Abstract
Background
The growth modulation index (GMI) is the ratio of time to progression with the nth line (TTPn) of therapy to the TTPn−1 with the n-1th line. GMI >1.33 is considered as a sign of activity in phase II trials.
Patients and Methods
This retrospective analysis evaluated the concordance between the GMI and the efficacy outcomes in 279 patients with advanced soft tissue sarcoma (ASTS) treated with trabectedin 1.5 mg/m² (24-h infusion every 3 weeks) in four phase II trials.
Results
One hundred and forty-two (51%) patients received one prior line and 137 ≥2 lines. The median TTPn was 2.8 months (range 0.2–26.8), whereas the median TTPn−1 was 4.0 months (0.3–79.5). The median GMI was 0.6 (0.0–14.4). Overall, 177 patients (63%) had a GMI <1; 21 (8%) a GMI equal to 1–1.33 and 81 (29%) a GMI >1.33, which correlated with the median overall survival in those patients (9.1, 13.9 and 23.8 months, respectively, P = 0.0005). A high concordance rate between the GMI and response rate (P < 0.0001) and progression-free survival (PFS, P < 0.0001) was observed. Good performance status (PS) was the only factor associated with GMI >1.33 (PS = 0; P < 0.04).
Conclusions
A high GMI was associated with favorable efficacy outcomes in patients treated with trabectedin. Further research is needed to assess GMI as an indicator in this setting.
Keywords: growth modulation index, sarcoma, time-to-progression ratio, trabectedin
introduction
Despite the use of doxorubicin (Adriamycin)-based regimen as the first-line treatment, <5% of patients with advanced/metastatic soft tissue sarcoma (ASTS) can be ‘cured’ [1, 2]. A majority of these patients have eventually experienced a disease progression. In this situation, the primary aim of a second-line treatment is to prolong overall survival (OS) with acceptable toxic effects and quality of life [1]. There is no consensual second-line treatment for ASTS patients [3]. In this setting, trabectedin (Yondelis®) had constantly reached the predefined level of activity fixed in phase II trials [4–7]. The schedule with a dose of 1.5 mg/m² given as a 24-h intravenous (i.v.) infusion every 3 weeks (q3w) provided a higher overall response rate and longer median progression-free survival (PFS) than the weekly schedule where trabectedin 0.58 mg/m2 was given as a 3-h weekly infusion for 3 weeks of a 4-week cycle [8–10]. Regarding activity assessment of second-line treatment, the tumor growth delay appears a more appropriate objective than tumor shrinkage [11–13]. Therefore, 3-month or 6-month PFS rates sound better study end-points than objective response achieved at the same time points. However, these PFS-based end-points do not take into account the extraordinary heterogeneity of STS [1]. More than 50 different subtypes of STS have been described, exhibiting some major differences in their clinical course and their chemosensitivity. For example, the term ‘vascular sarcomas’ gather at least two different entities with opposite behaviors; metastatic epitheloid hemangioendotheliomas are usually indolent with spontaneous long-lasting stabilizations [14] and on the opposite, angiosarcomas are usually very aggressive [15]. This heterogeneity could be partially taken into account in single-arm stratified clinical trials. In the most recently published trials, at least four different subgroups of ASTS have been separated: liposarcomas, leiomyosarcomas, synovial sarcoma and other sarcomas [16, 17]. Nevertheless, this partition remains debatable because, for instance, the first group gathers several entities (myxoid liposarcomas, round cell liposarcomas, well-differentiated/dedifferentiated liposarcomas….), which substantially differ in their clinical courses, their molecular signatures and their intrinsic chemosensitivity [18, 19]. Moreover, most of clinical trials exploring the activity of second-line treatment are phase II trials [3].
The use of randomized phase II trials is the most common method to manage tumor heterogeneity in designing phase II trials [9]. An internal comparator (based on the standard treatment) is used to verify the objective response rates, the time to progression (TTP) and the OS observed in study population as defined by eligibility criteria. Nevertheless, this method is hardly feasible in the case of refractory ASTS because there is no consensual standard second-line treatment and because the use of a placebo, without crossover, is ethically debatable. In 1998, Von Hoff has proposed another approach, which consisted on the use of intra-patient comparison of successive TTP [20]. The ratio of the TTP with the second (or later) line (TTPn) divided by the TTPn−1 with the first line treatment was termed growth modulation index (GMI). Each couple tumor/patient is its own control. The everyday observation that underscores this approach is that TTP tends to become shorter with successive chemotherapy lines. This approach is appealing because it could achieve the dual goals of having a controlled, TTP-based and single treatment design. Despite its potential utility, GMI remains rarely used [21–23]. There are two major pitfalls in using GMI as primary end-point in phase II screening trials: the need to know a priori the correlation between TTPn−1 and TTPn and the choice of the appropriate threshold to define ‘responders’ [24, 25]. Since successive TTPs tend to become shorter, a GMI >1.0 (or, more conservatively >1.33 to eliminate chance fluctuations) should be considered as a sign of activity [20].
In the present exploratory retrospective analysis, we aimed to explore the interest of GMI in ASTS patients receiving an active drug, hereby being trabectedin as second or later line treatment after failure or intolerance to doxorubicin and ifosfamide [4–6, 9, 26].
patients and methods
patients
We analyzed pooled data from 279 adult STS patients who received single-agent trabectedin 1.5 mg/m2 given as a 24-h continuous i.v. infusion q3w in four phase II completed clinical trials [4–6, 9].
primary end-point
The primary end-point was the GMI, as defined by Von Hoff: GMI=TTPn/TTPn−1. [20]. We have explored the interest of two potential thresholds: GMI > 1.0 and GMI > 1.33. GMI > 1.33 was defined by Von Hoff as the sign of drug activity. For GMI calculation, TTP(s) were used as a unidimensional variable. The GMI was available in all cases. In this exploratory retrospective analysis, both TTPs have been supplied by investigator centers, and there was no central review.
statistical analysis
A description of the study population used classical descriptive statistics: percentages for categorical data, median and range for continuous data. Correlations between variables were measured by usual statistics, Pearson's and Spearman's coefficients for continuous variables and Fisher's exact test for categorical variables. Indeed associations between the GMI and time to event variables (e.g. PFS and OS) were assessed by means of the Kaplan–Meier method and comparisons between them were done by means of the log-rank test. For the assessment of possible prognostic factors for GMI logistics regressions have been used.
results
patients
Analysis population was comprised of 170 women (60.9%) and 109 men (39.1). The median age was 52 (19–81). The performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG) was 0 in 137 cases (49.1%), 1 in 140 cases (50.2%) and 2 in 1 case (0.4%), respectively. The histological subtypes were leiomyosarcoma in 145 cases (52.0%), liposarcoma in 59 cases (21.1%), synovial sarcoma in 23 cases (8.2%) and other subtypes in 52 cases (18.6%). Tumour grade was 1 in 27 cases (9.7%), 2 in 71 cases (25.4), 3 in 130 cases (46.6), and this grade was unknown or not assessable in 51 cases (18.3%). The most common primary sites were uterus (53 cases, 19.0%) and limb/superficial trunk (98 cases, 35.1%). Liver, lung and bone metastasis were present in 69 (24.7%), 178 (63.8%) and 23 (8.2%) patients, respectively. Two hundred and seventy-two (97.5%), 230 (82.4%), 55 (19.7%) and 41 (14.7%) patients have previously received doxorubicin, ifosfamide, dacarbazine and gemcitabine, respectively. One hundred and forty-two patients (50.9%) have had received trabectedin as a second-line treatment
growth modulation with trabectedin
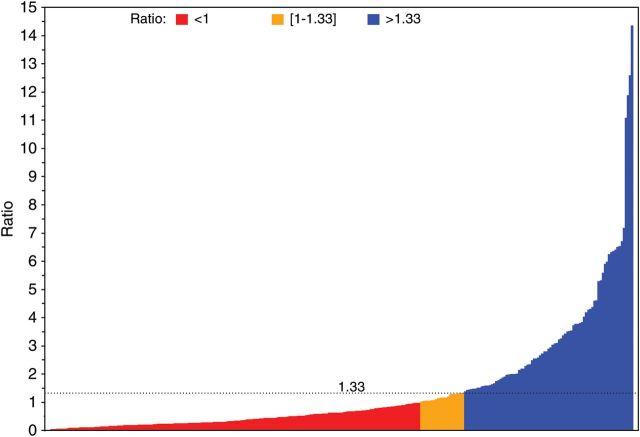
The median TTPn was 2.8 (range 0.2–26.8). The median TTPn−1 was 4.0 months (range 0.3–79.5). The Spearman Rho between TTPn and TTPn−1 was 0.07 (P = 0.2293). The median GMI was 0.6 (range 0.0–14.4). One hundred and seventy-seven patients (63.4%) experienced a GMI <1, 21 (7.5%) a GMI = (1–1.33) and 81 (29.0%) a GMI > 1.33 (Figure 1).
Figure 1.
Waterfall plot depicting the growth modulation index (GMI) in patients with metastatic soft tissue sarcoma receiving trabectedin as salvage chemotherapy.
relation between the GMI and other activity end-points
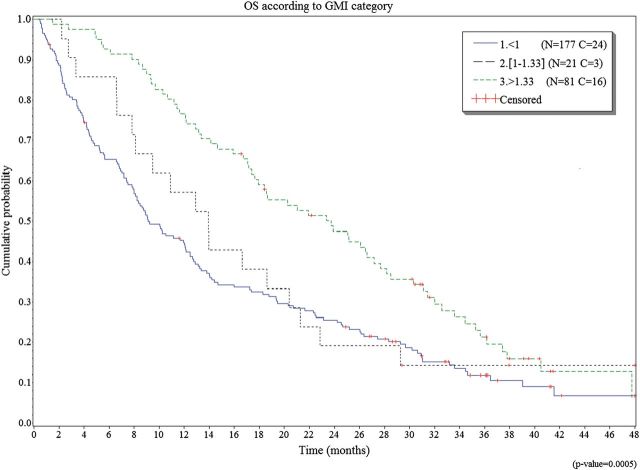
As expected, there was a strong relation between the GMI and PFS (log-rank test = <0.0001; Table 1). There was also a strong relation between the best objective response and the GMI (Fisher's exact test =< 0.0001; Table 1). The median OS was 12.9 months. There was a strong correlation between OS and GMI (log-rank test 0.0005). Table 1 and Figure 2 describe OS for the different values of GMI.
Table 1.
Relation between the GMI and other activity end-points.
| Categories | GMI < 1.0 (n = 177) | GMI=(1.00–1.33) (n = 21) | GMI > 1.33 (n = 81) | P value |
|---|---|---|---|---|
| PFS | ||||
| Censored | 18 (10.2%) | 2 (9.5%) | 21 (25.9%) | <0.0001* |
| Median PFS (months) | 1.8 | 3.2 | 7.6 | |
| PFS at 3 months | 29.6% | 52.4% | 82.6% | |
| PFS at 6 months | 12.3% | 28.6% | 56.8% | |
| Best objective response | ||||
| Assessable | 177 (100.0%) | 21 (100.0%) | 79 (97.5%) | <0.0001** |
| Progressive disease | 107 (60.5%) | 9 (42.9%) | 12 (14.8%) | |
| Stable disease | 67 (37.9%) | 12 (57.1%) | 50 (61.7%) | |
| Partial response | 3 (1.7%) | 0 | 17 (21.0%) | |
| Overall survival | ||||
| Censored | 24 (13.6%) | 3 (14.7%) | 16 (19.8%) | =0.0005* |
| Median OS (months) | 9.1 | 13.9 | 23.8 | |
| OS at 12 months | 44.7% | 57.1% | 76.5% | |
| OS at 24 months | 25.5% | 19.0% | 47.5% | |
| OS at 36 months | 11.8% | 14.3% | 21.3% | |
*Log-rank test; **Fisher's exact test.
GMI, growth modulation index; OS, overall survival; PFS, progression-free survival.
Figure 2.
Median overall survival according the different values of growth modulation index (GMI).
factors associated with GMI >1.33
The univariate logistic analysis identified one factor associated with GMI >1.33, which was PS = 0 [OR = 1.76 (1.04–2.98)] (Table 2). Tumor grade and histological subtypes were not associated with GMI > 1.33.
Table 2.
Factors associated with the GMI >1.33
| Parameters | Categories | GMI > 1.33 n (%) | Odds ratio (OR, 95% CI) | P value |
|---|---|---|---|---|
| Age | <52 | 38 (27.9) | 0.90 (0.54; 1.51) | 0.6954 |
| ≥52 | 43 (30.1) | 1 | ||
| Tumor grade | Other | 48 (32.2) | 1 | 0.2108 |
| High | 33 (25.4) | 1.40 (0.83–2.36) | ||
| Histological subtypes | Lipo- and Leiomyo-sarcomas | 64 (31.4) | 1.56 (0.84–2.89) | 0.1576 |
| Other subtypes | 17 (22.7) | 1 | ||
| Performance status (Eastern Cooperative Oncology Group, ECOG) | 0 | 48 (35.0) | 1.76 (1.04–2.98) | 0.0388 |
| 1 & 2 | 33 (23.4) | 1 | ||
| Ifosfamide | No prior treatment | 14 (28.6) | 0.97 (0.49–1.92) | 0.9378 |
| Prior treatment | 67 (29.1) | 1 | ||
| Dacarbazine | No prior treatment | 65 (29.0) | 1.00 (0.52–1.91) | 0.9915 |
| Prior treatment | 16 (29.1) | 1 | ||
| Gemcitabine | No prior treatment | 71 (29.8) | 1.32 (0.61–2.83) | 0.4794 |
| Prior treatment | 10 (24.4) | 1 | ||
| Number of previous lines of chemotherapy | 1 | 41 (28.9) | 0.98 (0.59; 1.65) | 0.9525 |
| ≥2 | 40 (29.2) | 1 | ||
| Lung metastasis | Absence | 34 (33.7) | 1.41 (0.83–2.4) | 0.2001 |
| Presence | 47 (26.4) | 1 | ||
| Liver metastasis | Absence | 59 (28.4) | 0.91 (0.50–1.64) | 0.7425 |
| Presence | 21 (30.4) | 1 | ||
| Bone metastasis | Absence | 73 (28.5) | 0.75 (0.30–1.84) | 0.5270 |
| Presence | 8 (34.8) | 1 | ||
| Other metastases | Absence | 25 (29.4) | 1.03 (0.59–1.80) | 0.9262 |
| Presence | 56 (28.9) | 1 | ||
| Hemoglobin (g/dl) | <12 | 35 (28.9) | 0.98 (0.58; 1.66) | 0.9458 |
| ≥12 | 46 (29.3) | 1 | ||
| Albumin (g/l) | <35 | 8 (19.5) | 0.52 (0.23; 1.18) | 0.1171 |
| ≥35 | 72 (31.9) | 1 |
GMI, growth modulation index.
discussion
The key findings of this exploratory retrospective analysis could be summarized as follows: (i) 29.0% of ASTS patients receiving trabectedin as a salvage therapy experienced a GMI >1.33, (ii) there were strong correlations between the GMI and other classical end-points for phase II screening trials: PFS and best objective response rate, (iii) patients with GMI > 1.33 experienced longer OS (24 months); this observation corroborating the hypothesis that a decrease of tumor growth may positively affect the OS, (iv) there was no correlation between TTPn and TTPn−1; in other words, patients benefiting from trabectedin were not systematically benefiting from the prior line and (v) as observed in first line [26], the main prognostic factor remains PS [26].
The GMI, first proposed by Von Hoff, is an appealing end-point for primarily evaluating cytostatic cancer treatments in phase II trials [20]. This progression-based measure has the dual advantages of being a more suitable indicator of delayed growth than traditional response measures and is a more controlled outcome than marginal TTP. This criterion remains rarely used. Von Hoff et al. have used the GMI to measure the activity of targeted therapies selected by molecular profiling in patients having failed to all effective treatments [27]. Eighteen out of 86 patients [20.9%; (12.3–29.5)] experienced GMI ≥ 1.3; this targeted therapeutic approach was considered as promising. Comella et al. have measured the activity of the combination oxaliplatin–raltitrexed–levo-folinic acid–5-fluorouracil in pretreated colorectal cancer patients. The GMI was ≥ 1.33 in 16 out of 50 patients [32.0% (19.0–44.9)] [23]. Bonetti et al. have used GMI as metric for measuring the activity of the combination oxaliplatin–5-fluorouracil in pretreated colorectal cancer patients [22]. Seventeen out of 34 patients showed a GMI ≥1.33 [50.0% (33.1–66.8)]. Both the regimens were considered as promising on the basis of the response rate. In the literature, there is only one report of GMI in sarcoma patients. Zalcberg et al. have measured the activity of imatinib at a dose of 800 mg/day in gastrointestinal stromal tumor patients experiencing progression after administration of imatinib 400 mg/day [21]. One hundred patients were assessable for GMI. Twenty seven patients showed a GMI ≥1.33 [24.5% (16.5–32.6)]. This increasing dose of imatinib is considered as an active treatment, especially in patients with exon 9 kit mutations [28]. The literature is scarce on GMI, but it appears that the rate of patients experiencing GMI ≥ 1.33 depends on the tumor type. In the present report, the rate of GMI ≥ 1.33 was 29.0% (23.7–34.3). There are no published data estimating this proportion in patients with refractory ASTS receiving another second-line treatment. The concept of GMI appears particularly appealing in the case of drug providing a low rate of objective response but associated with a long-lasting tumor growth delay, such as current molecular targeted therapy. GMI appears useful for the assessment of trabectedin activity in patients with sarcoma. Because, this drug acts as well as a classical DNA-binding cytotoxic agent, but also as a complex cytostatic agent (impact on the expression of oncogenic transcripts in translocation-related sarcoma, impact on the microenvironment) [29].
There are some limitations in this retrospective analysis. Whereas TTPn was precisely calculated from data generated by clinical trials, TTPn−1 was retrospective data, given by the physician. In the present study, there was no central review for both TTPs. Nevertheless, as recommended by Von Hoff [20], in order to take into account the fluctuations in TTPn−1 estimations, we have considered that a large improvement of the TTP (+ 33%) was a subjective sign of drug activity. The ideal situation to precisely measure TTPn−1 as well as TTPn should be a randomized clinical trial with cross-over evaluating trabectedin versus, for example, best physician's choice second-line chemotherapy in ASTS. Second, the TTP is influenced by frequency of the follow-up evaluation (the ‘time-assessment bias’) and the clinical perception by the physician of tumor progression before formal tumor assessment (e.g. symptomatic deterioration suggesting disease progression and leading to drug discontinuation for ‘symptomatic progression’). Nevertheless, the ‘time-assessment bias’ also effects both the estimation of PFS and PFS rate at fixed time-points. Third, precautions in the interpretation of the results might be taken into account because one part of the ratio calculation (Trabectedin TTP) is obviously correlated with the results in RR/PFS/OS. Lastly, the correlation between TTPn−1 and TTPn was low, and this could constitute an obstacle to design a clinical trial with GMI as primary end-point [24, 25]. The main reason explaining the low correlation between TTP(s) is certainly the heterogeneity of both subtypes of sarcoma and the nature of the prior chemotherapy line (single-agent versus combination therapies, doxorubicin-based versus non-doxorubicin-based regimens). This needs further exploratory analysis on larger cohorts of patients.
The GMI appears as an appealing complementary end-point for screening phase II trials. This end-point measures the tumor growth delay. This end-point takes into account the heterogeneity of ASTS, which could not be summarized by four histological subtypes and tumor grade (Table 2). This study confirms that trabectedin is an active drug in pretreated ASTS. This is the first report setting up the rate of patients experiencing GMI ≥ 1.33 in these circumstances and the correlation between TTPn−1 and TTPn. This could be useful to assess GMI as exploratory end-points in further clinical trials. However, at that time, despite its appealing aspects, there is no sufficient evidence for the use of GMI as primary end-point when designing clinical trial in sarcoma patients.
disclosures
AC, JG, AN, PZ and MJP are employed by PharmaMar SA. NP, GDD, JYB, RGM, SPC, IJ, MVM, PS, JV, PC, SR, HJS and ALC are investigators in the clinical trials funded by PharmaMar SA and have received research grant from PharmaMar SA.
acknowledgements
The authors would like to acknowledge Séverine Marchant and Adnan Tanović for providing writing assistance for the manuscript. This work is presented in part at the ASCO 2012 Annual meeting, 01–05 June 2012, Chicago, USA (Abstract 10013).
references
- 1.Clark MA, Fisher C, Judson I. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. doi:10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Blay JY, van Glabbeke M, Verweij J, et al. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer. 2003;39:64–69. doi: 10.1016/s0959-8049(02)00480-x. doi:10.1016/S0959-8049(02)00480-X. [DOI] [PubMed] [Google Scholar]
- 3.Penel N, Van Glabbeke M, Marreaud S, et al. Testing new regimens in patients with advanced soft tissue sarcoma: analysis of publications from the last 10 years. Ann Oncol. 2011;22:1266–1272. doi: 10.1093/annonc/mdq608. doi:10.1093/annonc/mdq608. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. doi:10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 5.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. doi:10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 6.Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. doi:10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 7.Van Glabbeke M, Hogendoorn PCW, Mouridsen H, et al. Progression free rate as the principal end-point for phase II trial on soft tissue sarcoma: what should be the target? A retrospective study of the EORTC soft tissue and bone sarcoma (STBSG) database. J Clin Oncol. 2001;20:354. (abstract 1413) [Google Scholar]
- 8.Krasner CN, McMeekin DS, Chan S, et al. A phase II study of trabectedin single agent in patients with recurrent ovarian cancer previously treated with platinum-based regimens. Br J Cancer. 2007;17:1618–1624. doi: 10.1038/sj.bjc.6604088. doi:10.1038/sj.bjc.6604088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. doi: 10.1200/JCO.2008.21.0088. doi:10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 10.del Campo JM, Roszak A, Bidzinski M, et al. Phase II randomized study of trabectedin given as two different every 3 weeks dose schedules (1.5 mg/m2 24 h or 1.3 mg/m2 3 h) to patients with relapsed, platinum-sensitive, advanced ovarian cancer. Ann Oncol. 2009;20:1794–1802. doi: 10.1093/annonc/mdp198. doi:10.1093/annonc/mdp198. [DOI] [PubMed] [Google Scholar]
- 11.Van Glabbeke M, Verweij J, Judson I. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. doi:10.1016/S0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 12.Cousin S, Taieb S, Penel N. A paradigm shift in tumour response evaluation of targeted therapy: the assessment of novel drugs in exploratory clinical trials. Curr Opin Oncol. 2012;24:338–344. doi: 10.1097/CCO.0b013e3283528b73. doi:10.1097/CCO.0b013e3283528b73. [DOI] [PubMed] [Google Scholar]
- 13.Verweij J. Other endpoints in screening studies for soft tissue sarcomas. Oncologist. 2008;13(Suppl 2):27–31. doi: 10.1634/theoncologist.13-S2-27. doi:10.1634/theoncologist.13-S2-27. [DOI] [PubMed] [Google Scholar]
- 14.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. doi:10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 15.Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26:5269–5274. doi: 10.1200/JCO.2008.17.3146. doi:10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 16.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. doi:10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 17.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. doi:10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. doi:10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 19.Italiano A, Toulmonde M, Cioffi A, et al. Advanced well-differentiated/dedifferentiated liposarcomas: role of chemotherapy and survival. Ann Oncol. 2011;23:1601–1607. doi: 10.1093/annonc/mdr485. [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs––twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998;4:1079–1086. [PubMed] [Google Scholar]
- 21.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–1757. doi: 10.1016/j.ejca.2005.04.034. doi:10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Bonetti A, Zaninelli M, Leone R, et al. Use of the ratio of time to progression following first- and second-line therapy to document the activity of the combination of oxaliplatin with 5-fluorouracil in the treatment of colorectal carcinoma. Ann Oncol. 2001;12:187–191. doi: 10.1023/a:1008354909478. doi:10.1023/A:1008354909478. [DOI] [PubMed] [Google Scholar]
- 23.Comella P, Casaretti R, Crucitta E, et al. Oxaliplatin plus raltitrexed and leucovorin-modulated 5-fluorouracil i.v. bolus: a salvage regimen for colorectal cancer patients. Br J Cancer. 2002;86:1871–1875. doi: 10.1038/sj.bjc.6600414. doi:10.1038/sj.bjc.6600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mick R, Crowley JJ, Carroll RJ. Phase II clinical trial design for noncytotoxic anticancer agents for which time to disease progression is the primary endpoint. Control Clin Trials. 2000;21:343–359. doi: 10.1016/s0197-2456(00)00058-1. doi:10.1016/S0197-2456(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 25.Kovalchik S, Mietlowski W. Statistical methods for a phase II oncology trial with a growth modulation index (GMI) endpoint. Contemp Clin Trials. 2011;32:99–107. doi: 10.1016/j.cct.2010.09.010. doi:10.1016/j.cct.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Penel N, Glabbeke MV, Mathoulin-Pelissier S, et al. Performance status is the most powerful risk factor for early death among patients with advanced soft tissue sarcoma: the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (STBSG) and French Sarcoma Group (FSG) study. Br J Cancer. 2011;104:1544–1550. doi: 10.1038/bjc.2011.136. doi:10.1038/bjc.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Hoff DD, Stephenson JJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. doi:10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 28.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. doi:10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxioid liposarcoma cells. Cancer Res. 2010;70:2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. doi:10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]