Abstract
Background:
Self-collected human papillomavirus (HPV) testing could reduce barriers to cervical cancer screening, with performance comparable to clinician-collected specimens. The ability of self-collected specimens to cross-sectionally and prospectively detect precursor lesions was investigated in an HPV vaccine randomized trial in Costa Rica.
Methods:
In the trial, 7466 women age 18 to 25 years received an HPV16/18 or control vaccine and were followed at least annually for four years. In this secondary analysis, we included all women who provided a self-collected cervicovaginal specimen six months after enrollment (5109 women = full analytical cohort). A subset (615 women = restricted cohort) also had clinician-collected specimens at the six-month postenrollment visit. High-grade squamous intraepithelial lesion or repeat low-grade squamous intraepithelial lesion prompted colposcopic referral throughout the study. HPV testing was performed with SPF10PCR/DEIA/LiPA25. Cross-sectional and prospective sensitivity, specificity, and predictive values were estimated.
Results:
In the full cohort, one-time HPV testing on self-collected samples detected prevalent CIN2+ with a sensitivity of 88.7% (95% confidence interval [CI] =77.0% to 95.7%) and a specificity of 68.9% (95% CI = 67.6% to 70.1%). For predicting incident CIN2+ in the subsequent four years, sensitivity was 73.9% (95% CI = 65.8% to 81.0%) and specificity 69.4% (95% CI = 68.1% to 70.7%). In the restricted cohort, for incident CIN2+, self-collected HPV was much more sensitive than cytology (80.0% vs 10.0%); relative sensitivity was 0.1 (95% CI = 0.03% to 0.5%). Furthermore, three times more women with normal baseline cytology developed incident CIN2+ than those with negative self-collected HPV. Self-collected and clinician-collected HPV testing had comparable performance. Agreement between self- and clinician-collected samples was 89.7% (kappa = 0.78, McNemar χ2 = 0.62) for carcinogenic HPV types.
Conclusions:
Self-collected specimens can be used for HPV-based screening, providing sensitivity and specificity comparable with clinician-collected specimens and detecting disease earlier than cytology.
Persistent infection with oncogenic human papillomavirus (HPV) causes cervical cancer (CC) through a series of steps: transmission, persistence, progression to precancer, and invasion (1,2). Most HPV infections clear within two years after acquisition (3), with viral peristence and progression occurring in a small subset of women.
Cytology-based screening programs have reduced CC in developed countries, with limited impact in developing countries. Cytology is insensitive, poorly reproducible, and affected by specimen collection, processing, and interpretation. Thus, one normal cytology result does not exclude underlying cervical disease and the test needs to be repeated to potentially detect missed abnormalities and capture incipient disease (4).
HPV testing is more sensitive than cytology for detection of cervical neoplasia, as multiples studies demonstrate (5–13). In a pooled analysis of European cohorts, a single cytology at baseline had a sensitivity of 60% (95% CI = 35% to 68%) and a specificity of 95% (95% CI = 93% to 98%) for incident CIN3+, while HPV testing had a sensitivity of 90% (95% CI = 80% to 95%) and a specificity of 89% (95% CI = 83% to 94%) (14). Thus, many countries are introducing HPV testing for primary screening, including the United States (15). Another advantage of HPV testing is that women can self-collect the sample, which can reduce cost, is noninvasive, and is generally better accepted (16–18). Self-collected HPV testing might increase screening participation in settings with limited resources, access to health services, or where cultural barriers exist.
In studies comparing HPV testing on self- vs clinician-collected samples, HPV prevalence and genotype distribution is almost equivalent (17,19). In a recent meta-analysis, self-collected HPV testing was 12% less sensitive than clinician-collected HPV testing for CIN2+ detection. However, in polymerase chain reaction (PCR)–based studies, test performance based on the two collection methods was comparable (20). HPV testing on self-collected samples has been shown to perform better than cytology (20–22).
The self-collected HPV test has only been evaluated cross-sectionally. Still needed are longitudinal evaluations of its ability to capture disease missed by colposcopy or disease that will become detectable in the future (23) and to reassure that among HPV-negative women at baseline the cumulative incidence of precancer remains low. The performance of HPV testing in vaccinated women is also of interest, because vaccination alters HPV type distribution and reduces incidence of precancer (24,25).
In an effort to guide clinical practice, we performed a secondary analysis within a large HPV vaccine trial in Costa Rica to evaluate cross-sectional and prospective clinical accuracy of one-time HPV testing on self-collected samples to detect CIN2+ cases, stratified by vaccination arm. Additionally, we compared its accuracy with clinician-collected HPV testing and cytology and agreement for detection of individual HPV genotypes.
Methods
This is a secondary analysis of data from the publicly funded Costa Rica Vaccine Trial, registered with Clinicaltrials.gov NCT00128661, whose primary aim was to evaluate efficacy of Cervarix® (HPV16/18) for prevention of cervical HPV16/18 infection and related precancerous lesions (26).
Population and Procedures
Between June 2004 and December 2005, 7466 women age 18 to 25 years were enrolled. Protocols were approved by the Ethical Committees in Costa Rica and the United States.
At enrollment, participants gave consent and a pelvic examination was performed on sexually experienced women. Exfoliated cervical cells were collected using a Cervex brush rinsed in PreservCyt solution, from which aliquots were drawn for HPV testing by PCR (SPF10/LiPA25) and ThinPrep cytology slides prepared. At enrollment and at the four-year visit, HPV DNA detection by Hybrid Capture 2 (HC2) was performed on all specimens. Eligible women were randomized to receive three doses of Cervarix® or control (hepatitis A) vaccine.
At the six-month visit (V06), women provided a cervicovaginal self-collected sample at the clinic for HPV testing by PCR (SPF10/LiPA25); this sample was not collected at any other visit. Collection consisted of inserting a dry Dacron swab as high as possible into the vagina, trying to avoid touching the external genitalia and rotating it 5 times. The swab was placed into 3mL of PreservCyt solution and immediately frozen. At V06, no pelvic exam was done, except among the subset of women who, at enrollment, had inadequate cytology, low-grade squamous intraepithelial lesion (LSIL), or atypical squamous cells of unknown biological significance (ASC-US) with positive HPV testing by HC2.
Women were scheduled for four annual (or semi-annual if necessary) follow-up visits including pelvic exams (see below).
Management of Abnormal Cytology Results
Women with LSIL, HPV-positive ASC-US, or inadequate cytology at any visit were rescreened semi-annually and returned to yearly follow-up after three normal cytologies or, if the lesion persisted, to colposcopy. High-grade squamous intraepithelial lesion (HSIL) at any time was referred to colposcopy. After colposcopy and/or treatment, women continued study-screening visits semi-annually, they returned to yearly follow-up after three normal cytologies, or they were referred again to colposcopy if they had ASCUS/HPV+ or worse.
ThinPrep slides were double-screened by cytotechnologists and adjudicated by one cytopathologist in Costa Rica. Abnormal slides (ASCUS or worse) and a 10% random sample of negatives were reread in the United States by one cytotechnologist and one pathologist. At the four-year visit, the US laboratory reinterpreted all slides with reactive changes that also had HC2 HPV–positive results. Clinical management of women was based on CR cytology. If cytology was upgraded in the US, it also led to colposcopic referral.
Biopsy and loop electrosurgical excision procedure specimens were interpreted by a Costa Rican pathologist, who determined clinical management. In addition, one National Cancer Institute (NCI) pathologist blindly diagnosed all histological specimens. Discrepancies led to review by another US expert pathologist. Final diagnosis was CIN2+ if two or more pathologists gave a CIN2 or worse diagnosis, and <CIN2 otherwise.
HPV Testing by PCR
HPV DNA detection/genotyping was performed by SPF10-DEIA/HPVLiPA25 (version 1, Labo Bio-Medical Products, Rijswijk, the Netherlands) at DDL Diagnostic Laboratory (DDL, Voorburg, the Netherlands), as described (27). Extracted DNA was used for amplification with SPF10 primers followed by DNA enzyme immunoassay detection of amplimers (DEIA). The same amplimers were used on SPF10-DEIA–positive samples to identify genotype by reverse hybridization on a line probe assay (LiPA) that detects 25 HPV genotypes. Specimens positive by SPF10/DEIA but negative for HPV-16 or HPV-18 by LiPA25 were tested for HPV-16 and HPV-18 using type-specific PCR primers (28).
Statistical Analysis
From the 7466 women recruited (Figure 1), the following were excluded: 983 who did not attend the V06 (133 because of colposcopy referral at enrollment), 1253 with no prior sexual experience at V06, 103 women with no follow-up visits, 13 with missing self-collected HPV-PCR results, and five for other reasons. Thus, the full analytical cohort (n = 5109) included all women who, at V06, had a self-collected sample and available HPV-PCR results. In this cohort, clinical accuracy of one-time HPV-PCR testing on self-collected samples was evaluated overall and stratified by vaccination arm. Additionally, PCR (SPF10/LiPA25) and HC2-HPV results from the enrollment visit (collected by clinicians approximately six months before self-sample) were used to compare performance of HPV testing on self- vs clinician-collected samples.
Figure 1.
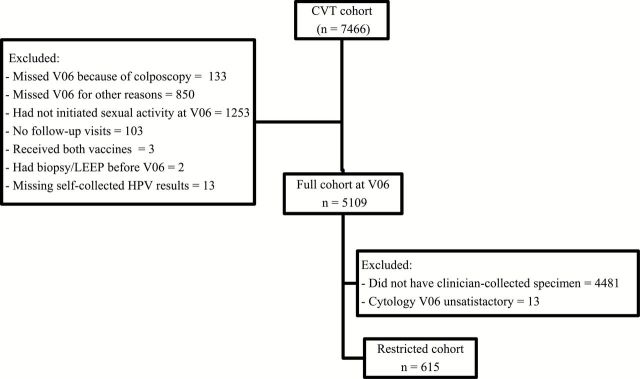
Consort diagram of women in the Costa Rica Vaccine Trial. CVT = Costa Rica Vaccine Trial; HPV = human papillomavirus.
The restricted cohort (Figure 1) included the subset of women with a pelvic exam at V06 (n = 615) (see above). Among this subset of women, we directly compared the accuracy of HPV-PCR testing on self- vs clinician-collected samples and cytology.
Our gold standard was histologically confirmed CIN2, CIN3, or more severe diagnosis, based on colposcopic referral after yearly (or six-monthly) cytology (29). Case patients were considered as having prevalent CIN2+ (Figure 2) if the diagnosis occurred after V06 (when the self-sample was collected) and before the first annual follow-up visit (V12); otherwise, it was considered incident CIN2+. Case counting began the day after self-collection at V06. Women attended between one and seven screening visits (24.2% had one to three and 57.4% had four). HPV detection was not a colposcopy referral criterion, except that HPV-HC2 was used to triage an ASC-US interpretation.
Figure 2.
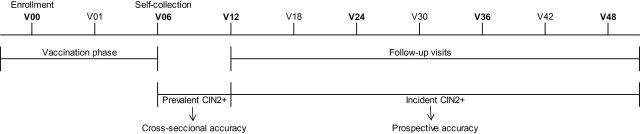
Timeline of the Costa Rica Vaccine Trial study visits. CVT = Costa Rica Vaccine Trial.
We calculated cross-sectional (gold standard = prevalent CIN2+) and prospective (gold standard = incident CIN2+) sensitivity, specificity, positive predictive value (PPV), and the complement of the negative predictive value (cNPV = 1-NPV) for detection of CIN2+. All proportions were calculated with exact 95% binominal confidence intervals (CIs). The PPV represents risk of CIN2+ associated with a positive test. Thus, a low PPV would reflect the unnecessary procedures induced by screening. The cNPV represents risk of CIN2+ with a negative test. Thus, a high cNPV indicates that the test was not sufficient to exclude underlying disease and that screening should be repeated within a short interval. Accuracy estimates by vaccination arm were compared by use of the Chi-square and Fisher’s exact tests; a P value under .05 (two-tailed) was considered statistically significant.
In the restricted cohort, accuracy estimates of cytology and HPV testing on clinician- vs self-samples were also compared by calculating ratios with 95% confidence intervals. If the ratio of the accuracy estimate was under one, the interpretation was that the self-collected sample used for HPV testing was better; if over one, the self-collected sample was viewed as less accurate; and if the confidence interval included 1.0, there was no difference in the accuracy between the two sample collection methods.
Also, in the restricted cohort, HPV type distribution was compared between specimen collection methods by calculating percent overall agreement, kappa, and the exact McNemar χ2 test. Interpretation of the kappa values vary, but typically a kappa value under 0.4 represents slight to fair agreement, 0.4 to 0.6 indicates moderate agreement, and greater than 0.6 indicates substantial-to-perfect agreement (30).
Cytology results were categorized as normal (normal, reactive, ASC-US–HPV negative) and greater than or equal to ASC-US (ASC-US–HPV positive, LSIL, HSIL, and glandular lesions). Only the Costa Rican interpretation was used for these analyses, although upgrades also prompted colposcopy referral. HPV-PCR testing by SPF10/LiPA25 was categorized as positive for carcinogenic HPV types (HPV) if there was at least one of the 12 Class I carcinogenic HPV types, as defined by the International Agency for Research on Cancer categorization (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) (1).
Results
In the full cohort, the median age at V06 was 22.0 years, 39.8% reported one lifetime sexual partner, and 40.0% reported two to three. Overall carcinogenic HPV-PCR positivity using the self-collected sample was 31.7%. Only five women out of 5230 who attended V06 and were eligible for self-collection refused it (99.9% compliance).
A total of 533 women were referred to colposcopy because of enrollment or V06 cytology results. Prevalent disease was defined as a CIN2+ diagnosed in this group. One hundred thirty-three of these did not have a self-collected sample, because at V06 they attended colposcopy after an HSIL or worse result in the enrollment cytology (Figure 1). Thus, we estimate that the analysis did not include approximately a quarter of the disease prevalent when the self-sample was collected. The median follow-up time for the full cohort from enrollment was 54 months, range nine to 76 months, and for the restricted cohort it was 55 months, range 10 to 74 months. It was similar between arms for both cohorts.
Table 1 shows the accuracy of one-time, self-collected HPV-PCR testing to detect prevalent (cross-sectional accuracy) and incident (prospective accuracy) CIN2+ cases, as well as the performance of HPV testing by HC2 and PCR using clinician-collected samples approximately six months before the self-sample was collected (at enrollment into the study).
Table 1.
Accuracy of HPV testing on self-collected and clinician-collected samples to detect high-grade disease (CIN2+) ascertained based on cytology referral to colposcopy*
| Accuracy estimates | 6-month visit, % (95% CI) | Enrollment visit, % (95% CI) | |
|---|---|---|---|
| Self-HPV (PCR)† (n = 5109) | Clinician-HPV (PCR)† (n = 4949) | Clinician-HPV (HC2)‡ (n = 4789) | |
| Prevalent CIN2+§ | |||
| Sensitivity | 88.7 (77.0 to 95.7) | 92.5 (81.8 to 97.9) | 96.1 (86.5 to 99.5) |
| Specificity | 68.9 (67.6 to 70.1) | 70.3 (69.0 to 71.6) | 68.2 (66.9 to 69.5) |
| Risk of CIN2+ if positive test (PPV)ǁ | 2.9 (2.1 to 3.8) | 3.3 (2.4 to 4.3) | 3.2 (2.3 to 4.1) |
| Risk of CIN2+ if negative test (cNPV)¶ | 0.2 (0.1 to 0.4) | 0.1 (0.03 to 0.3) | 0.06 (0.007 to 0.2) |
| Incident CIN2+ | |||
| Sensitivity | 73.9 (65.8 to 81.0) | 72.4 (64.0 to 79.8) | 68.7 (60.0 to 76.5) |
| Specificity | 69.4 (68.1 to 70.7) | 70.8 (69.5 to 72.1) | 68.5 (67.2 to 69.9) |
| Risk of CIN2+ if positive test (PPV)ǁ | 6.3 (5.2 to 7.6) | 6.5 (5.3 to 7.8) | 5.8 (4.7 to 7.1) |
| Risk of CIN2+ if negative test (cNPV)¶ | 1.0 (0.7 to 1.4) | 1.1 (0.8 to 1.5) | 1.3 (0.9 to 1.7) |
* Disease was ascertained based on cytologic referral; human papillomavirus (HPV) was used to triage atypical squamous cells of unknown biological significance. Number of CIN2+ included: Self-collection = 53 prevalent, 138 incident; Clinician–polymerase chain reaction (PCR) = 53 prevalent, 134 incident; Clinician-HC2 = 51 prevalent, 131 incident. CI = confidence interval; cNPV = complement of the negative predictive value; HPV = human papillomavirus; PCR = polymerase chain reaction; PPV = positive predictive value.
† HPV DNA testing by SPF10/LiPA25 was categorized as positive if there was at least one of the 12 Class I carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59).
‡ HPV DNA testing by Hybrid capture 2 (HC2) detects: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, with some cross reactivity for other related types.
§ For prevalent CIN2+, the estimates of performance for Clinician-HPV might be underestimated given that the samples were collected at enrollment and prevalent case count for this analysis after V06 and before the first annual follow-up visit.
ǁ PPV: positive predictive value represents the risk of disease if the test was positive.
¶ cNPV: complement of the negative predictive value (1-NPV) represents the risk of disease if the test was negative.
We observed that 88.7% (95% CI = 77.0% to 95.7%) of women with prevalent CIN2+ (detected mainly by cytologic referral) had a positive self-collected HPV-PCR test (Table 1), the specificity was 68.9% (95% CI = 67.6% to 70.1%), and the cNPV for CIN2+ was 0.2% (95% CI = 0.1% to 0.4%). The self-collected HPV-PCR test was positive in 73.9% (95% CI = 65.8% to 81.0%) of the CIN2+ cases detected during the follow-up period; the specificity was 69.4% (95% CI = 68.1% to 70.7%), and the PPV for CIN2+ detection during the follow up was 6.3% (95% CI = 5.2% to 7.6%) compared with a cNPV of 1.0% (95% CI = 0.7% to 1.4%) if the test was negative. For incident CIN2+, the self-collected sample missed 12 incident cases, while the clinician sample at enrollment missed 15 cases (McNemar χ2 = 1.0). Self-collected HPV-PCR testing had comparable performance to the HPV-PCR and HPV-HC2 using clinician-collected samples.
In the subset of women (n = 615) with a pelvic exam performed at V06 (restricted cohort) (Table 2), there was no difference in the performance of self-collected HPV-PCR vs clinician-collected HPV-PCR for detection of prevalent or incident CIN2+. Instead, for incident CIN2+, self-collected HPV was much more sensitive than cytology (80.0% vs 10.0% ); relative sensitivity was 0.1 (95% CI = 0.03 to 0.5). Cytology was 90.0% less sensitive for incident CIN2+ than self-collected HPV-PCR (relative sensitivity = 0.1, 95% CI = 0.03 to 0.5) but 50.0% more specific (relative specificity = 1.5, 95% CI = 1.3 to 1.7). Also, the risk of CIN2+ during follow-up was three times higher for women with a normal cytology at baseline than for women with negative self-collected HPV testing (relative cNPV = 2.9, 95% CI = 1.0 to 8.3). Self-collected samples showed good concordance with clinician-collected samples; only one incident CIN2+ case was missed by the self-sample and none by the clinician-sample (McNemar χ2 = 1.0).
Table 2.
Performance of HPV testing on self-collected samples vs clinician-collected samples and cytology to detect prevalent and incident CIN2+*, among the 615 women with concurrent results for the three screening tests (restricted cohort)†
| Accuracy estimates | Clinician- vs self-collection, HPV testing‡ | |||||
|---|---|---|---|---|---|---|
| Prevalent CIN2+, % (95% CI) | Incident CIN2+, % (95% CI) | |||||
| Clinician | Self-collection | Ratio, clinician to self-collection | Clinician | Self-collection | Ratio, clinician to self-collection | |
| Sensitivity | 92.0 (80.8 to 97.8) | 88.0 (75.7 to 95.5) | 1.0 (0.9 to 1.2) | 85.0 (62.1 to 96.8) | 80.0 (56.3 to 94.3) | 1.1 (0.8 to 1.4) |
| Specificity | 39.6 (35.6 to 43.8) | 38.4 (34.4 to 42.6) | 1.0 (0.9 to 1.2) | 37.8 (33.9 to 41.8) | 36.8 (32.9 to 40.8) | 1.0 (0.9 to 1.2) |
| PPV§ | 11.9 (8.8 to 15.5) | 11.2 (8.3 to 14.8) | 1.1 (0.7 to 1.6) | 4.3 (2.6 to 6.9) | 4.1 (2.4 to 6.5) | 1.1 (0.6 to 2.1) |
| cNPVǁ | 1.8 (0.5 to 4.4) | 2.7 (1.0 to 5.8) | 0.7 (0.2 to 2.3) | 1.3 (0.3 to 3.8) | 1.8 (0.5 to 4.5) | 0.7 (0.2 to 3.2) |
| Cytology vs self-collection | ||||||
| Prevalent CIN2+, % (95% CI)¶ | Incident CIN2+, % (95% CI) | |||||
| Accuracy estimates | Cytology | Self-collection | Ratio, cytology to self-collection | Cytology# | Self-collection | Ratio, cytology to self-collection |
| Sensitivity | – | – | – | 10.0 (1.2 to 31.7) | 80.0 (56.3 to 94.3) | 0.1 (0.03 to 0.5) |
| Specificity | – | – | – | 56.0 (51.9 to 60.0) | 36.8 (32.9 to 40.8) | 1.5 (1.3 to 1.7) |
| PPV§ | – | – | – | 0.8 (0.09 to 2.7) | 4.1 (2.4 to 6.5) | 0.2 (0.04 to 0.8) |
| cNPVǁ | – | – | – | 5.1 (3.1 to 8.0) | 1.8 (0.5 to 4.5) | 2.9 (1.0 to 8.3) |
* Disease was ascertained based on cytologic referral; human papillomavirus (HPV) was used to triage atypical squamous cells of unknown biological significance (ASC-US). Number of CIN2+ included: Prevalent = 50, Incident = 20. CI = confidence interval; cNPV = complement of the negative predictive value; HPV = human papillomavirus; PCR = polymerase chain reaction; PPV = positive predictive value.
† Women in the restricted cohort had a pelvic exam performed at the six-month visit, given that at enrollment they had inadequate cytology or low-grade squamous intraepithelial lesion or ASC-US with positive HPV test.
‡ HPV DNA testing was categorized as positive if there was at least one of the 12 Class I carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59).
§ PPV: positive predictive value, which is the risk of disease after a positive test.
ǁ cNPV: complement of the negative predictive value (1-NPV), which is the risk of disease after a negative test.
¶ The estimates and the ratio cytology to self-collection is not shown for prevalent CIN2+, because at baseline only cytology was used to refer women to colposcopy.
# Cytology was considered abnormal if ≥ ASC-US.
No statistically significant differences were observed in the HPV genotype distribution by specimen collection in the restricted cohort (Table 3); for detection of carcinogenic HPV types overall or by individual types, the agreement was 89.7% and the kappa was 0.78 (McNemar χ2 = 0.62). Agreement between individual carcinogenic HPV types was 95.5% or higher, and kappas were 0.74 or higher, except for HPV-59 (kappa = 0.64). Prevalence of noncarcinogenic HPV type was statistically significantly lower in clinician-collected samples (self-collection 42.4% vs clinician-collection 38.4%, P = .01). Self-collected samples detected statistically significantly more HPV40, HPV42, HPV43, and HPV74.
Table 3.
Prevalence of HPV infection on self- and clinician-collected specimens among women for whom both specimens were collected (restricted cohort*)
| Category HPV type | Prevalence self-collection | Prevalence clinician-collection | Percentage agreement | Kappa | McNemar χ2 |
|---|---|---|---|---|---|
| HPV positive (any type) | 85.5 | 83.4 | 89.7 | 0.61 | 0.14 |
| Any carcinogenic | 63.7 | 62.8 | 89.7 | 0.78 | 0.62 |
| HPV-16 | 17.5 | 16.9 | 96.5 | 0.88 | 0.52 |
| HPV-18 | 5.9 | 5.9 | 97.8 | 0.80 | 1.00 |
| HPV-31 | 9.4 | 9.7 | 97.8 | 0.87 | 0.79 |
| HPV-33 | 3.0 | 3.3 | 98.4 | 0.74 | 0.75 |
| HPV-35 | 3.7 | 3.5 | 98.9 | 0.84 | 1.00 |
| HPV-39 | 10.7 | 9.7 | 96.2 | 0.79 | 0.30 |
| HPV-45 | 4.8 | 4.9 | 97.9 | 0.78 | 1.00 |
| HPV-51 | 8.9 | 9.2 | 95.9 | 0.75 | 0.85 |
| HPV-52 | 12.1 | 12.4 | 95.5 | 0.79 | 0.85 |
| HPV-56 | 9.6 | 8.7 | 96.0 | 0.76 | 0.42 |
| HPV-58 | 9.1 | 9.7 | 96.5 | 0.79 | 0.52 |
| HPV-59 | 4.3 | 4.0 | 97.1 | 0.64 | 0.81 |
| Any noncarcinogenic | 42.4 | 38.4 | 84.9 | 0.69 | 0.01 |
| HPV-6 | 6.1 | 5.7 | 98.7 | 0.89 | 0.73 |
| HPV-11 | 1.3 | 1.4 | 99.2 | 0.70 | 1.00 |
| HPV-34 | 1.1 | 0.8 | 99.4 | 0.66 | 0.63 |
| HPV-40 | 3.0 | 1.9 | 98.6 | 0.70 | 0.04 |
| HPV-42 | 2.1 | 0.6 | 98.6 | 0.47 | 0.04 |
| HPV-43 | 3.2 | 1.6 | 97.5 | 0.46 | 0.02 |
| HPV-44 | 3.8 | 2.7 | 97.3 | 0.57 | 0.14 |
| HPV-53 | 7.5 | 7.5 | 95.9 | 0.70 | 1.00 |
| HPV-54 | 3.3 | 3.0 | 97.5 | 0.59 | 0.80 |
| HPV-66 | 8.3 | 8.9 | 97.8 | 0.86 | 0.42 |
| HPV-70 | 3.8 | 4.8 | 98.4 | 0.81 | 0.11 |
| HPV-74 | 5.3 | 3.3 | 97.1 | 0.65 | 0.008 |
| HPV-68_73 | 6.1 | 7.0 | 96.2 | 0.69 | 0.31 |
| Uncharacterized | 7.0 | 7.0 | 95.2 | 0.63 | 1.00 |
* Women in the restricted cohort had a pelvic exam performed at the 6-month visit given that at enrollment had inadequate cytology or low-grade squamous intraepithelial lesion or atypical squamous cells of unknown biological significance with positive human papillomavirus test. HPV = human papillomavirus.
Table 4 summarizes performance of HPV testing by vaccination arm in the full cohort. As expected, for prevalent disease, self-collected samples tested by HPV-PCR had similar sensitivity in both arms (P = .68), but specificity was lower in the control arm (71.1%, HPV versus 66.7% control arm; p=0.001). For detection of incident CIN2+, sensitivity was 81.4% (95% CI = 69.1% to 90.3%) in the HPV arm and 68.4% (95% CI = 56.9% to 78.4%) in the control arm (P = .09), the corresponding specificities were 71.7% and 67.2% (P = .001). The same measures were calculated using the clinician-collected samples for HPV testing: sensitivity for detection of incident CIN2+ by HPV-PCR was 84.7% (95% CI = 73.0% to 92.8%) for the HPV arm and 62.7% (95% CI = 50.7% to 73.6%) for the control arm (P = .005); the HPV-HC2 test sensitivity was 81.4% (95% CI = 69.1% to 90.3%) for the HPV arm and 58.3% (95% CI = 46.1% to 69.8%) for the control arm (P = .005).
Table 4.
Sensitivity and specificity of HPV testing by vaccination arm on self-collected and clinician-collected samples
| Screening method | Prevalent CIN2+*, % (95% CI) | Incident CIN2+*, % (95% CI) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Self-collection, HPV PCR† (n = 5109) | ||||
| HPV arm | 86.2 (68.3 to 96.1) | 71.1 (69.3 to 72.9) | 81.4 (69.1 to 90.3) | 71.7 (69.9 to 73.4) |
| Control arm | 91.7 (73.0 to 99.0) | 66.7 (64.8 to 68.5) | 68.4 (56.9 to 78.4) | 67.2 (65.3 to 69.1) |
| P | .68 | .001 | .09 | .001 |
| Clinician-collection, HPV PCR† (n = 4949)‡ | ||||
| HPV arm | 86.2 (68.3 to 96.1) | 70.9 (69.1 to 72.7) | 84.7 (73.0 to 92.8) | 71.6 (69.7 to 73.4) |
| Control arm | 100.0 (85.8 to 100.0) | 69.7 (67.8 to 71.5) | 62.7 (50.7 to 73.6) | 70.0 (68.1 to 71.8) |
| P | .12 | .34 | .005 | .22 |
| Clinician-collection, HPV HC2§ (n = 4789)‡ | ||||
| HPV arm | 92.9 (76.5 to 99.1) | 68.6 (66.7 to 70.4) | 81.4 (69.1 to 90.3) | 69.1 (67.2 to 71.0) |
| Control arm | 100.0 (85.2 to 100.0) | 67.8 (65.9 to 69.7) | 58.3 (46.1 to 69.8) | 68.0 (66.0 to 70.0) |
| P | .50 | .57 | .005 | .41 |
* Disease was ascertained based on cytologic referral; human papillomavirus (HPV) was used to triage atypical squamous cells of unknown biological significance. CI = confidence interval; HPV = human papillomavirus; PCR = polymerase chain reaction.
†SPF10/LiPA25 was categorized as positive if there was at least one of the 12 Class I carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59).
‡ The clinician samples were collected at enrollment (approximately six months before the self-sample).
§ HPV DNA testing by Hybrid capture 2 (HC2) detects: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, with some cross reactivity for other related types.
Discussion
We assessed performance of one-time self-collected PCR-based HPV testing to detect CIN2+ among women age 18 to 25 years in Costa Rica. The HPV test on self-collected samples was positive in 89% of women with prevalent CIN2+. The test was also positive in 74% of women with a CIN2+ lesion detected in the following three years (incident disease). In this same population, the HPV test on the clinician-collected sample taken approximately six months earlier (enrollment visit of the trial) was positive in 93% and 72% of women with prevalent and incident CIN2+, respectively. Corresponding figures for clinician-HC2 test were 96% and 69%, respectively. Women with negative self-collected HPV had very low risk of prevalent or incident CIN2+, which is remarkable in this young, high-prevalence population.
Performance of HPV testing on self-collected samples was equivalent to clinician-collected samples, despite the use of different collection devices (dacron vs brush). One-time self-collected HPV predicted 80% of CIN2+ lesions not detected by cytology in the first screening but detected by subsequent cytologies over the next three years. Thus, if HPV-positive women had been referred to colposcopy or followed more closely, many of the incident lesions would likely have been detected earlier. In contrast, one-time cytology predicted only 10% of incident disease, confirming superiority of HPV testing for primary screening.
We noted high agreement in HPV genotype distribution between self- and clinician-collected samples, with similar proportions of overall and individual carcinogenic HPV types. In contrast, noncarcinogenic HPV detection was higher in self-collected specimens, consistent with previous reports that noncarcinogenic types have tropism for vaginal epithelium, over-represented in self-collected samples (31,32).
Some studies reported reduced sensitivity for self-collected specimens (17,20,33), but our results corroborate that when using PCR detection methods, the results are comparable for both sampling approaches (20,34).
We also evaluated performance of self-collected HPV testing in a vaccinated cohort. Sensitivity for incident CIN2+ was higher among vaccinated than among unvaccinated women, regardless of collection method and HPV test (SPF10/LiPA25 or HC2). Vaccinated women are less likely to acquire new HPV16/18 infections during follow-up, and HPV16/18 infections tend to progress to CIN2+ faster than other HPV types (35,36). Thus, vaccinated women with a negative baseline HPV test could acquire a non-HPV16/18 infection during follow-up but not have enough time to develop CIN2+ during follow-up. In contrast, unvaccinated women with a negative HPV test at baseline could acquire a rapidly progressing HPV16/18 infection and develop CIN2+ during follow-up. This may have decreased sensitivity to predict future disease among unvaccinated women and may have important implications for vaccinated cohorts if confirmed in older women.
Our analysis has the strength of a robust gold standard with adjudicated histology and the use of multiple cytologies over four years for colposcopic referral.
Our study is not without limitations. The low sensitivity of cytology limits the analyses of test performance for detection of prevalent disease, given that HPV is more sensitive than cytology and we did not refer women based on HPV testing at enrollment. As a result, the true prevalence of baseline disease is probably higher than we observed with our cytology-only referral algorithm. Another limitation is that we were only able to directly compare performance of collection methods in the subset of women with previous low-grade cytology (restricted cohort), a subgroup with a higher HPV prevalence. However, this should not have affected the comparison of collection methods focused on evaluation of incident CIN2+. Also, about 25% of the disease prevalent when the self-sample was collected was not included in the analysis, because some women missed visits while attending colposcopy triggered by enrollment HSIL.
Our results should be interpreted carefully, given that our population is younger than the recommended age for HPV-based screening. This age group has higher HPV prevalence, and transient infections are more common than in older women and this could affect performance of the tests. On the other hand, the potential impact of reduced exfoliation in older women on the performance of self-collection has not been investigated.
The high acceptance of self-collection is noteworthy (99%) and promising in terms of its utility in settings like Costa Rica. However, younger women might be more comfortable self-collecting a cervicovaginal sample, and this could have produced specimens of better quality, partially explaining performance of the test. In addition, clinicians instructed participants on how to collect the self-sample at the clinics.
The similar performance of the self-collected and the clinician-collected HPV test for detection of prevalent and incident disease indicate that this could represent an invaluable tool for improving screening coverage. In settings without cervical cancer screening programs, a one-time self-HPV test would be preferable to screening with one-time cytology. However, the limited specificity of the HPV test still requires the use of additional triage tests among HPV positives. The most commonly used triage method is currently cytology, but several molecular tests are under investigation for this purpose (37).
In conclusion, our data indicate that PCR HPV testing on a self-collected sample is feasible and well accepted and provides sensitivity and specificity comparable with clinician-collected specimens. In addition, it detects disease earlier than cytology and should be considered in cervical cancer screening programs to reduce cost and increase coverage.
Funding
The Costa Rica Human Papillomavirus (HPV) Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study.
The NCI and Costa Rica investigators are responsible for the design and conduct of the study, the collection, management, analysis, and interpretation of the data, and the preparation of the manuscript. On efficacy-related manuscripts, GSK has the right to review and comment.
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, and Sarah Thomas).
Names and Affiliations of Investigators in the Costa Rica Vaccine Trial (CVT) Group: Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica—Mario Alfaro (cytopathologist), Manuel Barrantes (field supervisor), M. Concepción Bratti (coinvestigator), Fernando Cárdenas (general field supervisor), Bernal Cortés (specimen and repository manager), Albert Espinoza (head, coding, and data entry), Yenory Estrada (pharmacist), Paula González (coinvestigator), Diego Guillén (pathologist), Rolando Herrero (co-principal investigator), Silvia E. Jiménez (trial coordinator), Jorge Morales (colposcopist), Luis Villegas (colposcopist), Lidia Ana Morera (head study nurse), Elmer Pérez (field supervisor), Carolina Porras (coinvestigator), Ana Cecilia Rodríguez (coinvestigator), Libia Rivas (clinical coordinator).
University of Costa Rica, San José, Costa Rica—Enrique Freer (director, HPV diagnostics laboratory), José Bonilla (head, HPV immunology laboratory), Alfonso García-Piñeres (immunologist), Sandra Silva (head microbiologist, HPV diagnostics laboratory), Ivannia Atmella (microbiologist, immunology laboratory), Margarita Ramírez (microbiologist, immunology laboratory).
United States National Cancer Institute, Bethesda, MD—Allan Hildesheim (co-principal investigator and NCI co-project officer), Hormuzd Katki (statistician), Aimée R. Kreimer (coinvestigator), Douglas R. Lowy (HPV virologist), Nora Macklin (trial coordinator), Mark Schiffman (medical monitor and NCI co-project officer), John T. Schiller (HPV virologist), Mark Sherman (QC pathologist), Diane Solomon (medical monitor and QC pathologist), Sholom Wacholder (statistician).
SAIC, NCI-Frederick, Frederick, MD—Ligia Pinto (head, HPV immunology laboratory), Troy Kemp (immunologist).
Women’s and Infants’ Hospital, Providence, RI—Claire Eklund (QC cytology), Martha Hutchinson (QC cytology).
Georgetown University, Washington, DC—Mary Sidawy (histopathologist),
DDL Diagnostic Laboratory, Netherlands—Wim Quint (virologist, HPV DNA testing), Leen-Jan van Doorn (HPV DNA testing).
References
- 1. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 2. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–819. [DOI] [PubMed] [Google Scholar]
- 5. Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. [DOI] [PubMed] [Google Scholar]
- 6. Castle PE, Stoler MH, Wright TC, Jr, et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12(9):880–890. [DOI] [PubMed] [Google Scholar]
- 7. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66(21):10630–10636. [DOI] [PubMed] [Google Scholar]
- 9. Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. [DOI] [PubMed] [Google Scholar]
- 10. Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. [DOI] [PubMed] [Google Scholar]
- 11. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13(1):78–88. [DOI] [PubMed] [Google Scholar]
- 12. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. [DOI] [PubMed] [Google Scholar]
- 13. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–1394. [DOI] [PubMed] [Google Scholar]
- 14. Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. [DOI] [PubMed] [Google Scholar]
- 16. Arriba LN, Enerson CL, Belinson S, et al. Mexican Cervical Cancer Screening Study II: acceptability of human papillomavirus self-sampler. Int J Gynecol Cancer. 2010;20(8):1415–1423. [DOI] [PubMed] [Google Scholar]
- 17. Gravitt PE, Belinson JL, Salmeron J, et al. Looking ahead: a case for human papillomavirus testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer. 2011;129(3):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scarinci IC, Litton AG, Garces-Palacio IC, et al. Acceptability and usability of self-collected sampling for HPV testing among African-American women living in the Mississippi Delta. Womens Health Issues. 2013;23(2):e123–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petignat P, Faltin DL, Bruchim I, et al. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105(2):530–535. [DOI] [PubMed] [Google Scholar]
- 20. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. [DOI] [PubMed] [Google Scholar]
- 21. Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011;378(9806):1868–1873. [DOI] [PubMed] [Google Scholar]
- 22. Snijders PJ, Verhoef VM, Arbyn M, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer. 2013;132(10):2223–2236. [DOI] [PubMed] [Google Scholar]
- 23. Arbyn M, Ronco G, Cuzick J, et al. How to evaluate emerging technologies in cervical cancer screening? Int J Cancer. 2009;125(11):2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377(9783):2085–2092. [DOI] [PubMed] [Google Scholar]
- 25. Tabrizi SN, Brotherton JM, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14(10):958–966. [DOI] [PubMed] [Google Scholar]
- 26. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Doorn LJ, Molijn A, Kleter B, et al. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–1297. [DOI] [PubMed] [Google Scholar]
- 30. Tisnado DM, Adams JL, Liu H, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44(2):132–140. [DOI] [PubMed] [Google Scholar]
- 31. Castle PE, Rodriguez AC, Porras C, et al. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34(11):849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gravitt PE, Lacey JV, Jr, Brinton LA, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10(2):95–100. [PubMed] [Google Scholar]
- 33. Cuzick J, Bergeron C, von Knebel Doeberitz M, et al. New technologies and procedures for cervical cancer screening. Vaccine 2012;30 Suppl 5:F107–F116. [DOI] [PubMed] [Google Scholar]
- 34. Belinson JL, Du H, Yang B, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int J Cancer. 2012;130(8):1855–1860. [DOI] [PubMed] [Google Scholar]
- 35. Brotherton JM, Tabrizi SN, Garland SM. Does HPV type 16 or 18 prevalence in cervical intraepithelial neoplasia grade 3 lesions vary by age? An important issue for postvaccination surveillance. Future Microbiol. 2012;7(2):193–199. [DOI] [PubMed] [Google Scholar]
- 36. Porras C, Rodriguez AC, Hildesheim A, et al. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]


