Abstract
Background:
In the United States, patients who enroll in chemotherapy trials seldom reflect the attributes of the general population with cancer, as they are often younger, more functional, and have less comorbidity. We compared survival following three chemotherapy regimens according to the setting in which care was delivered (ie, clinical trial vs usual care) to determine the generalizability of clinical trial results to unselected elderly Medicare patients.
Methods:
Using SEER-Medicare data, we estimated survival for elderly patients (ie, age 65 years or older, n = 14097) with advanced pancreatic or lung cancer following receipt of one of three guideline-recommended first-line chemotherapy regimens. We compared their survival to that of similarly treated clinical trial enrollees, without age restrictions, with the same diagnosis and stage (n = 937). All statistical tests were two-sided.
Results:
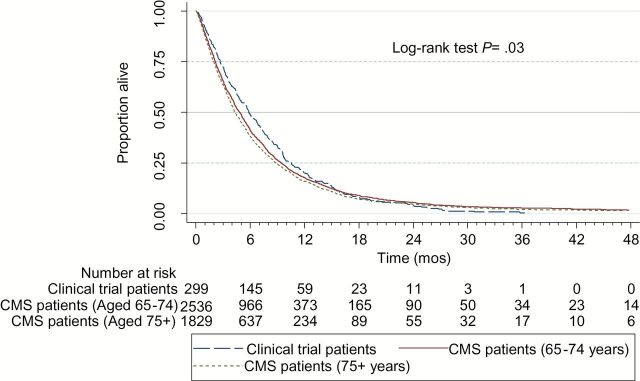
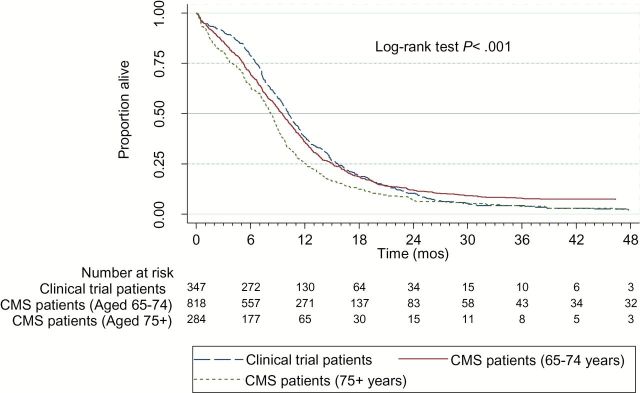
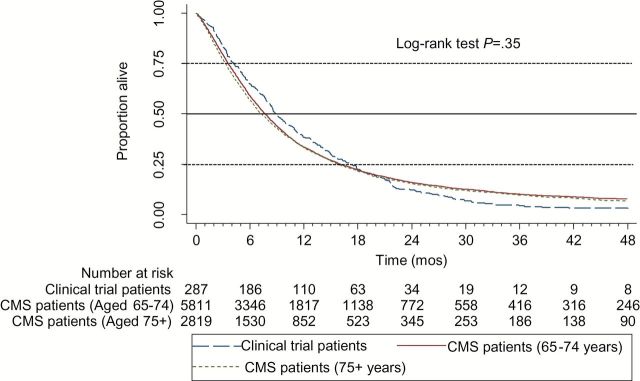
Trial patients were 9.5 years younger than elderly Medicare patients. Medicare patients were more often white and tended to live in areas of greater educational attainment than trial enrollees. For each tumor type, Medicare patients who were 75 years or older had median survivals that were six to eight weeks shorter than those of trial patients (4.3 vs 5.8 months following treatment with single agent gemcitabine for advanced pancreatic cancer, P = .03; 7.3 vs 8.9 months following treatment with carboplatin and paclitaxel for stage IV non–small cell lung cancer, P = .91; 8.2 vs 10.2 months following treatment with CDDP/ VP16 for extensive stage small cell lung cancer, P ≤ .01), whereas younger Medicare patients had survival times that were similar to those of trial patients.
Conclusions:
Results of clinical trials for advanced pancreatic cancer and lung cancers tended to correctly estimate survival for Medicare patients aged 65 to 74 years, but to overestimate survival for older Medicare patients by six to eight weeks. These estimates of Medicare patients’ survival may aid subsequent patients and their oncologists in treatment decision-making.
Medicare spends billions of dollars annually on chemotherapy for treatment of elderly cancer patients, yet surprisingly little is known about its effectiveness for this large patient population (1). National guidelines that identify first-line cancer site and stage-specific “standard of car” chemotherapy regimens are primarily informed by results of large, randomized clinical trials, which often enroll comparatively young and healthy patients (2,3). These guidelines are then applied, often without evidence, to treatment of age-eligible (or “elderly”) Medicare patients with cancer who are treated in the usual-care setting (ie, outside of clinical trials). The dearth of realistic estimates of chemotherapy-related benefits may hinder informed treatment decision-making for such patients and their oncologists. Authors of the recent Institute of Medicine (IOM) report entitled “Delivering high-quality cancer care: Charting a new course for a system in crisis” highlight the critical need to “expand the breadth of data collected on cancer interventions for older adults and individuals with multiple comorbid conditions” (4).
Consistent with the IOM’s charge, we sought: 1) to estimate the generalizability of trial results to usual-care elderly Medicare patients and 2) to describe “real world” survival estimates following treatment of elderly Medicare patients with guideline-recommended regimens. Specifically, we compared the survival of patients with advanced pancreatic and lung cancers following treatment with the standard first-line chemotherapy regimens according to the setting in which treatment was delivered (ie, clinical trial vs Medicare usual care). These are rapidly fatal cancers and reporting survival time according to treatment setting provides needed prognostic information regarding these patients who are nearing the end of life at the time of diagnosis.
Methods
The research was approved by the Harvard Medical School Committee on Human Subjects, the Dana-Farber Harvard Cancer Center Institutional Review Board, the Duke University Institutional Review Board, and the Principal Investigators of each of the Surveillance, Epidemiology, and End Results (SEER) Registries.
SEER-Medicare Data
We used observational data from the National Cancer Institute’s (NCI’s) SEER-Medicare program to identify elderly Medicare patients who were treated with standard first-line chemotherapy regimens in the usual-care setting. Through the creation of a unique patient identifier, the NCI has made possible patient-level linkage of SEER cancer registry information to Centers for Medicare and Medicaid Services (CMS) health care utilization information for Medicare beneficiaries. The SEER program collects information regarding patients from geographically diverse registries in order to monitor trends in incidence and survival (5). The program collects patient demographics and detailed information about the cancer diagnosis, including date of diagnosis, site, histology, stage, and date of death. Data include certain initial anticancer therapies.
Medicare is a federally sponsored health insurance program administered by CMS, the beneficiaries of which include more than 96% of all US citizens aged 65 years and older (1). CMS maintains billing records of outpatient, inpatient, home health, hospice, and other claims for all beneficiaries not enrolled in risk contract health maintenance organizations (HMOs). For chemotherapy to be identifiable, patients must be eligible for Medicare part B. We required that between the time period of the first day of the month and year of diagnosis through six months following that date, SEER-Medicare patients could not be enrolled in an HMO. In addition, they had to be continuously enrolled in Medicare parts A and B for those six months to ascertain first-line intravenous chemotherapy use in the outpatient setting.
We used SEER data to identify all Medicare patients diagnosed alive in SEER registries at or after age 65 between 1993 and 2009 with advanced pancreatic cancer, extensive stage small cell lung cancer (ES SCLC), or stage IV non–small cell lung cancer (NSCLC). Patients were staged according to the SEER historic stage convention (6). The sample was limited to patients with no known prior cancers documented in SEER. Supplementary Table 1 and Supplementary Figure 1 (available online) describe treatment restrictions. We then studied the Medicare patients’ claims to identify those receiving first-line treatment with one of three chemotherapy regimens of interest (ie, gemcitabine (n = 4365), cisplatin/etoposide (CDDP/VP16) (n = 1102), or carboplatin and paclitaxel (n = 8630) using previously validated methods (7). Supplementary Table 2 (available online) defines the treatment schedules. In an intent-to-treat approach, we required that the regimen of interest was the first chemotherapy regimen SEER-Medicare patients received following their cancer diagnosis (mirroring clinical trial requirements) and that all of the day-one chemotherapy drugs were administered on the same day using an approach we have described previously (7). This eliminated patients treated on apparently nonstandard schedules. Supplementary Table 3 (available online) contains a description of the Medicare files and codes that we used to identify the regimens of interest.
Cancer and Leukemia Group B (CALGB) Data
We used data from the NCI-sponsored cooperative clinical trial group CALGB, now a part of the Alliance for Clinical Trials in Oncology, to identify patients with advanced pancreatic or lung cancers who were treated with standard first-line chemotherapy regimens in the clinical trial setting. All trial participants had signed informed consent for trial treatment. The CALGB represented a geographically disparate network of physicians, academic medical centers, and community hospitals. Data from the trials were collected and maintained centrally at the CALGB Statistical Center. Among the data collected in a consistent manner across all therapeutic trials was registration information, which included study number, participant identifiers, demographic and disease information, treatment information, and survival endpoints. We used data from the CALGB to identify clinical trial patients (of all ages) who were treated with standard first-line chemotherapy regimens as part of the control arm of phase III clinical trials. That is, we identified all CALGB study subjects with the same cancer sites, histology, and stages who were treated between 1998 and 2006 on a phase III trial containing a control arm that included the standard first-line chemotherapy regimens of interest. The corresponding studies are CALGB 80303, which included a single agent gemcitabine arm (n = 300) (8), CALGB 9732, which included a CDDP/VP16 arm (n = 349) (9), and CALGB 9730, which included a carboplatin and paclitaxel arm (n = 288) (10).
Variables
All-cause mortality, which was available in both data sources, served as the outcome variable. The essential predictor variable was treatment setting: clinical trial vs usual care. Demographic and disease variables were used to compare the cohorts by venue.
Statistical Approach
For each of the three tumor site-, histology-, stage-, and treatment-specific cohorts, we visualized and compared survival by treatment setting and usual-care Medicare patient age using the Kaplan-Meier (K-M) method and log-rank tests. All statistical tests were two-sided. Because the age distributions of clinical trial patients and elderly Medicare usual-care patients did not fully overlap, and because there were few and often incomplete covariables common to both data sources, multivariable analyses were not undertaken.
All analyses were performed using STATA 10 (STATA, College Station, TX) at Harvard Medical School. A P value of less than or equal to .05 was considered statistically significant.
Results
Covariables
On average, 42.5% of the usual-care elderly SEER-Medicare patients (43397/102046) received some chemotherapy during the six months following cancer diagnosis in the ambulatory setting. Among those patients, 32.5% (14 097/43397) received one of the first-line regimens of interest, though there was considerable variation across cohorts in the percent of patients who received the regimen. Ten point five percent (1102/10731) of ES SCLC patients received first-line CDDP/VP16, 32.5% (8633/26256) of NSCLC patients received first-line carboplatin and paclitaxel, and 68.1% (4365/6410) of advanced pancreatic cancer patients received first-line single-agent gemcitabine. Consistent with the intent-to-treat approach, 100% of each of the three cancer site-, stage-, and treatment-specific usual care cohorts were assumed to have received the treatment of interest if they had evidence of having received the drug combination of interest on day one.
Table 1 shows that, compared with usual-care elderly Medicare patients, the clinical trial patients were on average approximately ten years younger. Supplementary Figure 2 (available online) contains a histogram depicting this difference. The usual-care and trial cohorts were similar with respect to patient sex, except that members of the NSCLC clinical trial cohort were more likely to be male than the usual-care Medicare cohort. On average, patients treated in the clinical trial setting were more likely to be African American and to reside in neighborhoods where a smaller proportion of residents had attended college.
Table 1.
Attributes of patients with advanced pancreatic and lung cancers according to treatment regimen and setting (n = 15034)
| Variable | Advanced pancreatic cancer | Stage IV NSCLC | ES SCLC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gemcitabine n = 4665 | Carboplatin/paclitaxel n = 8918 | CDDP/VP16 n = 1451 | |||||||
| T+* (n = 300) | T-† (n = 4365) | P‡ | T+* (n = 288) | T-† (n = 8630) | P | T+* (n = 349) | T-† (n = 1102) | P‡ | |
| Age, mean, y | 64.4 | 73.7 | .001 | 63.3 | 72.5 | .001 | 61.5 | 71.4 | .001 |
| Age, range, y | 39–86 | 65–96 | - | 42–83 | 65–93 | - | 39–82 | 65–92 | - |
| Male | 0.51 | 0.49 | .52 | 0.68 | 0.59 | .001 | 0.55 | 0.54 | .71 |
| Race | .001 | .001 | .28 | ||||||
| White | 0.88 | 0.85 | 0.78 | 0.87 | 0.91 | 0.91 | |||
| Black | 0.08 | 0.09 | 0.14 | 0.08 | 0.07 | 0.05 | |||
| Other | 0.04 | 0.06 | 0.08 | 0.05 | 0.02 | 0.04 | |||
| Marital Status | § | .001 | .001 | § | .001 | ||||
| Married | - | 0.63 | 0.56 | 0.63 | - | 0.60 | |||
| Widowed | - | 0.21 | 0.08 | 0.19 | - | 0.22 | |||
| Single | - | 0.07 | 0.08 | 0.07 | - | 0.05 | |||
| Divorced | - | 0.06 | 0.18 | 0.08 | - | 0.09 | |||
| Separated | - | 0.01 | 0.02 | <0.01 | - | <0.01 | |||
| Unknown | 1.00 | 0.03 | 0.08 | 0.03 | 1.00 | 0.03 | |||
| College education | 0.27 | 0.27 | .71 | 0.21 | 0.25 | .001 | 0.21 | 0.24 | .001 |
| Treatment years | 2004–2006 | 1997–2009 | 1998–2000 | 1993–2009 | 1998–2001 | 1993–2009 | |||
* Clinical trial patient. CDDP/VP = cisplatin and etoposide; EC SCLC = extensive stage small cell lung cancer; NSCLC = non–small cell lung cancer. Values are presented as proportions unless defined otherwise.
† Usual-care elderly patient.
‡ We compared demographic and treatment variables for trial- and nontrial-treated patients using the chi-squared test (for categorical variables) and the t-test (for continuous variables). All statistical tests were two-sided.
§ Marital status was not collected as part of 80303 nor 9732.
Survival
The survival of Medicare patients relative to that of trial patients varied by age for each of the three cohorts as described in Table 2 and Figures 1–3. Among elderly Medicare patients aged 65 to 74 years who were treated in the usual-care setting, median survival times did not differ in a statistically significant manner from those of clinical trial patients (ie, 4.8 vs 5.8 months following treatment with single agent gemcitabine for advanced pancreatic cancer, P = .37; 7.7 vs 8.9 months for advanced NSCLC, P = .70; and 9.5 vs 10.2 months following treatment with CDDP/VP16 for ES SCLC, P = .99). Supplementary Figure 3 (available online) contains the survival distribution of the SEER-Medicare patients by site, histology, stage, and treatment cohort.
Table 2.
Survival distribution (months) for cancer site, stage, and treatment-specific cohort according to treatment setting and age (n = 15034)
| Cohort | Standard tegimen | N | Treatment setting | Age, y | Median, mos | LRT P* |
|---|---|---|---|---|---|---|
| Advanced PC | Gemcitabine | 300 | Clinical trial | All | 5.8 | .03 |
| 2536 | CMS usual care | 65–74 | 4.8 | |||
| 1829 | CMS usual care | 75+ | 4.3 | |||
| Stage IV NSCLC | Carboplatin/paclitaxel | 288 | Clinical trial | All | 8.9 | .35 |
| 5811 | CMS usual care | 65–74 | 7.7 | |||
| 2819 | CMS usual care | 75+ | 7.3 | |||
| ES SCLC | CDDP/VP16 | 349 | Clinical trial | All | 10.2 | .001 |
| 818 | CMS usual care | 65–74 | 9.5 | |||
| 284 | CMS usual care | 75+ | 8.2 |
*The two-sided log-rank test was used to compare the survival distributions of the three patient groups. CDDP = cisplatin; CMS = Centers for Medicare and Medicaid Services; EC SCLC = extensive stage small cell lung cancer; LRT = log-rank test; NSCLC = non–small cell lung cancer; PC = pancreatic cancer; VP16 = etoposide.
Figure 1.
All-cause mortality following treatment with gemcitabine for clinical trial patients and elderly usual care medicare patients with advanced pancreatic cancer. A two-sided log-rank test was used to compare the survival distributions of patients according to group membership. CMS = Centers for Medicare and Medicaid Services.
Figure 3.
All-cause mortality following treatment with cisplatin and etoposide for clinical trial patients and elderly usual care medicare patients with extensive stage small cell lung cancer. A two-sided log-rank test was used to compare the survival distributions of patients according to group membership. CMS = Centers for Medicare and Medicaid Services.
Figure 2.
All-cause mortality following treatment with carboplatin and paclitaxel for clinical trial patients and elderly usual care medicare patients with stage IV non–small cell lung cancer. A two-sided log-rank test was used to compare the survival distributions of patients according to group membership. CMS = Centers for Medicare and Medicaid Services.
However, following the same treatments, the Medicare patients aged 75 years or older who were treated in the usual-care setting generally had shorter median survival times (Table 2). That is, among Medicare patients aged 75 years or older who were treated in the usual-care setting, median survival times were six to eight weeks shorter than those of clinical trial patients (ie, 4.3 vs 5.8 months following treatment with single agent gemcitabine for advanced pancreatic cancer, P = .03; 7.3 vs 8.9 months following treatment with carboplatin and paclitaxel for stage IV NSCLC, P = .91; 8.2 vs 10.2 months following treatment with CDDP/VP16 for ES SCLC, P ≤ .001). These P values pertain only to log-rank test survival distribution comparisons of clinical trial patients with usual-care Medicare patients aged 75 and older. The differences were statistically significant for the pancreatic and ES SCLC, but not for the NSCLC, patients.
Sensitivity Analyses
We reevaluated survival differences across cohorts after limiting SEER-Medicare patients to just those patients whose treatment was started in the years in which their corresponding trial patients’ treatment was started (Supplementary Table 4). For the pancreatic cancer cohort and the ES SCLC cohort, median survival times associated with the limited years of data were generally similar to the survival times from the full years of analyses, with the exception of loss of statistical significance, which is likely because of the power to detect meaningful differences with smaller sample sizes. However, for the stage IV NSCLC cohort, survival was appreciably shorter for the Medicare patients, particularly the patients who were aged 75 years or older at the time of treatment, where their median survival was 6.0 months and the trial patients 8.9 months, almost three months longer. Medicare patients aged 65 to 74 years had median survival times of 6.9 months. For these analyses, P = .02 for the comparison across the three groups, whereas for the full analysis the clinical differences were smaller and not statistically significant.
Discussion
The median survival times of usual-care elderly Medicare patients, as compared with those of trial patients, often differed clinically and statistically significantly by Medicare patient age at the time of treatment. That is, for Medicare patients who were aged 65 to 74, median survival times were not statistically significantly shorter than those of trial patients. In contrast, for Medicare patients who were aged 75 or older, median survival times were approximately six to eight weeks shorter than those of trial patients in each of the three cohorts; in two of the three cohorts (ie, advanced pancreatic cancer treated with gemcitabine and ES SCLC treated with cisplatin/VP16), these differences were clinically and statistically significant (ie, P ≤ .05). In short, the results: 1) suggest that clinical trial results may overestimate the expected survival for older Medicare patients with advanced pancreatic cancer and lung cancers and 2) may provide potentially useful prognostic information for future Medicare patients’ and their oncologists’ treatment decision-making. Prognostic misunderstanding in clinical oncology is rife among patients, physicians, and families, particularly as patients near the end of life, and these estimates may be better tailored for this population of patients (11–16). Therefore, this study and these results “expand the breadth of data collected on cancer interventions for older adults” as called for by the IOM and therefore have the potential to better inform the clinical care delivered to elderly Medicare patients with cancer (17).
Importantly, for the Medicare lung cancer cohorts who were known to receive some form of chemotherapy, only 10% of Medicare patients with ES SCLC and only 30% of Medicare patients with stage IV NSCL cancer were treated with the first-line, guideline-recommended therapies we evaluated. This is not entirely surprising, as a prior meta-analysis showed that patients with ES SCLC who were over 70 years of age and treated with carboplatin-based therapies had a trend toward a lower hazard of death compared with those treated with carboplatin-based therapies, something that may have influenced treating oncologists in the community (18). Nor is it surprising that 30% (rather than 100%) of patients with stage IV NSCLC received carboplatin/paclitaxel given that there are multiple guideline-endorsed first-line platinum-based doublet therapies that have been shown to be equivalent to carboplatin/paclitaxel in clinical trials (19). In addition, single agents such as navelbine have been shown to be an efficacious alternative in elderly patients (19,20).
An important limitation of this study is that the factors mediating the survival differences between patients treated in these different settings are not discernible through examination of unadjusted Kaplan-Meier curves. However, based on the samples’ age differences and the well-described force of mortality associated with age, it is not surprising that elderly Medicare patients have shorter survival times (21). Age is also related to comorbidity and declining functional status, which can exert their own forces of mortality on individuals with or without cancer (22–24). Elting and colleagues previously compared attributes of trial and nontrial patients and found that trial patients were younger, had better performance status, had less medical comorbidity, and were more often male (25). Gross and colleagues showed that participants in cooperative group trials had higher SES and were less often African American than unselected elderly cancer patients from SEER-Medicare data (26,27). Because Gross did not require SEER-Medicare comparators to have been treated with chemotherapy, our findings that usual-care Medicare patients who were treated with guideline-recommended chemotherapy resided in higher SES areas than trial patients and that, in two of the cohorts, they were more likely to be African American does necessarily not contravene his findings. These differences in the sociodemographic characteristics of the cohorts may arise if patients who live in lower SES areas or who are African American are less likely to receive standard chemotherapy as we defined it. In fact, undertreatment of patients from resource-poor communities and from disadvantaged minority groups, including African American patients, is well described in observational research with SEER-Medicare data (28–36).
Another limitation of the study is that we did not measure use of second-line therapies. Attributing survival differences between trial and nontrial patients exclusively to first-line therapies may be incorrect if trial patients are more likely than usual-care patients to subsequently receive efficacious second-line chemotherapy. Additionally, study patients may derive clinical benefit from their participation in trials (the so-called “trial effect”), or from treatment at centers of excellence that are more likely to conduct and enroll patients in clinical trials (37–39).
Finally, because individual drug names are not included in Medicare inpatient files, we studied only those usual care patients who underwent their first treatment in the ambulatory setting. Our results therefore may only generalize to the more than 85% of Medicare patients whose treatment is initiated in the ambulatory setting (7).
In applying these findings to any age group, it is essential to recognize that there is heterogeneity in important attributes like functional status, comorbidity, and general health status. Experts in geriatric oncology therefore advocate for comprehensive geriatric assessments that take into account many of these factors and that can assist in selecting patients for treatment, including participation in clinical trials (40,41). Given the similarly in survival between Medicare patients aged 65 to 74 years and the trial patients we studied, physician selection for usual-care treatment appears to have been reasonable.
In summary, our results suggest results of clinical trials in advanced pancreatic and lung cancer patients are generalizable to Medicare patients aged 65 to 74 years who are deemed candidates for chemotherapy by their treating oncologist. In contrast, for Medicare patients 75 years or older with the three diseases studied here, trial results tend to overestimate survival by six to eight weeks. These “real world” results may help inform treatment discussions between older patients with these common advanced cancers and their oncologists.
Funding
The research for 70802 (Alliance) was supported, in part, by grants from the National Cancer Institute (NCI) (R01 CA132900) to Harvard Medical School (Elizabeth B. Lamont, MD, Principal Investigator), (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair), and (CA33601) to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD). The authors also acknowledge K07 CA9389204 (Lamont), without which this paper would not have been possible.
Supplementary Material
Funding sources were not involved in the design, collection, analyses, interpretation of the data, drafting of the manuscript, nor the decision to submit the manuscript to this journal.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
We would like to acknowledge Dr. Joan Warren, an NCI Population Science Investigator who arranged the linkage of Surveillance Epidemiology and End Results data to Medicare data and has remained an invaluable asset to researchers using these data. We would like to acknowledge the expert programming skills of Ms. Laurie Meneades within the Department of Health Care Policy, whose talent and scientific insights were great additions to the project.
References
- 1. Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 3. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 4. IOM (Institute of Medicine). 2013. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 5. National Cancer Institute. SEER: Surveillance Epidemiology and End Results. Available at: http://seer.cancer.gov/ Accessed May 10, 2007.
- 6. Young JL, Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA. (eds). SEER Summary Staging Manual - 2000: Codes and Coding Instructions., National Cancer Institute, NIH Pub. No. 01-4969, Bethesda, MD2001. [Google Scholar]
- 7. Lamont EB, Lan L. Sensitivity of Medicare claims data for measuring use of standard multi-agent chemotherapy regimens. Medical Care. 2014;52(3):e15–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niell HB, Herndon II JE, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol. 2005;23(16):3752–3759. [DOI] [PubMed] [Google Scholar]
- 10. Lilenbaum RC, Herndon II JE, List MA, Desch C, Watson DM. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (study 9730). J Clin Oncol. 2005;23(1):190–196. [DOI] [PubMed] [Google Scholar]
- 11. Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320(7233):469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Int Med. 2001;134(12):1096–1105. [DOI] [PubMed] [Google Scholar]
- 13. Lennes IT, Temel JS, Hoedt C, Meilleur A, Lamont EB. Predictors of newly diagnosed cancer patients’ understanding of the goals of their care at initiation of chemotherapy. Cancer. 2013;119(3):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. New Engl J Med. 2012;367(17):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mack JW, Cook EF, Wolfe J, Grier HE, Clearly PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25(11):1357–1362. [DOI] [PubMed] [Google Scholar]
- 16. Mackillop WJ, Stewart WE, Ginsburg AD, et al. Cancer patients’ perceptions of their disease and its treatment. Br J Cancer. 1998;50 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. IOM (Institute of Medicine). 2013. Delivering high-quality cancer care: Charting a new course for a system in crisis. http://www.iom.edu/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf Published September 10th Accessed September 8, 2014. [PubMed]
- 18. Mavroudis D, Papadakis E, Veslemes M, et al. Greek Lung cancer Cooperative Group. A multicenter randomized clinical trial comparing paclitaxel-cisplatin-etoposide versus cisplatin-etoposide as first-line treatment in patients with small-cell lung cancer. Ann Oncol. 2001;12 463–470. [DOI] [PubMed] [Google Scholar]
- 19. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. [DOI] [PubMed] [Google Scholar]
- 20. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst. 1999;91(1):66–72. [DOI] [PubMed] [Google Scholar]
- 21. Period Life Table, 2010. Social Security Administration Website. Available at: http://www.ssa.gov/oact/STATS/table4c6.html Accessed June 23, 2014.
- 22. National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying. Available at: http://www.cdc.gov/nchs/data/hus/hus10.pdf Published February 2011. Accessed July 7, 2014. [PubMed]
- 23. Ervin RB. Prevalence of functional limitations among adults 60 years of age and over: United States, 1999–2002. Advance data from vital and health statistics; no 375. Hyattsville, MD: National Center for Health Statistics, 2006. [PubMed] [Google Scholar]
- 24. Søgaard M1, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106(11):2452–2458. [DOI] [PubMed] [Google Scholar]
- 26. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 27. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483–491. [DOI] [PubMed] [Google Scholar]
- 28. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. [DOI] [PubMed] [Google Scholar]
- 29. Murphy MM, Simons JP, Ng SC, et al. Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(11):2968–2977. [DOI] [PubMed] [Google Scholar]
- 30. Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–2211. [DOI] [PubMed] [Google Scholar]
- 31. Baldwin L, Dobie SA, Billingsley K, et al. Explaining Black–White Differences in Receipt of Recommended Colon Cancer Treatment. J Natl Cancer Inst. 2005;97(16):1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamont EB, Zaslavsky AM, Subramanian SV, Meilleur A, He Y, Landrum MB. Elderly breast and colorectal cancer patients’ clinical course: patient and contextual influences. Med Care. 2014;52(9):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson DR, Martinez ME, Gupta S, et al. Racial disparity in consultation, treatment, and the impact on survival in metastatic colorectal cancer. J Natl Cancer Inst. 2013;105(23):1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du XL1, Sun CC, Milam MR, Bodurka DC, Fang S. Ethnic disparities in socioeconomic status, diagnosis, treatment, and survival among older women with epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18(4):660–669. [DOI] [PubMed] [Google Scholar]
- 35. Bhargava A, Du XL. Racial and socioeconomic disparities in adjuvant chemotherapy for older women with lymph node-positive, operable breast cancer. Cancer. 2009;115(13);2999–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsieh MC, Chiu YW, Velasco C, Wu XC, O’Flarity MB, Chen VW. Impact of race/ethnicity and socioeconomic status on adjuvant chemotherapy use among elderly patients with stage III colon cancer. J Registry Manag. 2013;40(4):180–187. [PubMed] [Google Scholar]
- 37. Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363 263–270. [DOI] [PubMed] [Google Scholar]
- 38. Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54 217–224. [DOI] [PubMed] [Google Scholar]
- 39. Vist G, Hagen KB, Devereaux PJ, Bryant D, Kristoffersen DT, Oxman AD. Systematic review to determine whether participation in a trial influences outcome. BMJ. 2005;330(7501):1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walters LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. [DOI] [PubMed] [Google Scholar]
- 41. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


