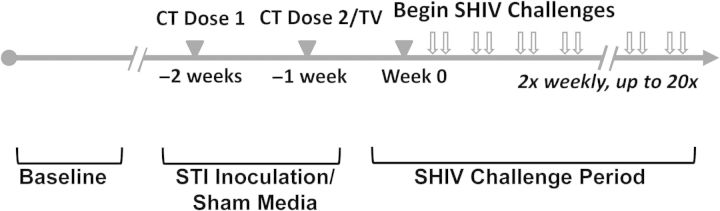
Study design. Analyses focused on 3 primary study stages: baseline, sexually transmitted pathogen (or sham media) inoculation period, and simian/human immunodeficiency virus (SHIV) challenge period. Abbreviations: CT, Chlamydia trachomatis; TV, Trichomonas vaginalis. Progesterone levels were measured once or twice weekly for 8–12 weeks during baseline (and throughout SHIV challenge period) to accurately assess menstrual cycle phase.
During the STI inoculation period, 9 macaques received inoculations of C. trachomatis and T. vaginalis, and 7 macaques received sham inoculation medium (control macaques). SHIV challenges began a median of 9 days after the start of the menstrual cycle (range, 5–18 days), and STI inoculations preceded challenge start by 2 weeks (Figure 1). Specimens of blood and genital secretions were collected, and STI diagnostic tests and colposcopy were performed.
Sample Collection
Blood was collected in cell preparation tubes and fractionated by centrifugation. Plasma aliquots were stored at −80°C. Genital secretions were collected twice weekly by placing ophthalmic sponges at the cervical os for 3 minutes to absorb secretions and were processed as described by Castle et al [36]. Eluates were stored at −80°C. Sponge eluates were available for analysis from 7 STI-positive macaques and 5 control macaques. Genital secretions were also collected for STI diagnostic tests, performed according to respective kit protocols.
STI Inoculation and Diagnostic Tests
C. trachomatis serovar D (1 × 106 inclusion-forming units) was applied twice, at a one-week interval, directly to the cervical mucosa, as previously described [19] (Figure 1). Control animals received sham inoculation media (sucrose-phosphate glutamate) [19]. C. trachomatis infection was monitored with the APTIMA Combo 2 test (Gen-Probe, San Diego, CA). T. vaginalis was propagated in culture and applied directly to the vaginal mucosa (5 × 106 trichomonads), as previously described [19]. T. vaginalis infection was monitored with the Trichomonas InPouch culture system (BioMed Diagnostics, White City, OR). Clinical signs of both STIs (mucosal erythema, edema, petechiae, abrasions, ulcerations, and/or discharge) were observed by colposcopy and documented with a standardized questionnaire [19]. At study conclusion, STI-positive macaques were administered azithromycin and metronidazole to clear C. trachomatis and T. vaginalis infections, respectively.
SHIVSF162p3 Challenge, Detection, and Virus Level Measurement
SHIVSF162p3 was provided by the National Institutes of Health AIDS Research and Reference Reagent Program (catalog no. 6526) and propagated at the CDC on pig-tailed macaque peripheral blood mononuclear cells. Macaques were challenged twice weekly in a repeat low-dose challenge model [37] for up to 20 challenges or until SHIV infection was confirmed. Previous studies used this virus stock at a challenge dose of 50 tissue culture infective doses (TCID50), but in this study a 5-fold lower, suboptimal challenge dose (10 TCID50) was used to delay infections in control macaques to potentially better observe enhanced susceptibility with STIs.
An in-house real-time polymerase chain reaction (PCR) assay was used to measure SHIV RNA levels, as previously described [19, 38]. Levels were reported as the number of SHIV RNA copies per milliliter of plasma. Two subsequent SHIV RNA–positive plasma samples were required before ceasing challenges. Infections were confirmed with an in-house SHIV proviral real-time PCR assay and enzyme immunoassay (EIA) detection of HIV envelope–specific antibodies (Bio-Rad HIV-1/HIV-2 Plus O EIA, Hercules, CA).
Analysis of Infections, Using Completed Menstrual Cycles During Virus Exposures
Pig-tailed macaques have lunar menstrual cycles, and their susceptibility to vaginal SHIV infection varies during the menstrual cycle [14, 15]. Thus, in this repeated low-dose vaginal challenge study, infection data and susceptibility were evaluated by analyzing the number of completed menstrual cycles during virus exposures (thus, 1 full menstrual cycle is equivalent to 1 SHIV susceptibility period). Menstrual cycle patterns of each macaque were determined by analyzing progesterone levels (Figure 2 and Supplementary Materials). For the analysis, a completed menstrual cycle begins on the start day of SHIV challenges in 1 cycle and ends on that same day in the following menstrual cycle. Subsequent complete cycles were similarly determined. We then determined the first day of SHIV RNA detection and the deduced day of SHIV infection by subtracting a 7-day period from the SHIV RNA detection date, to correct for the period between infection and quantifiable RNA detection (often referred to as a viral eclipse period; Figure 2 and Supplementary Figure 1). For example, in Figure 2, challenges began in macaque DA53 on day 5 of the menstrual cycle, with the first complete cycle ending on day 5 of next cycle. In this animal, because plasma SHIV RNA was detected on day 14 of the second cycle, SHIV infection was deduced as having occurred on day 7 of that cycle. Because the deduced day of infection (day 7) occurred outside the first completed menstrual cycle, we concluded that 2 cycles were required for SHIV infection (Figure 2). The same rationale was applied to all macaques, and descriptions are provided for each macaque in Supplementary Figure 1 and summarized in Supplementary Table 1.
Figure 2.
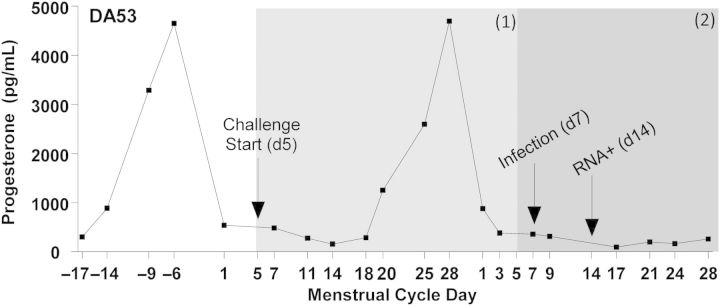
Representative menstrual cycle time line, relative to simian/human immunodeficiency virus (SHIV) susceptibility periods. Progesterone levels were plotted and used to determine menstrual cycle day, with day 1 of each cycle designated as the day following the steep decline in the hormone level. For analysis purposes, 1 menstrual cycle equals 1 SHIV susceptibility period, and during SHIV exposures, a single susceptibility period was defined from the menstrual cycle day on which SHIV challenges began until the same respective day in the following cycle, as indicated by gray shading. Macaques which became infected within 1 menstrual cycle had a deduced viral eclipse–corrected day of infection within the first susceptibility period, or in the case of DA53 (as shown), SHIV infection occurred in the second menstrual cycle, requiring 2 susceptibility periods for infection. For uninfected macaques in the control arm, up to 3 susceptibility periods were observed without infection (Supplementary Materials).
Measurement of Cytokine and Chemokine Levels
Cytokine and chemokine levels were assayed from both plasma and genital secretion eluates, using a Luminex assay format. Levels of interferon γ (IFN-γ), interleukin 2, interleukin 4, interleukin 10 (IL-10), tumor necrosis factor α, interleukin 8, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α and 1β, and RANTES were measured with the Invitrogen Monkey Cytokine and Chemokine 5-Plex panels. Interleukin 6 (IL-6), interleukin 12, interleukin 17, and granulocyte colony-stimulating factor (G-CSF) levels were determined with single-plex kits (Invitrogen catalog nos. LPC0001, LPC0002, LPC0061, LPC0121, LPC0171, and LPC2031, respectively; Carlsbad, CA). For genital secretions, levels were normalized on the basis of the weight of collected secretions. Levels were reported as picograms per milliliter of plasma or secretion. Statistical analysis of median levels per subject was performed with the Holm-Bonferroni correction [39].
RESULTS
Confirmation and Management of STIs
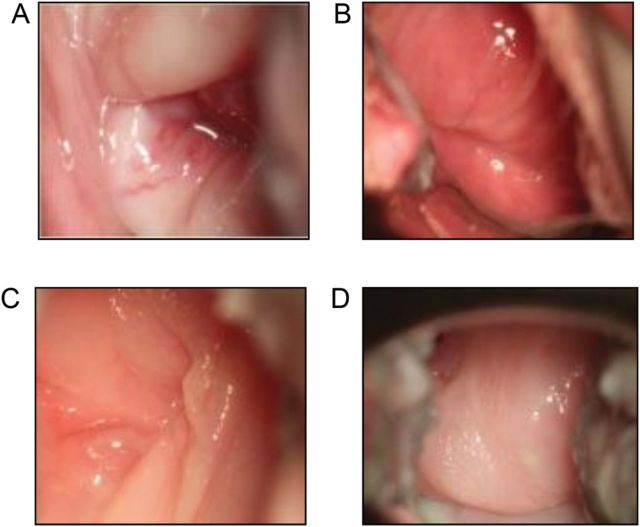
Dual C. trachomatis and T. vaginalis infections were confirmed in each STI-inoculated macaque (n = 9) within one week of inoculation. STI-positive animals had clinical signs such as mucosal erythema, edema, and discharge, and in 3 macaques, petechiae on the cervical mucosa were observed. Colposcopy images of representative STI-positive and control macaques are shown in Figure 3. In STI-positive macaques, clinical signs were most severe 7–10 days after inoculation with C. trachomatis and T. vaginalis, with erythema and discharge persisting to varying degrees throughout the study. Normal, menstrual-related discharge was observed in both groups.
Figure 3.
Representative colposcopy images. Tissue edema, erythema, vascularization (A), and petechiae (B) of 2 representative sexually transmitted infection–positive animals (DA53 and FJ55). Normal cervical mucosa of 2 representative control macaques (DK52 and PYH2; C and D).
Increased Susceptibility to SHIV Infection in STI-Positive Macaques
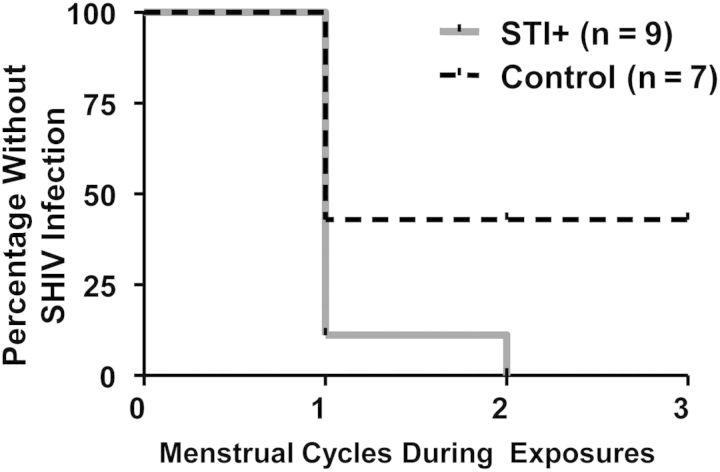
The start of virus exposure varied between days and 5–18 of the menstrual cycle, but the range of menstrual cycle challenge start days was similar by study group (see methods). All (9 of 9) STI-positive animals became SHIV infected, and 89% (8 of 9) were infected within 1 menstrual cycle. The remaining STI-positive animal (animal DA53) was SHIV infected within 2 menstrual cycles (Figure 2 and Supplementary Materials). Only 57% of controls (4 of 7) became SHIV infected, despite up to 20 SHIV challenges over the course of 3 menstrual cycles. For uninfected macaques, only completed menstrual cycles were assessed, regardless of additional exposures in subsequent, incomplete cycles (eg, animals DK52, PHQ1, and 96Po17; Supplemental Figure,). Figure 4 depicts the Kaplan–Meier survival analysis among STI-positive macaques, compared with controls. STI-positive macaques were infected within fewer menstrual cycles, compared with controls, indicating an enhanced susceptibility to SHIV infection due to STI coinfection (P = .04, by the log–rank test). These data are corroborated by Fisher's exact analyses comparing the number of cycles with and the number without SHIV infection between the 2 study groups (P = .02) The ratio of infection probabilities (ie, the number infected per total number of menstrual cycles), or relative risk, in STI-positive animals, compared with control animals, was 2.5 (95% confidence interval, 1.1–5.6).
Figure 4.
Susceptibility periods during simian/human immunodeficiency virus challenges/exposures. Kaplan–Meier survival analysis comparing the number of susceptibility periods, or completed menstrual cycles, required for infection among sexually transmitted infection (STI)–positive (gray line) versus control (dashed black line) animals. STI-positive animals were infected within fewer menstrual cycles, compared with controls (P = .04, log–rank test).
Association of SHIV RNA Detection With Menstrual Cycle Phase
Plasma SHIV RNA detection was plotted in temporal relation to the respective menstrual cycle day for all SHIV-positive macaques. SHIV RNA detection was more commonly observed later in a menstrual cycle in STI-positive macaques, compared to controls (P = .01, by the Wilcoxon test; Figure 5). The median day of SHIV RNA detection for STI-positive animals was day 19 of the menstrual cycle, compared with day 4 among 4 control animals. The observation among control animals was similar to our previous mean viremia detection on day 6 in 19 STI-naive pig-tailed macaques in a previous study [14].
Figure 5.
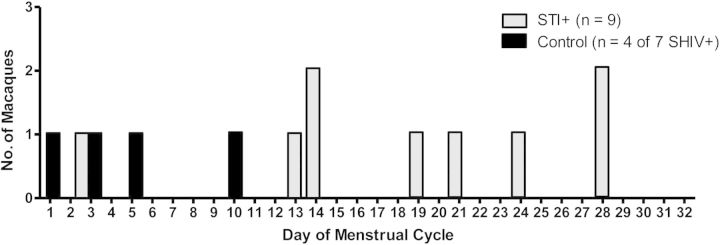
For each simian/human immunodeficiency virus (SHIV)–positive animal (9 of 9 in the sexually transmitted infection [STI]–positive group and 4 of 7 in the control group), the sampling time point at which plasma SHIVSF162p3 RNA was first detected was plotted by the respective day of the menstrual cycle. Plasma progesterone levels were monitored throughout the study course, and day 1 was defined as the time point immediately following the steep progesterone decline (as described in Figure 2). Plasma SHIVSF162p3 RNA in STI-positive animals (grey bars) was detected later in the menstrual cycle, compared with controls (black bars; P = .01, by the Wilcoxon test).
Effect of STI Coinfection on SHIV Plasma Viremia
Plasma SHIV RNA levels were monitored twice weekly. Longitudinal plasma virus levels were not significantly different between study groups (P > .05, by area under the curve and generalized estimating equations analyses; Figure 6A). For SHIV-uninfected macaques 96Po17, DK52, and PHQ1, levels were undetectable and were excluded from analyses. For STI-infected macaques, the median peak plasma virus level was 7.7 log10 (range, 5.69–9.20 log10 copies/mL), compared to 6.4 log10 among controls (range, 5.42–7.02 log10 copies/mL; Figure 6B). This difference was not significant (P = .2, by the Wilcoxon test).
Figure 6.
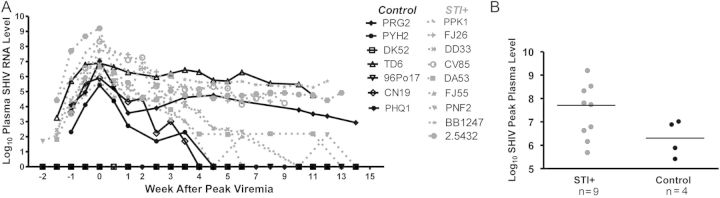
A, Longitudinal plasma simian/human immunodeficiency virus (SHIV) RNA levels. Log10 SHIV RNA levels plotted (y-axis) for sexually transmitted infection (STI)–positive (grey dashed) and control (black solid) animals, relative to the time of peak viremia (x-axis). Differences in levels in SHIV-infected animals between the 2 study groups were not statistically significant (96Po17, DK52, and PHQ1 were excluded from analyses as uninfected macaques). B, Peak plasma SHIV RNA levels. No significant difference was noted between SHIV-infected, STI-positive (grey) and control macaques. Hash indicates median levels.
Cytokine and Chemokine Analyses
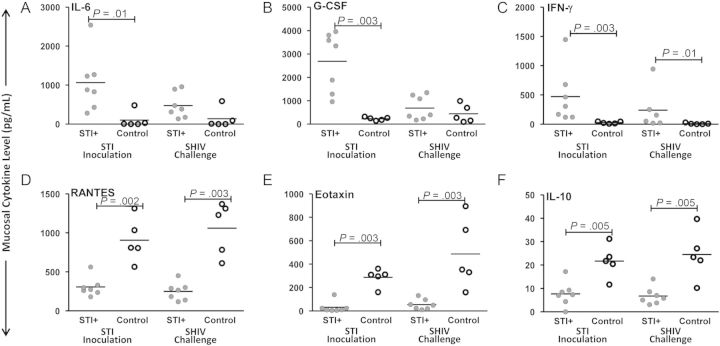
In genital mucosal secretions, significant differences between study groups were observed for levels of both inflammatory (Figure 7A–C) and HIV-inhibitory/anti-inflammatory (Figure 7D–F) mediators during the STI inoculation and SHIV challenge periods (Figure 1). Inflammatory cytokines IL-6, G-CSF, and IFN-γ (Figure 7A–C; P = ≤.05, by the Wilcoxon test) were higher in the STI-positive group, compared with the control group, during the STI inoculation phase. Levels of IFN-γ remained significantly higher in the STI-positive group through the SHIV challenge period (P = .01, by the Wilcoxon test). Control animals had higher levels of HIV-inhibitory chemokines RANTES and eotaxin and higher levels of antiinflammatory cytokine IL-10 during both the STI inoculation and SHIV challenge phases, compared with STI-positive animals (Figure 7D–F; P ≤ .002, by the Wilcoxon test). Cytokine and chemokine levels were intermittently detectable and lower in plasma, with only modest differences between study groups (P > .05) for IL-6 and IFN-γ during the SHIV challenge period (data not shown).
Figure 7.
Median per-subject levels of cervicovaginal (mucosal) cytokines. Analytes measured from available samples of 7 sexually transmitted infection (STI)–positive and 5 control macaques. Median levels of the inflammatory markers interleukin 6 (IL-6; A), granulocyte colony-stimulating factor (G-CSF; B), and interferon γ (IFN-γ; C) were significantly higher during the sexually transmitted pathogen inoculation period in STI-positive animals, compared with control animals (P = ≤ .01, by the Wilcoxon test); levels of IFN-γ remained higher during the SHIV challenge period (P = .01). Median levels of the human immunodeficiency virus–inhibitory chemokines RANTES (D) and eotaxin (E) and the downregulatory cytokine interleukin 10 (IL-10; F) were significantly higher in control animals versus STI-positive animals during both the STI inoculation and SHIV challenge phases (P ≤ .002, by the Wilcoxon test).
DISCUSSION
This study is the first to determine the effect of C. trachomatis and T. vaginalis infection on the risk of HIV infection in a nonhuman primate model, and we observed a 2.5-fold risk of infection among macaques coinfected with these sexually transmitted pathogens. This difference in susceptibility was similar to those found by Crostarosa et al in a study of HSV-2/SIV coinfection, in which 100% of Depo-Provera–treated, HSV-2–infected macaques (6 of 6) became SIV infected, compared with only 46% of controls (6 of 13; P < .05) [20], equating to approximately twice the risk. With the exception of HSV-2 infection, limited animal data are available on effects of STIs on HIV susceptibility. Our data describe a biological link between 2 non-ulcerative genital tract infections and an enhanced risk of HIV infection. Although epidemiological associations between STIs and the risk of HIV transmission have been reported [1, 3], the role of STIs, particularly non-ulcerative STIs, in HIV risk has been debated because of sexual and behavioral confounders. However, this study's findings, coupled with those of the study by Crostarosa et al [20], definitively associate STIs with an increased risk of HIV infection and provide plausible mechanisms of susceptibility.
We hypothesize that the mechanism of increased susceptibility is genital tract inflammation and potential recruitment of SHIV target cells, and evidence of cervicovaginal inflammation was observed in this cohort. In the HSV-2 study by Crostarosa et al, increased susceptibility was noted after lesions healed, suggesting that ulcerations may not be the only factor contributing to the increased risk [20]. Instead, resulting inflammation may underlie enhanced susceptibility to SHIV infection. In this study, clinical STI signs peaked 7–10 days after infection and then waned by 4–6 weeks after infection. Related to this, we observed increased levels of inflammatory mediators in STI-positive macaques during the STI inoculation period, which persisted during SHIV challenges (Figure 7). These inflammatory mediators elicit the recruitment and activation of potential viral target cells and increase blood permeability in tissues, which could enhance susceptibility to mucosal SHIV transmission. Moreover, Poli et al described the ability of IL-6 to induce HIV replication and propagate an inflammatory state [40], while others reported an association between HIV infection and elevated systemic and genital IL-6 levels [41, 42]. Additionally, the suppression of RANTES may also increase susceptibility by increasing concentrations of unbound CCR5 viral coreceptors [43]. Even STI treatment may not fully reduce inflammatory effects on susceptibility. Crostarosa et al observed an increased susceptibility to SIV, despite resolution of herpetic lesions [20]. Fichorova et al noted that even the common treatment for trichomoniasis, metronidazole, may amplify the parasitic inflammatory response [44]. Also, Garcia-Lerma et al reported that natural dATP substrate levels in target tissues affected the efficacy of tenofovir-based prevention methods [45]; this balance of substrate levels could be disrupted by inflammation and cell infiltrate. It is plausible that STI-induced inflammation and cell recruitment may alter drug substrate concentrations, thereby decreasing the efficacy of reverse transcriptase–based prevention approaches. These collective data provide new insight for the consideration of STI confounders in clinical HIV studies, microbicide trials, or vaccine trials because of the likelihood of lingering inflammation after acute infection or treatment increasing HIV susceptibility. Moreover, the targeted demographic for these interventions experiences a high STI prevalence with possible recurring infections amplifying risk. We intend to use this concomitant STI-HIV coinfection model to address these questions and rigorously test PrEP and microbicide modalities to ensure broad efficacy. The use of nonhuman primate STI coinfection models for evaluation of biomedical preventions may better inform the selection of microbicides or vaccines for human clinical trials.
To our knowledge, this is the first report on SHIV susceptibility studies using a STI coinfection and SHIV repeat low-dose model in pig-tailed macaques. The study by Crostarosa et al used noncycling, progesterone-treated macaques receiving single SHIV challenge doses [20]. This model does not account for varying susceptibility due to the menstrual cycle or for effects of physiologically relevant repeated, low-dose virus exposures. In cycling pig-tailed macaques, SHIV susceptibility varies during the cycle; therefore, differences in susceptibility per SHIV challenge precluded analyses of the numbers of exposures to infection, as was previously performed [37]. Instead, we considered numbers of completed menstrual cycles during exposures to assess increased risk or susceptibility to infection, as has been recently implemented [13, 16–18]. Also, to better observe enhanced susceptibility, we used a suboptimal virus dose, with the hope of delaying infections in the controls, relative to the expected accelerated onset of infections in STI-positive animals. We found that control macaques either became infected later in their first cycles or remained uninfected over multiple cycles, rather than experiencing delayed infections throughout. This finding was unexpected and raises the possibility that minor variability in individual animal susceptibility may be exacerbated when very low-dose virus challenges are used, thus reducing power. Alternative macaque model designs to evaluate susceptibility, such as an animal infectious dose, as described in the study of Schistosoma mansoni–SIV coinfection performed by Chenine et al [46], could be explored.
Interestingly, SHIV RNA in STI-positive animals was more often detected later in a menstrual cycle (Figure 5), compared with real-time controls and with 19 historic controls (data not shown [14]), when SHIV RNA was detected earlier in a menstrual cycle. If a 7-day correction factor is used to account for the time between exposure and our detection of viral RNA, STI-positive animals would have primarily been infected earlier in the menstrual cycle, compared with controls. This was an unexpected finding. It is unclear why STIs might widen the window of susceptibility to SHIV infection, relative to the menstrual cycle. Our data suggest that STIs may negate the hypothesized innate level of resistance during the follicular phase [14]. STIs may induce a chemokine and cytokine milieu (decreased RANTES, eotaxin, and IL-10 expression and increased IL-6, IFN-γ, and IL-1β expression; Figure 7) that recruits and activates viral target cells and also increases blood vessel density to a level typically seen only in the late luteal phase. Thus, the milieu could convert this thick epithelium from a protective barrier to a tissue that facilitates virus entry and infection. Another possibility is that a synergistic relationship may exist between STI-induced discharge and menstrual cycle–related discharge that more efficiently transports virus to target cells and tissues. Further research is needed to characterize target cell infiltration, mucus, and mucosal immunity during STIs to explain early transmission events when STIs are present. Additionally, most STI-positive animals went through a luteal phase without becoming SHIV infected (For example, Supplementary Figure 1D). Again, this was an unexpected finding and is an area for future study to better understand dynamics of SHIV infection in the context of STI coinfection.
Our data show that C. trachomatis and T. vaginalis coinfection increases the risk of SHIV infection in pig-tailed macaques. A similar susceptibility enhancement may also exist for other highly prevalent, non-ulcerative STI pathogens, such as Neisseria gonorrhoeae or Mycoplasma genitalium. Furthermore, our model of concomitant STI and HIV infection can be used to comprehensively evaluate the efficacy of HIV prevention modalities in the context of STIs, as it is possible that PrEP efficacy may be diminished in the presence of genital tract infections. The collective goals from these data are to both inform public health policy and implement highly effective intervention methods for a combined approach to HIV prevention.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Debra Adams, for her assistance in the propagation of the SHIVSF162p3 challenge stocks; Dr Michael Hendry and Dr Kevin Karem, for their support of these studies; and Dr Ajay Vishwanathan, Dr. Charles Dobard, and Dr. David Garber, for their consultations and input on study design.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC) and by the National Institutes of Health and CDC (interagency agreement Y1-AI-0681-02).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 2.Gray RH, Wawer MJ, Sewankambo NK, et al. Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai District, Uganda. Rakai Project Team. AIDS. 1999;13:2113–23. doi: 10.1097/00002030-199910220-00015. [DOI] [PubMed] [Google Scholar]
- 3.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC. STDs, viral load, circumcision, and the transmission of HIV. In: Braun JF, Horn T, editors. The PRN handbook. New York City: Physicians Research Network; 2000. [Google Scholar]
- 5.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–7. doi: 10.1097/01.olq.0000154504.54686.d1. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Global Prevalence and Incidence of Selectable Curable Sexually Transmitted Infections Overview and Estimates. www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf. Accessed 13 November 2012.
- 7.Lifson JD, Haigwood NL. Lessons in nonhuman primate models for AIDS vaccine research: from minefields to milestones. Cold Spring Harb Perspect Med. 2012;2:a007310. doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR. 2011;60:65–8. [PubMed] [Google Scholar]
- 9.Microbicide Trials Network. MTN statement on decision to discontinue use of Tenofovir gel in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3909. Accessed 21 May 2013.
- 10.Microbicide Trials Network. MTN statement on decision to discontinue use of oral Tenofovir tablets in VOICE, a major HIV prevention study in women. http://www.mtnstopshiv.org/node/3619. Accessed 21 May 2013.
- 11.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One. 2012;7:e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vishwanathan SA, Guenthner PC, Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–4. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 15.Kersh E, Henning T, Vishwanathan SA, et al. SHIV Susceptibility Changes during the Menstrual Cycle of Pigtail Macaques. J Med Primatol. 2014 doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86:718–25. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobard C, Sharma S, Parikh UM, et al. Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007701. 227ra35. [DOI] [PubMed] [Google Scholar]
- 18.Smith JM, Rastogi R, Teller RS, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A. 2013;110:16145–50. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henning T, Fakile Y, Phillips C, et al. Development of a pigtail macaque model of sexually transmitted infection/HIV coinfection using Chlamydia trachomatis, Trichomonas vaginalis, and SHIV(SF162P3) J Med Primatol. 2011;40:214–23. doi: 10.1111/j.1600-0684.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crostarosa F, Aravantinou M, Akpogheneta OJ, et al. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One. 2009;4:e8060. doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton DL, Cosgrove-Sweeney Y, Agnew K, Hillier SL. Concurrent STI: development of a macaque model for C. trachomatis and T. vaginalis infections. Proceedings of the Eleventh International Symposium on Human Chlamydial Infections; Niagara-on-the-Lake, Canada: 2006. pp. 417–20. [Google Scholar]
- 22.Patton DL, Sweeney YT, Agnew KJ, Balkus JE, Rabe LK, Hillier SL. Development of a nonhuman primate model for Trichomonas vaginalis infection. Sex Transm Dis. 2006;33:743–6. doi: 10.1097/01.olq.0000218871.89901.61. [DOI] [PubMed] [Google Scholar]
- 23.Patton DL, Sweeney YT, Paul KJ. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009;36:350–6. doi: 10.1097/OLQ.0b013e318195c31a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton D, Butler K, Cosgrove-Sweeney YT, Tsai CC, Doncel G, Saifuddon M. Chlamydia trachomatis plus SHIV: co-infection model development in the pigtailed macaque. In: Chernesky M, editor. Chlamydia infections. Ontario, Canada: Elsevier Biomedical Press; 2006. pp. 412–24. [Google Scholar]
- 25.Plummer FA, Simonsen JN, Cameron DW, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–9. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 26.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 27.Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(suppl 2):S114–25. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks E, Tam MA, Lycke NY. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog. 2010;6:e1001179. doi: 10.1371/journal.ppat.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear GT, Kendrick SR, Chen HY, et al. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PLoS One. 2011;6:e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65:89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 31.Ficarra M, Ibana JS, Poretta C, et al. A distinct cellular profile is seen in the human endocervix during Chlamydia trachomatis infection. Am J Reprod Immunol. 2008;60:415–25. doi: 10.1111/j.1600-0897.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol. 2012;189:2441–9. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Schust DJ, Ibana JA, Buckner LR, et al. Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Curr HIV Res. 2012;10:218–27. doi: 10.2174/157016212800618093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim. 1981;15:351–3. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 36.Castle PE, Rodriguez AC, Bowman FP, et al. Comparison of ophthalmic sponges for measurements of immune markers from cervical secretions. Clin Diagn Lab Immunol. 2004;11:399–405. doi: 10.1128/CDLI.11.2.399-405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otten RA, Adams DR, Kim CN, et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191:164–73. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 38.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–11. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 39.Holm S. A simple sequentially rejective multiple test procedure. J Scand Stat. 1979;6:65–70. [Google Scholar]
- 40.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–4. [PubMed] [Google Scholar]
- 42.Spear GT, Zariffard MR, Chen HY, et al. Positive association between HIV RNA and IL-6 in the genital tract of Rwandan women. AIDS Res Hum Retroviruses. 2008;24:973–6. doi: 10.1089/aid.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 44.Fichorova RN, Lee Y, Yamamoto HS, et al. Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One. 2012;7:e48418. doi: 10.1371/journal.pone.0048418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Lerma JG, Aung W, Cong ME, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011;85:6610–7. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chenine AL, Shai-Kobiler E, Steele LN, et al. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. 2008;2:e265. doi: 10.1371/journal.pntd.0000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.