Abstract
Introduction:
Individuals with substance use disorders (SUDs) experience increased smoking-related morbidity and mortality but severely compromised smoking treatment benefits. Residential SUD treatment settings may be particularly positioned to target smoking, with ever-increasing smoking bans and culture shifts, but most smokers continue smoking. This study examined the effects of contingency management (CM) for increasing smoking abstinence in residential patients.
Methods:
Smokers interested in quitting were recruited from a residential SUD program for men and were randomized to frequent smoking monitoring with behavioral support (monitoring; n = 21) or that plus smoking abstinence–contingent (expired carbon monoxide [CO] ≤ 6 ppm; urinary cotinine ≤ 30ng/ml) incentives (CM, n = 24) for 4 weeks. After setting a quit date, procedures included daily behavioral support and smoking self-reports, 2 CO samples (a.m./p.m.) Monday through Friday, and cotinine tests on Mondays. CM participants received escalating draws for prizes ($1, $20, and $100 values) for negative tests; positive and missed samples reset draws. Follow-ups involved samples, self-reported smoking, and self-efficacy (weeks 4, 8, 12, and 24).
Results:
Percent days CO-negative was higher with CM (median [interquartile range] 51.7% [62.8%]) compared to monitoring (0% [32.1%]) (p = .002). Cigarettes per day declined and point-prevalence abstinence increased through follow-up (p < .01), without significant group by time effects (p > .05). Abstinence self-efficacy increased overall during the intervention and more with CM compared to monitoring and was associated with abstinence across conditions through follow-up.
Conclusions:
CM improved some measures of response to smoking treatment in residential SUD patients.
INTRODUCTION
Cigarette smoking remains a leading cause of preventable morbidity, mortality, and lost productivity (World Health Organization, 2011). Individuals with additional non-nicotine substance use disorders (SUD) are disproportionately affected. They have higher smoking rates, at about 90% (Kalman, 1998) compared to 19% of U.S. adults (Schiller, Lucas, & Peregoy, 2012) and 30% (1.2 billion smokers) worldwide (World Health Organization). They smoke more heavily (Lasser et al., 2000), are more likely to die from smoking-related complications than SUD problems (Hurt et al., 1996), and have mortality rates four times higher than nonsmokers with SUD (Hser, McCarthy, & Anglin, 1994).
One barrier to treating smoking with SUD is a perceived lack of demand, but up to about 80% of SUD smokers contemplate quitting smoking (Richter, Gibson, Ahluwalia, & Schmelze, 2001). Another barrier is concern that treating smoking might jeopardize SUD recovery. However, smoking abstinence is associated with decreased illicit drug and alcohol use (Baca & Yahne, 2009), and 25% decreased likelihood of SUD relapse (Prochaska, Delucchi, & Hall, 2004). Currently, addressing smoking with SUD is prescribed widely and efforts are on the rise (Guydish et al., 2012; Sherman, 2008; Williams et al., 2005). Nevertheless, smoking quit rates in SUD smokers are low, with or without behavioral and pharmacological smoking treatment (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Hays, Croghan, Schroeder, Ebbert, & Hurt, 2011; Lasser et al., 2000; Richter, McCool, Catley, Hall, & Ahluwalia, 2006; Stein et al., 2006).
Residential SUD treatment settings may be especially positioned to address smoking. The context of high-intensity SUD treatment services may similarly support intensive quit smoking services and may act synergistically with effective smoking interventions to improve smoking outcomes. Further, the accelerating pace of smoking bans and related policies creates a pressing need to identify effective smoking treatments for these patients.
To date, most research on smoking bans in these settings focuses predominantly on attitudes pre-, post-, or without policy changes. Reports that do examine smoking behavior indicate most patients continue smoking despite bans (Callaghan et al., 2007; Guydish et al., 2012). Those who quit, even with counseling and pharmacotherapy, precipitously return to smoking following SUD treatment (Guydish et al., 2012; Prochaska et al., 2004). Intensive and multimodal strategies are likely needed to tackle these disparities in smoking-related harm and treatment efficacy and to effectively treat smoking in this population.
Contingency management (CM) is the most effective psychosocial treatment for SUD (Dutra et al., 2008) and may improve smoking outcomes for SUD smokers. CM involves identifying a clinically relevant, objectively defined target behavior (e.g., expired carbon monoxide [CO] reading ≤ 6 ppm, indicating smoking abstinence), frequently monitoring that behavior, and providing tangible incentives (vouchers and prizes) when it occurs. CM improves abstinence in alcohol, cocaine, marijuana, and opioid-maintained treatment patients (Higgins, Silverman, & Heil, 2008; Petry, 2012). Most evidence supporting CM for smoking comes from tightly controlled quasi-laboratory-based studies, often in nontreatment seekers (e.g., Roll, Higgins, & Badger, 1996; Stitzer, Bickel, Bigelow, & Liebson, 1986).
Research on CM for treating smoking in SUD patients has focused primarily on opioid maintenance settings, in part because clinic attendance is daily, coinciding with the ability to assess smoking with CO tests and reinforce abstinence (Dunn et al., 2010; Dunn, Sigmon, Thomas, Heil, & Higgins, 2008; Shoptaw et al., 2002; Shoptaw, Jarvik, Ling & Rawson, 1996). Two demonstration projects examined CM for smoking abstinence in nonopioid medication settings. Robles et al. (2005) invited 16 women in residential SUD treatment who continued smoking more than 10 cigarettes daily despite smoking cessation counseling to receive education, brief support, and 4 weeks of thrice-daily CO tests Monday–Friday and a urinary cotinine (COT) test each Monday, with abstinent-contingent reinforcement. Abstinence increased during CM compared to pre- and postintervention. We (Alessi, Petry, & Urso, 2008) recruited 24 men in residential SUD treatment to receive CO monitoring four times weekly, a random COT test weekly, brief behavioral support, and abstinence-contingent incentives (n = 12) or the same without incentives (n = 12) for 4 weeks, with CO testing tapering thereafter. CO-based abstinence increased with CM relative to the control condition. Overall, these studies are promising but indicate wide variability in response to CM and are few in number.
The construct of self-efficacy (Bandura, 1977) may help explain variability in treatment response. Abstinence self-efficacy mediates the relation between smoking treatment and increased abstinence (Hendricks, Delucchi, & Hall, 2010; McCarthy et al., 2010; Stanton, Lloyd-Richardson, Papandonatos, de Dios, & Niaura, 2009), and abstinence increases self-efficacy (Perkins, Parzynski, Mercincavage, Conklin, & Fonte, 2012). In three studies with all participants receiving CM, smoking abstinence and self-efficacy were positively related (Amodei & Lamb, 2010; Lamb, Morral, Galbicka, Kirby, & Iguchi, 2005; Romanowich, Mintz, & Lamb, 2009), but whether CM differentially impacts self-efficacy to affect abstinence could not be evaluated without a non-CM condition.
The purpose of this study was to conduct a randomized clinical trial of CM for smoking abstinence in residential SUD treatment patients interested in quitting. This population is increasingly required to adhere to smoke-free policies but has very low smoking cessation rates, and the setting may allow for twice-daily CO testing, a schedule critical to the success of CM for smoking abstinence. We hypothesized that CM would increase smoking abstinence compared to behavioral support and monitoring without CM. We also assessed relations between during treatment self-efficacy and abstinence.
METHODS
Participants and Setting
Participants (N = 45) were men entering long-term (>6 months) adult (age 18 minimum) residential SUD treatment in southern Connecticut. Inclusion criteria were smoking 10 cigarettes per day minimum, study intake CO ≥8 ppm (biochemical confirmation of smoking status), and self-reported interest in quitting smoking. Individuals receiving or expecting outside smoking treatment, with serious and uncontrolled psychiatric illness (e.g., acute schizophrenia, suicide risk) or with substantial cognitive impairment (<23 on the Mini-Mental State Examination [MMSE]; Folstein, Folstein, & McHugh, 1975), were excluded. Pathological gamblers in recovery were also excluded because of concern that prize CM might jeopardize gambling recovery (but see Petry & Alessi, 2010; Petry et al., 2006). Research staff referred ineligible patients to Connecticut’s QuitLine.
Residential treatment services included individual and group therapy, psychoeducational groups, self-help and 12-step meetings, and other resources as needed. Treatment did not include opiate substitution or other medications for treating SUD. Substance use was monitored using urine tests, instant swabs, and breathalyzers, with positive tests typically handled clinically by staff. Study participation did not affect standard treatment services. Smoking was prohibited indoors, and smoking breaks occurred about every other hour during treatment programming. This residential program did not include any smoking cessation treatment; neither the program nor study provided smoking cessation medication. A bachelor’s-level trained research assistant administered all study procedures following completion of a University of Massachusetts online workshop on basic skills for working with smokers and with direct supervision by the principal investigator (SMA). The University Institutional Review Board approved this study. Recruitment and follow-up occurred from June 2008 through October 2010, through funding cycle.
Procedures
Baseline
Following written informed consent and determining eligibility, participants completed two quit smoking preparation sessions. Study visit Day 1 involved submitting two CO samples (5hr apart minimum) and completing a 30-min counseling session based on clinical practice guidelines and a consumer guide on quitting (Agency for Health Care Policy and Research, 1996). A quit plan was developed, including anticipating potential challenges and identifying emotional, environmental, social support, and other coping strategies. 4.8 (3.9) days later on visit Day 2, progress and obstacles were discussed, the quit plan updated, and a target quit date scheduled for within 1 week.
Intervention
Following baseline, randomization to one of two conditions occurred using an urn procedure (Stout, Wirtz, Carbonari, & Del Boca, 1994) and stratifying on at least one CO ≤ 6 ppm during baseline (yes/no) (73.3% submitted no baseline CO < 6 ppm).
Monitoring (n = 21). Starting on the quit date, CO samples were collected twice-daily (5hr apart minimum) Monday through Friday during Weeks 1–4. Research staff discussed test results with participants. Brief (about 5min) behavioral support was delivered to encourage achieving and maintaining smoking abstinence. Topics discussed included personal reasons for quitting, skill-based items, and craving control tips. Individualized discussion occurred about challenges and successes.
Cigarettes smoked and use of alcohol, drugs, and medication were tracked at every session. COT tests occurred each Monday. Breath alcohol and urinary cocaine, marijuana, and opiate tests occurred weekly, on a random day.
Monitoring Plus CM (CM; n = 24). For CM participants, in addition to the procedures above, abstinence-contingent incentives were available Weeks 1–4 for CO tests ≤ 6 ppm and COT ≤ 30ng/ml.
During Week 1, each smoking-negative CO test was reinforced to encourage even short periods of abstinence during the critical initial days (e.g., Romanowich & Lamb, 2010). A “Guaranteed Prize” bowl contained 70 cards, with 64 worth a prize about $1 in value (e.g., toiletries, sports drink, and gum), 5 worth a $20 prize (exercise weights, portable games, Barnes, and Noble gift cards), and 1 worth a $100 prize (linens, TV, and DVD player). Draws for CO-negative tests started at one and increased by one for each consecutive negative test, capping at five (40 draws maximum in Week 1). A positive test (or unexcused missed sample) reset draws to one for the next negative test, and missed samples cleared in advance (court appearance, doctor appointment) did not reset draws. Each session, participants received prizes earned and a written reminder about draws available next time.
Standard Prize Bowl. During Weeks 2–4, the bowl for CO-negative tests contained 500 cards, 50% worth a prize. Of those, 219 were $1 prizes, thirty $20 prizes, and one $100 prize. Five bonus draws were available per COT-negative test. In total, patients could earn 150 draws from this prize bowl in Weeks 2–4 for all CO samples submitted and negative, and 15 draws for three COT-negative tests. Transition from the Guaranteed to Standard Prize Bowl did not affect the number of draws available, so that patients at the cap of five draws per CO-negative test continued drawing five times per negative test when they proceeded to Week 2.
Follow-up
Follow-up interviews occurred at Weeks 4, 8, 12, and 24 relative to the quit date. Assessments of smoking, self-efficacy, substance use, and medications were completed, and CO, COT, breath alcohol, and urine drug tests conducted.
See Figure 1 for the Consolidating Standards of Reporting Trials (CONSORT) diagram of participant flow recruitment through follow-up and Supplementary Table 1 for the CONSORT checklist.
Figure 1.
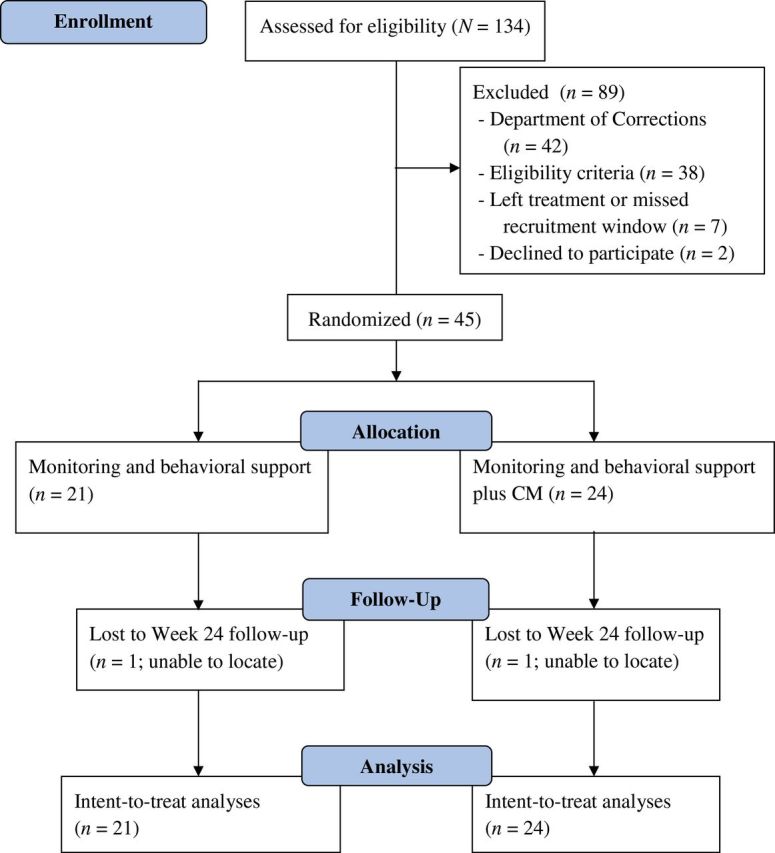
The flow of participants from the point of initial contact through data analysis per Consolidating Standards of Reporting Trials (CONSORT) guidelines. CM = contingency management.
Assessments
At intake, a case report form captured sociodemographic and smoking history. The MMSE (Folstein et al., 1975) evaluated cognitive impairment. Diagnostic and Statistical Manual of Mental Disorders, fourth edition TR criteria (American Psychological Association, 2000) determined alcohol, cocaine, marijuana, and opiate abuse/dependence. The NORC DSM Screen for Gambling Problems (NODS; Gerstein et al., 1999; National Opinion Research Center, 1999) identified pathological gambling, with a question about recovery status (no/yes). The Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was also completed, with scores ranging 0 (none) to 10 (most severe).
The Relapse Situation Efficacy Questionnaire (RSEQ; Gwaltney et al., 2001) and timeline followback (Sobell & Sobell, 1992) were administered at intake and each follow-up. The RSEQ asks about confidence in ability to abstain in specific contexts, with items rated 1–4 (“Not at all confident” to “Extremely confident”); total scores range 1–4. The timeline followback captured daily frequency and intensity of cigarette smoking and use of alcohol, cocaine, marijuana, opiates, sedatives/benzodiazepines, and smoking cessation and other medication use.
CO was tested with a calibrated PicoSmokerlyzer® CO monitor (Bedfont Scientific Ltd.) and urinary COT via the on-site immediate-results Accutest® NicAlert™ (JANT Pharmacal Corporation). Urinary cocaine, opiate, and marijuana tests were conducted with OnTrak TesTsticks (Varian), and breath alcohol tested with an Alco-Sensor® IV Altometer (Intoximeters).
Compensation
Participants received $15 for the intake, $25 per follow-up, and a $1 gift certificate or item (snacks and gum) per CO and COT sample, independent of results. CM participants could earn up to 190 draws for CO-negative tests, with average expected maximum earnings of $426.56 in prizes, and 15 draws for COT-negative tests, for an expected additional $46.43 in prizes on average.
Data Analysis
Intent-to-treat analyses (N = 45) were conducted for primary and secondary outcomes, except 43 cases contributed to the self-efficacy over time analysis due to two missed Week 4 follow-ups. Initially, baseline data were examined for differences between conditions (none found). For effects of CM on smoking during the intervention, outcomes were percent of days CO-negative and self-reported cigarettes per day. Percent of days CO-negative was computed with the numerator as number of days with at least one CO test and all CO ≤ 6 ppm and analyzed twice, first with days of no CO samples coded positive (denominator: 20 days) and again with no-sample days omitted from the denominator. Seven-day point-prevalence abstinence (PPA; defined as CO- and COT-verified self-reported no smoking) and self-reported cigarettes per day were examined through follow-up. Associations between self-efficacy and abstinence outcomes were also examined (but mediation tests were not possible due to the distribution of abstinence outcomes). Secondary outcomes were frequency of drug/alcohol-positive tests, self-reported non-nicotine substance use, study attendance, and treatment retention.
For all analyses, differences between conditions on single timepoint outcomes were examined with analysis of variance (ANOVA), Mann–Whitney U, or Pearson chi-square tests, as appropriate. The variables of past 30-day work income (U.S. dollars), number of voluntary quit attempts, percent days CO-negative, and days of self-reported substance use were examined with nonparametric tests because transformations did not correct nonnormal distributions. Changes over time intake-Week 4 were examined with repeated measures ANOVA (self-reported cigarettes per day, self-efficacy). Remaining changes over time were modeled with hierarchical mixed models, specified for dichotomous (PPA) or continuous (cigarettes per day) outcomes, with subjects entered as random effects and condition, time (days; nested within person), and the condition by time interaction as fixed effects. The sample size was sufficient to detect an effect size of about d ≥ .80 on the primary CO-negative outcome; effect size estimates or relation magnitude accompany significance tests whenever possible. Statistical significance was determined at α ≤ .05. Hierarchical Linear and Nonlinear Modeling (HLM) V6.01 was used for hierarchical mixed models and IBM® SPSS® Statistics V19 for remaining analyses.
RESULTS
Demographic and Baseline Characteristics
Demographic and baseline characteristics did not differ significantly between conditions (Table 1). Most participants were cocaine and/or opiate dependent. Overall, participants had about a 20-year smoking history, most smoked more than 15 cigarettes daily, and a majority had one previous voluntary quit attempt.
Table 1.
Demographic and Baseline Characteristics
| Variables | Monitoring (n = 21) | Monitoring plus CM (n = 24) | Significance test value (df) | p value |
|---|---|---|---|---|
| Age (average, SD) | 37.4 (9.8) | 38.4 (9.9) | F(1, 43) = 0.13 | .73 |
| Years of education (average, SD) | 11.3 (2.7) | 11.5 (2.1) | F(1, 43) = 0.04 | .84 |
| Never been married (n) | 81.0% (17) | 58.3% (14) | χ2(df = 1) = 2.67 | .10 |
| Employed full-time (n) | 66.7% (14) | 58.3% (14) | χ2(df = 1) = 0.33 | .57 |
| Income from work past 30 days (median, IQR) | $0 ($1060) | $0 ($0) | U = 218.50 | .33 |
| Ethnicity (n) | χ2(df = 1) = 0.50 | .83 | ||
| Hispanic | 14.3% (3) | 16.7% (4) | ||
| Non-Hispanic | 85.7% (18) | 83.3% (20) | ||
| Race (n) | χ2(df = 3) = 7.44 | .06 | ||
| Black | 42.9% (9) | 16.7% (4) | ||
| European American | 47.6% (10) | 79.2% (19) | ||
| Native Hawaiian/Pacific Islander | 9.5% (2) | 0% (0) | ||
| More than one race | 0% (0) | 4.2% (1) | ||
| Cigarette smoking | ||||
| Age first smoked (average, SD) | 16.1 (9.0) | 14.0 (3.1) | F(1, 43) = 1.23 | .27 |
| Cigarettes per day (average, SD) | 18.1 (7.5) | 19.2 (5.2) | F(1, 43) = 0.65 | .42 |
| Fagerström score (average, SD) | 3.7 (1.1) | 3.9 (.97) | F(1, 43) = 0.68 | .42 |
| Minnesota Nicotine Withdrawal Scale (average, SD) | 8.5 (6.3) | 8.3 (5.8) | F(1, 43) = .01 | .92 |
| At least 1 baseline CO ≤ 6 ppm (n)a | 28.6% (6) | 25.0% (6) | χ2(df = 1) = 0.07 | .79 |
| CO value (average, SD) | 18.3 (6.2) | 15.1 (5.0) | F(1, 43) = 3.78 | .06 |
| Salivary cotinineb (median, IQR) | 6 (0) | 6 (0) | U = 245.00 | .81 |
| Quitting | ||||
| Number of voluntary attempts (median, IQR) | 1.0 (0) | 1.0 (1) | U = 251.5 | .99 |
| Abstinence self-efficacy (average, SD) | 2.1 (0.4) | 2.0 (0.4) | F(1, 43) = 0.76 | .39 |
| Substance dependence (n) | ||||
| Alcohol only | 0.0% (0) | 8.3% (2) | χ2(df = 1) = 1.83 | .18 |
| Cocaine only | 4.8% (1) | 12.5% (4) | χ2(df = 1) = 0.83 | .36 |
| Opiate only | 23.8% (5) | 12.5% (3) | χ2(df = 1) = 0.98 | .32 |
| Marijuana only | 0% (0) | 0% (0) | – | – |
| Poly-dependence | 71.4% (15) | 58.3% (14) | χ2(df = 1) = 0.84 | .36 |
Note. CM = contingency management; CO = carbon monoxide; IQR = interquartile range.
aStratification variable.
bCotinine equivalent of test stick result of 6 is >2,000ng/ml per manufacturer insert.
Attendance and Adherence
On average, CM and Monitoring participants attended 27.0 (8.8) and 28.1 (9.3) study sessions in Weeks 1–4 of 40 total, respectively (F(1, 43) = 0.17, p = .69, Cohen’s d = .00). There were no study withdrawals. Three individuals in each condition were discharged from SUD residential treatment prior to the end of study participation (χ2[df = 1] = 0.03, p = .86). CM and Monitoring participants did not differ on the number of CO and COT samples submitted, with on average (SD) 26.8 (8.2) and 28.1 (8.6) CO samples (F(1, 43) = 0.27, p = .61, d = .02) and 3.5 (1.0) and 3.6 (1.1) COT samples (F(1, 43) = 0.27, p = .61, d = .02), respectively.
Smoking Behavior
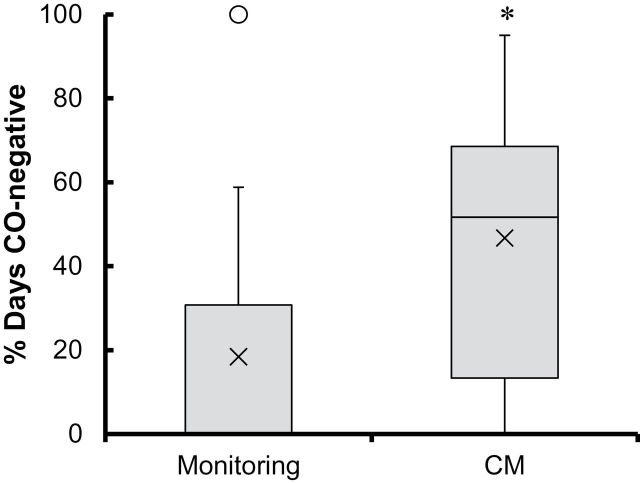
Percent of days CO-negative was greater with CM versus Monitoring, both when days of no samples were coded smoking-positive (U = 137.0, p = .008, η = 0.75) and omitting no-sample days from the denominator (U = 119, p = .002, η = 0.92) (Figure 2). In the CM and Monitoring condition, the median (interquartile range [IQR]) percent of days CO-negative was 32.5% (45%) and 0.0% (25%), respectively, with missed days coded positive, and 51.7% (55.2%) and 0.0% (30.8%), respectively, with missed days omitted from the denominator. Self-reported cigarettes per day decreased from intake through the 28-day period (F(1, 41) = 54.04, p = .00, d = .32) without differences by condition (p = .38, d = .0004) or condition by time (p = .30, d = .0009). COT-negative samples were rare, with one occurrence in the Monitoring condition and eight instances across three CM participants (p = .28).
Figure 2.
Box and whisker plot of percent of days CO-negative (CO ≤ 6 ppm) in the monitoring (n = 21) and CM (n = 24) conditions. In the monitoring condition, the median was zero. The ends of the whiskers are the minimum (bottom) and maximum (top) values save the outlier, defined as any value more than 1.5 times the length of the box from either end of the box and indicated here by an open circle for visual purposes only. All data were included in analyses. × indicates the mean; * indicates significant differences between study conditions at p = .002 for the analysis omitting days of missed samples, and p = .008 for the analysis coding days with missed samples smoking-positive. CM = contingency management; CO = carbon monoxide.
HLM analyses examined changes in smoking outcomes beyond Week 4, intake through Week 24 follow-up, and by study condition. There was a significant increase in the odds of CO- and COT-verified 7-day PPA (slope coefficient = 0.019 [SE = 0.003], T-ratio [approx. df = 43] = 4.95, p = .00, odds ratio 1.02, CI = 1.012–1.028), such that 15.6% (n = 7) of individuals were not smoking at Week 24; the condition by time interaction was not significant (p = .84). Over the same follow-up period, cigarettes smoked per day decreased (slope coefficient = −0.017 [SE = 0.007], T-ratio [approx. df = 43] = −2.59, p = .01), although again there were no differences by condition over time (p = .83). Supplementary Figure 1 depicts these trajectories over time and by study condition.
Self-Efficacy
There was a significant interaction between study condition and time (intake, Week 4) for abstinence self-efficacy (F(1, 41) = 5.70, p = .02, d = .24). Self-efficacy increased pre- to posttreatment to a greater extent on average (SD) with CM (2.0 [0.39] to 2.59 [0.61]) compared to Monitoring (2.1 [0.4] to 2.3 [0.5]). Week 4 self-efficacy was significantly correlated with percent of days CO-negative during the intervention (r = .60, p = .001), and self-efficacy and PPA measured at each follow-up were significantly correlated (r bs values = .52–.59, p = .001). PPA was not significantly correlated with changes in cigarettes smoked per day at follow-ups (p > .05).
Non-Nicotine Substance Use
Throughout, days of self-reported substance use were rare and without differences by condition (U = 192.0–219.5, p = .17–1.00), as was the number of drug-positive urine tests (χ2[df = 1] = 0.06–0.90, p = .34–.81). The number of drug-positive tests was 3 of 43 tests at Week 4, 6 of 41 tests at Week 8, 8 of 39 at Week 12, and 6 of 32 at Week 24.
Intervention and Reinforcement Exposure
CM patients earned a median (IQR) of 28 draws (89.5 draws) for CO-negative tests, resulting in $96.46 ($162.26) in prizes and constituting 20.5% (42.4%) of maximum expected earnings. They earned 0.0 (0.0) draws and $0.0 ($0.0) in prizes for COT-negative tests.
Adverse Events
The one serious adverse event involved a CM participant hospitalized after experiencing alcohol disease-related heart, liver, and lung problems. This event was deemed unrelated to study participation.
DISCUSSION
We examined a 4-week abstinence-based reinforcement intervention for reducing smoking in residential SUD treatment patients. Overall, frequent CO monitoring was feasible, but not without exception. During the incentive period, smoking abstinence increased with CM; self-efficacy increased in both groups but more so with CM. When examined through follow-up, across conditions, observed smoking reductions were generally on par with effects of psychosocial and pharmacotherapies for smoking cessation in SUD populations, and self-efficacy was associated with abstinence status.
This residential SUD treatment program approached smoking like most such settings in the United States and elsewhere. There was a partial smoking ban, but smoking treatment services were not available. Many staff and most residents smoked, and smoke breaks occurred throughout the day. Nonetheless, all but two individuals screened and eligible elected to participate, completion rates were high, and there were no study withdrawals. These patterns may reflect increasing acceptance of treating smoking along with SUD, consistent with current guidelines.
Achieving a full day of CO-negative tests was relatively common in CM participants and rare in Monitoring participants. However, neither condition engendered sustained abstinence. Cessation is often the goal, but a history of abstinence can benefit later attempts to cease smoking (Falba, Jofre-Bonet, Busch, Duchovny, & Sindelar, 2004; Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Kenford et al., 1994), and smoking reduction can increase the likelihood of future cessation (Hughes & Carpenter, 2006). Why effects of CM were modest herein is unclear. One possible explanation relates to reinforcement exposure, which is critical to effective CM but was relatively low. Forty percent of CM patients never earned up to the 5-draw cap, achievable after 2.5 days of all CO-negative readings, and missed sessions were one reason for lost reinforcement. Our expectations about the feasibility of twice-daily sessions exceeded observations. Individual and programmatic circumstances may restrict in-person monitoring, but technology can also monitor and reinforce smoking abstinence remotely (Alessi & Petry, 2013; ongoing clinical trial NCT01484717; Dallery, Glenn, & Raiff, 2007; Glenn & Dallery, 2007; Stoops et al., 2009).
Smoking-positive tests were another reason for missed reinforcement. The escalating reinforcement schedule (Roll et al., 1996), reset condition (Roll & Higgins, 2000), and higher reinforcement density during the critical first week (Ferguson, Gitchell, Shiffman, & Sembower, 2009; Kenford et al., 1994; Romanowich & Lamb, 2010) were parameters included because they are important in improving CM outcomes. Higher magnitude reinforcement also improves outcomes with CM across substances of abuse in meta-analysis (Lussier, Heil, Mongeon, Badger, & Higgins, 2006) and with CM for smoking abstinence (Stitzer & Bigelow, 1983, 1984). Incentives for each smoking-negative test were substantially reduced compared to reports in the literature (Alessi, Badger, & Higgins, 2004; Donatelle, Prows, Champeau, & Hudson, 2000; Higgins et al., 2004; Robles et al., 2005; Shoptaw et al., 2002); higher reinforcement magnitude may have increased response to CM.
Understanding the process variables underlying effects of CM may point to other modifiable targets to improve treatment response. Previous work demonstrates mediation effects of self-efficacy on the relation between traditional smoking cessation counseling and pharmacotherapy and abstinence. To our knowledge, this is the first randomized controlled trial on CM for smoking abstinence to examine these relations, and results support previous findings. Also consistent with prior work, self-efficacy assessed prequit date did not predict abstinence (Gwaltney, Metrik, Kahler, & Shiffman, 2009; Romanowich, Mintz, & Lamb, 2009). Importantly, there was no evidence that external reinforcers for abstinence jeopardized self-efficacy. Thus, additional methods to enhance self-efficacy during the quit attempt may further improve outcomes with CM and other treatments.
Interestingly and contrary to expectations, reductions in smoking during intervention and follow-up occurred across conditions, amounting to 15.6% of participants abstinent at month 6. This is in direct contrast with observations of increased smoking during residential SUD treatment (Kelly et al., 2012). Moreover, this level of abstinence, even in our control condition, is on par with abstinence rates achieved in other SUD smokers with smoking cessation pharmacotherapy (Kalman et al. 2011; Kalman, Kahler, Garvey, & Monti, 2006; Poling, Rounsaville, Gonsai, Severino, & Sofuoglu, 2010), even though the present study included no medications. Unlike other studies, we also observed increases in smoking abstinence over time. Why is uncertain. Smoking restrictions were not placed in effect, and no changes in smoking policies occurred. The extent to which the frequent CO monitoring, behavioral support, or both were active ingredients requires further investigation.
In this study, there was no nontreatment control condition, restricting our ability to attribute changes in abstinence over time to the monitoring condition, as well as detect group differences. The relatively small sample size, though appropriate for testing feasibility, can limit ability to detect effects. Furthermore, the sample was limited to men. Although no outcome differences by sex have been reported with CM, differences in smoking (Becker & Hu, 2008), smoking-related morbidity (Mucha, Stephenson, Morandi, & Dirani, 2006), and response to at least some smoking treatments (Perkins & Scott, 2008) exist.
Strengths of this study are that it is the first randomized clinical trial of CM for smoking abstinence in residential SUD treatment patients. Eligibility criteria were relatively liberal, increasing the generalizability of results. Further, collecting both self-reports and biochemical tests of smoking status allowed more comprehensive analysis of smoking than would fewer measures. Measurement of COT at follow-up, as done here, is especially important given limitations of self-report and CO testing.
Overall, SUD treatment programs have increasing demands to address cigarette smoking and interventions that enhance smoking treatment response rates are needed. Our results suggest that intensive monitoring and brief, standard smoking cessation counseling alone or in conjunction with CM for smoking abstinence may reduce smoking in at least a subset of residential SUD treatment patients.
SUPPLEMENTARY MATERIAL
Supplementary Table 1 and Figure 1 can be found online at http://www.ntr.oxfordjournals.org
FUNDING
This study and the preparation of this report were funded by National Institutes of Health grants R21-DA021836, R21-DA029215, R01-DA013444, R01-DA027615, R01-DA024667, P30-DA023918, and P50-DA092410.
DECLARATION OF INTERESTS
None declared.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and the staff at Liberation Programs, Inc., and the Clinical Research Center at the University of Connecticut Health Center for support of and involvement in this project. We are grateful to J. Urso and J. Tymoszczuk for implementing study procedures and E. Ciesielski, S. Sierra, and L. LeBlanc for assisting with study administration and reporting requirements.
REFERENCES
- Agency for Health Care Policy and Research. (1996). You can quit smoking: Consumers guide Retrieved September 22, 2005, from www.cdc.gov/tobacco/quit/canquit.htm
- Alessi S. M., Badger G. J., Higgins S. T. (2004).. An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental and Clinical Psychopharmacology, 12, 276–287. [DOI] [PubMed] [Google Scholar]
- Alessi S. M., Petry N. M. (2013). A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction, 108, 900–909. 10.1111/add.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi S. M., Petry N. M., Urso J. (2008). Contingency management promotes smoking abstinence in residential substance abuse treatment patients. Journal of Applied Behavior Analysis, 41, 617–622. 10.1901/jaba.2008.41-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. (2000). Diagnostic and statistical manual of mental disorders (DSM-IV-TR) (4th ed., text revision). Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Amodei N., Lamb R. J. (2010). The role of nicotine replacement therapy in early quitting success. Nicotine & Tobacco Research, 12, 1–10. 10.1093/ntr/ntp164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca C. T., Yahne C. E. (2009). Smoking cessation during substance abuse treatment: What you need to know. Journal of Substance Abuse Treatment, 36, 205–219. 10.1016/j.jsat.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Bandura A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84, 191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- Becker J. B., Hu M. (2008). Sex differences in drug abuse. Frontiers in Neuroendocrinology, 29, 36–47. 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan R. C., Brewster J. M., Johnson J., Taylor L., Beach G., Lentz T. (2007). Do total smoking bans affect the recruitment and retention of adolescents in inpatient substance abuse treatment programs? A 5-year medical chart review, 2001–2005. Journal of Substance Abuse Treatment, 33, 279–285. 10.1016/j.jsat.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Dallery J., Glenn I. M., Raiff B. R. (2007). An internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence, 86, 230–238. 10.1016/j.drugalcdep.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Donatelle R. J., Prows S. L., Champeau D., Hudson D. (2000). Randomized controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Supporter (SOS) program. Tobacco Control, 9(Suppl. 3), 11167–11169. 10.1136/tc.9.suppl_3.iii67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. E., Sigmon S. C., Reimann E., Badger G. J., Heil S., Higgins S. T. (2010). A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Experimental and Clinical Psychopharmacology, 18, 37–50. 10.1037/a0018649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K. E., Sigmon S. C., Thomas C. S., Heil S. H., Higgins S. T. (2008). Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavior Analysis, 41, 527–538. 10.1901/jaba.2008.41-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L., Stathopoulou G., Basden S. L., Leyro T. M., Powers M. B., Otto M. W. (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165, 179–187. 10.1176/appi.aji.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Falba T., Jofre-Bonet M., Busch S., Duchovny N., Sindelar J. (2004). Reduction of quantity smoked predicts future cessation among older smokers. Addition, 99, 93–102. 10.1111/j.1360-0443.2004.00574.x [DOI] [PubMed] [Google Scholar]
- Ferguson S. G., Gitchell J. G., Shiffman S., Sembower M. A. (2009). Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: Secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clinical Therapeutics, 31, 1957–1965. 10.1016/j.clinthera.2009.08.029 [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gerstein D. R., Volberg R. A., Toce M. T., Harwood H., Johnson R. A., Christiansen E., … Tucker A. (1999). Gambling impact and behavior study: Report to the National Gambling Impact Study Commission. Chicago, IL: National Opinion Research Center. [Google Scholar]
- Glenn I. M., Dallery J. (2007). Effects of internet-based voucher reinforcement and a transdermal nicotine patch on cigarette smoking. Journal of Applied Behavior Analysis, 40, 1–13. 10.1901/jaba.2007.40-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay S. G., Forbes A., Marriner T., Pethica D., McNeil J. J. (1994). Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal, 309, 842–846. 10.1136/bmj.309.6958.842 7950614 [Google Scholar]
- Grant B. F., Hasin D. S., Chou S. P., Stinson F. S., Dawson D. A. (2004). Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 61, 1107–1115. 10.1001/archpsyc.61.11.1107 [DOI] [PubMed] [Google Scholar]
- Guydish J., Tajima B., Kulaga A., Zavala R., Brown L. S., Bostrom A., … Chan M. (2012). The New York policy on smoking in addiction treatment: Findings after 1 year. American Journal of Public Health, 102, e17–e25. 10.2105/AJPH.2011.300590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney C. J., Shiffman S., Norman G. J., Paty J. A., Kassel J. D., Gyns M., … Balabanis M. (2001). Does smoking abstinence self-efficacy vary across situations? Identifying context-specificity within the Relapse Situation Efficacy Questionnaire. Journal of Consulting and Clinical Psychology, 69, 516–527. 10.1037/0022-006X.69.3.516 [PubMed] [Google Scholar]
- Gwaltney D. J., Metrik J., Kahler C. W., Shiffman S. (2009). Self-efficacy and smoking cessation: A meta-analysis. Psychology of Addictive Behaviors, 23, 56–66. 10.1037/a0013529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. T., Croghan I. T., Schroeder D. R., Ebbert J. O., Hurt R. D. (2011). Varenicline for tobacco dependence treatment in recovering alcohol-dependent smokers: An open-label pilot study. Journal of Substance Abuse Treatment, 41, 102–107. 10.1016/j.jsat.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hendricks P. S., Delucchi K. L., Hall S. M. (2010). Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug and Alcohol Dependence, 109, 114–119. 10.1016/j.drugalcdep.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. T., Heil S. H., Solomon L. J., Bernstein I. M., Lessier J. P., Abel R. L., … Badger G. J. (2004). A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research, 6, 1015–1020. 10.1080/14622200412331324910 [DOI] [PubMed] [Google Scholar]
- Higgins S. T., Silverman K., Heil S. H. (2008). Contingency management in substance abuse treatment. New York, NY: Guilford Press. [Google Scholar]
- Hser Y. L., McCarthy W. J., Anglin M. D. (1994). Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Preventive Medicine, 23, 61–69. [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Carpenter M. J. (2006). Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine & Tobacco Research, 8, 739–749. 10.1080/14622200600789726 [DOI] [PubMed] [Google Scholar]
- Hurt R. D., Offord K. P., Croghan I. T., Gomez-Dahl L., Kottke T. E., Morse R. M., Melton L. J. (1996). Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. Journal of the American Medical Association, 275, 1097–1103. 10.1001/jama.1996.03530380039029 [DOI] [PubMed] [Google Scholar]
- Kalman D. (1998). Smoking cessation treatment for substance misusers in early recovery: A review of the literature and recommendations for practice. Substance Use and Misuse, 33, 2021–2047. [DOI] [PubMed] [Google Scholar]
- Kalman D., Herz L., Monti P., Kahler C. W., Mooney M., Rodrigues S., Connor K. (2011). Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: Results from a randomized, double-blind, placebo-controlled study. Drug and Alcohol Dependence, 118, 111–118. 10.1016/j.drugalcdep.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D., Kahler C. W., Garvey A. J., Monti P. M. (2006). High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-Week outcomes. Journal of Substance Abuse Treatment, 30, 213–217. 10.1016/j.jsat.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Kelly P. J., Baker A. L., Deane F. P., Kay-Lambkin F. J., Bonevski B., Tregarthen J. (2012). Prevalence of smoking and other health risk factors in people attending residential substance abuse treatment. Drug and Alcohol Review, 31, 638–644. 10.1111/j.1465-3362.2012.00465.x [DOI] [PubMed] [Google Scholar]
- Kenford S. L., Fiore M. C., Jorenby D. E., Smith S. S., Wetter D., Baker T. B. (1994). Predicting smoking cessation: Who will quit with and without the nicotine patch. Journal of the American Medical Association, 271, 589–594. 10.1001/jama.1994.03510320029025 [DOI] [PubMed] [Google Scholar]
- Lamb R. J., Morral A. R., Galbicka G., Kirby K. C., Iguchi M. Y. (2005). Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology, 13, 83–92. 10.1037/1064-1297.13.2.83 [DOI] [PubMed] [Google Scholar]
- Lasser K., Boyd J. W., Woolhander S., Himmelstein D. U., McCormick D., Bor D. H. (2000). Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association, 284, 2606–2610. 10.1001/jama.284.20.2606 [DOI] [PubMed] [Google Scholar]
- Lussier J. P., Heil S. H., Mongeon J. A., Badger G. J., Higgins S. T. (2006). A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction, 101, 192–203. 10.1111/j.1360-0443.2006.01311.x [DOI] [PubMed] [Google Scholar]
- McCarthy D. E., Piasecki T. M., Jorenby D. E., Lawrence D. L., Shiffman S., Baker T. B. (2010). A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction, 105, 2195–2208. 10.1111/j.1360-0443.2010.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha L., Stephenson J., Morandi N., Dirani R. (2006). Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gender Medicine, 3, 279–291. [DOI] [PubMed] [Google Scholar]
- National Opinion Research Center. (1999). Gambling impact and behavior study. Chicago, IL: University of Chicago. [Google Scholar]
- Perkins K. A., Parzynski C. S., Mercincavage M., Conklin C. A., Fonte C. A. (2012). Is self-efficacy for smoking abstinence a cause of, or a reflection on, smoking behavior change? Experimental and Clinical Psychopharmacology, 21, 56–62. 10.1037/a0025482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. A., Scott J. (2008). Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine and Tobacco Research, 10, 1245–1251. 10.1080/14622200802097506 [DOI] [PubMed] [Google Scholar]
- Petry N. M. (2012). Contingency management for substance abuse treatment: A guide to implementing evidence-based practice. New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Petry N. M., Alessi S. M. (2010). Prize-based contingency management is efficacious in cocaine abusers with and without recent gambling participation. Journal of Substance Abuse Treatment, 39, 282–288. 10.1016/j.jsat.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N. M., Kolodner K. B., Li R., Peirce J. M., Roll J. M., Stitzer M. L., Hamilton J. A. (2006). Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence, 83, 269–273. 10.1016/j.drugalcdep.2005.11.023 [DOI] [PubMed] [Google Scholar]
- Poling J., Rounsaville B., Gonsai K., Severino K., Sofuoglu M. (2010). The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: A pilot study. American Journal on Addictions, 19, 401–408. 10.1111/j.1521-0391.2010.00066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. J., Delucchi K., Hall S. M. (2004). A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment recovery. Journal of Consulting and Clinical Psychology, 72, 1144–1156. 10.1037/0022-006X.72.6.1144 [DOI] [PubMed] [Google Scholar]
- Richter K. P., Gibson C. A., Ahluwalia J. S., Schmelze K. H. (2001). Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health, 91, 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. P., McCool R. M., Catley D., Hall M., Ahluwalia J. S. (2006). Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. Journal of Addictive Disorders, 24, 79–90. 10.1300/J069v24n04_06 [DOI] [PubMed] [Google Scholar]
- Robles E., Crone C. C., Whiteside-Mansell L., Conners N. A., Bokony P. A., Worley L. L., McMillan D. E. (2005). Voucher-based incentives for cigarette smoking reduction in a women’s residential treatment program. Nicotine and Tobacco Research, 7, 111–117. 10.1080/14622200412331328448 [DOI] [PubMed] [Google Scholar]
- Roll J. M., Higgins S. T. (2000). A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence, 58, 103–109. [DOI] [PubMed] [Google Scholar]
- Roll J. M., Higgins S. T., Badger G. J. (1996). An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis, 29, 495–505. 10.1901/jaba.1996.29-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P., Lamb R. J. (2010). The relationship between in-treatment abstinence and post-treatment abstinence in a smoking cessation treatment. Experimental and Clinical Psychopharmacology, 18, 32–36. 10.1037/a0018520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P., Mintz J., Lamb R. J. (2009). The relationship between self-efficacy and reductions in smoking in a contingency management procedure. Experimental and Clinical Psychopharmacology, 17, 139–145. 10.1037/a0015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. S., Lucas J. W., Peregoy J. A. (2012). Summary health statistics for U.S. adults: National Health Interview Survey, 2011. National Center for Health Statistics. Vital and Health Statistics, 10, 85–86. [PubMed] [Google Scholar]
- Sherman S. E. (2008). A framework for tobacco control: Lessons learnt from Veterans Health Administration. British Journal of Medicine, 336, 1016–1019. 10.1136/bmj.39510.805266.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S., Jarvik M. E., Ling W., Rawson R. A. (1996). Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors, 21, 409–412. [DOI] [PubMed] [Google Scholar]
- Shoptaw S., Rotheram-Fuller E., Yang X., Frosh D., Nahom D., Jarvik M. E., … Ling W. (2002). Smoking cessation in methadone maintenance. Addiction, 97, 1317–1328. 10.1046/j.1360-0443.2002.00221.x [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. (1992). Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In Litten R., Allen J. (Eds.), Measuring alcohol consumption (pp. 41–71). Totowa, NJ: Humana Press. [Google Scholar]
- Stanton C. A., Lloyd-Richardson E. E., Papandonatos G. D., de Dios M. A., Niaura R. (2009). Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Education and Prevention, 21(3 Suppl.), 65–80. 10.1521/aeap.2009.21.3_supp.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. D., Weinstock M. C., Herman D. S., Anderson B. J., Anthony J. L., Niaura R. (2006). A smoking cessation intervention for the methadone-maintained. Addiction, 101, 599–607. 10.1111/j.1360-0443.2006.01406.x [DOI] [PubMed] [Google Scholar]
- Stitzer M. L., Bickel W. K., Bigelow G. E., Liebson I. A. (1986). Effects of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug and Alcohol Dependence, 18, 341–348. [DOI] [PubMed] [Google Scholar]
- Stitzer M. L., Bigelow G. E. (1983). Contingent payment for carbon monoxide reduction: Effects of pay amount. Behavior Therapy, 14, 647–656. 10.1016/S0005-7894(83)80057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer M. L., Bigelow G. E. (1984). Contingent reinforcement for carbon monoxide reduction: Within-subject effects of pay amount. Journal of Applied Behavior Analysis, 17, 477–483. 10.1901/jaba.1984.17-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops W. W., Dallery J., Fields N. M., Nuzzo P. A., Schoenberg N. E., Martin C. A., … Wong C. J. (2009). An internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug and Alcohol Dependence, 105, 56–62. 10.1016/j.drugalcdep.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. L., Wirtz P. W., Carbonari J. P., Del Boca F. K. (1994). Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol, Supplement, 12, 70–75. [DOI] [PubMed]
- Williams J. M., Foulds J., Dwyer M., Order-Connors B., Springer M., Gadde P., Ziedonis D. M. (2005). The integration of tobacco dependence treatment and tobacco-free standards into residential addictions treatment in New Jersey. Journal of Substance Abuse Treatment, 28, 331–340. 10.1016/j.jsat.2005.02.010 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). WHO report on the global tobacco epidemic, 2011. Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.