Abstract
Impairment of hippocampal-dependent spatial learning and memory with aging affects a large segment of the aged population. Hippocampal subregions (CA1, CA3, and DG) have been previously reported to express both common and specific morphological, functional, and gene/protein alterations with aging and cognitive decline. To comprehensively assess gene expression with aging and cognitive decline, transcriptomic analysis of CA1, CA3, and DG was conducted using Adult (12M) and Aged (26M) F344xBN rats behaviorally characterized by Morris water maze performance. Each subregion demonstrated a specific pattern of responses with aging and with cognitive performance. The CA1 and CA3 demonstrating the greatest degree of shared gene expression changes. Analysis of the pathways, processes, and regulators of these transcriptomic changes also exhibit a similar pattern of commonalities and differences across subregions. Gene expression changes between Aged cognitively Intact and Aged cognitively Impaired rats often showed an inversion of the changes between Adult and Aged rats. This failure to adapt rather than an exacerbation of the aging phenotype questions a conventional view that cognitive decline is exaggerated aging. These results are a resource for investigators studying cognitive decline and also demonstrate the need to individually examine hippocampal subregions in molecular analyses of aging and cognitive decline.
Key Words: Gene expression, Hippocampus, Aging, Cognitive impairment.
With advanced aging, declines in cognitive function are a common complication impacting a variety of brain functions (1). Spatial learning and memory in particular is impaired in aged subjects with cognitive decline, potentially resulting in disability and loss of independence (1). With an aging population and the increasing prevalence of conditions such as vascular diseases and type 2 diabetes, which further increase the risk of cognitive impairment (2,3), the incidence of cognitive decline is expected to increase concomitantly.
The neurobiological basis of nonneurodegenerative cognitive decline remains undetermined, but research is aided by the fact that impaired hippocampal-dependent cognitive function with aging is evident in humans (4), monkeys (5), rats (6), and mice (7). From human and animal models, extensive data demonstrate that cognitive decline occurs in the absence of neuronal cell death (6–9). Instead, hippocampal-related cognitive decline may result from impaired hippocampal synaptic morphology, signaling, and regulation; decreased plasticity; impaired neurogenesis; and increased neuroinflammatory processes (10–14). Importantly, each specific process demonstrates hippocampal subregion specificity (for review, see (13)). We, and others, have used transcriptomic and proteomic technologies to identify changes in hippocampal gene and protein expression with aging and with cognitive decline that may underlie these functional changes (15–20). However, only a limited number of studies have examined changes with aging and/or cognitive decline with hippocampal subregion specificity (19,20). Gene expression changes restricted to specific subregions could remain undetected in studies using whole hippocampal tissue samples due to the unchanged expression levels in other subregions. To identify commonalities and differences in hippocampal subregion (CA1, CA3, and DG) gene expression with aging and/or cognitive decline, we applied a transcriptome profiling approach to hippocampal subregions isolated from Adult and Aged rats behaviorally phenotyped for performance in a spatial learning and memory task. These findings demonstrate shared and distinct differences in gene expression with aging and cognitive decline across hippocampal subregions. Additionally, Aged cognitively Impaired animals demonstrated, for many genes, a failure of the response observed with aging, indicating cognitive decline is not a result of a more exaggerated aging phenotype. These datasets also serve as a resource for other investigators examining hippocampal aging and cognitive deficits.
Methods
Animals
Two cohorts of male Fischer 344 × Brown Norway (F1) hybrid rats (Table 1) were purchased from the Harlan Industries (Indianapolis, IN) National Institute on Aging colony. Rats were acclimatized in quarantine for 2 weeks upon arrival and singly housed in laminar-flow cages in the OUHSC specific pathogen-free Barrier Facility with food (Purina Mills, Richmond, IN) and water available ad libitum.
Table 1.
Animal Cohorts
| Adult (12 mo) | Aged (26 mo) | Analyses Performed | |
|---|---|---|---|
| Cohort 1: hippocampal subregion analysis | n = 7 | n = 22 | Microarray, qPCR |
| Cohort 2: whole hippocampus analysis | n = 8 | n = 22 | Microarray, qPCR |
Behavioral Characterization
Hippocampus-dependent spatial learning and memory was evaluated by Morris water maze as previously described (18,21). The behavioral testing and results for Cohort 1 have been previously described (22,23). Mean proximity to platform values during probe trials were used to differentiate Aged rats into Aged Intact (probe trial performance within 1 SD below the best performing Adult rat and 1 SD above the worst performing Adult rat) and Aged Impaired (>1 SD above the worst performing Adult).
Dissections
Hippocampal CA1, CA3, and DG subregions were individually dissected from left and right hippocampi. Hippocampi were hemisected and the dorsomedial portion was further dissected into four blocks perpendicular to the longitudinal axis. From these blocks, CA3 was isolated by a cut connecting the ends of the inner and outer blades of the DG. CA1 and DG were isolated by cutting along the hippocampal fissure as described previously (21,24). For the purposes of this dissection, CA2 was included in the CA3. For whole hippocampal dissections, the entire hippocampus was removed as described previously (22,25).
RNA Isolation
Hippocampal samples were homogenized in 300 µL TriReagent (Molecular Research Center, Cincinnati, OH) by bead mill (Retsch TissueLyser II; Qiagen, Valencia, CA) and RNA isolated according to standard methods (21). RNA was purified from any trace organic contamination using Qiagen RNeasy Mini column purification (Qiagen). Quality and quantity were assessed by microfluidic chip (Agilent 2100 Expert Bioanalyzer Nano Chip; Agilent, Palo Alto, CA) and spectrometry (NanoDrop ND1000; Thermo Scientific, Wilmington, DE), respectively, with RNA integrity numbers less than 8 used as an exclusion criterion.
Microarray Analysis
Transcriptomic analysis of hippocampal samples derived from Adult and Aged rats (CA1: n = 3 Adult [after one microarray failed quality control], n = 8 Aged; CA3: n = 4 Adult, n = 8 Aged; DG: n = 4 Adult, n = 8 Aged; Whole Hipp.: n = 6 Adult, n = 16 Aged). For subregion analysis, Aged rats included n = 4 Aged Intact and n = 4 Aged Impaired. Gene expression analysis was performed using Illumina RatRef-12 microarrays (Illumina, San Diego, CA) according to standard methods and as previously described (26,27). For a detailed description of the microarray methods, see the Supplementary Methods. The full microarray dataset has been deposited in the Gene Expression Omnibus, accession# GSE55352.
Bioinformatic Analysis and Visualization
For potential function and transcriptional regulator analyses, the Ingenuity Pathway Analysis (Qiagen, Redwood City, CA) database was used. Bioinformatic analysis was conducted on both the set of genes differentially expressed with aging (Adult vs all Aged) and those differentially expressed with cognitive impairment (Aged Intact vs Aged Impaired). For process and regulatory analyses, an overlap p value and an activation z-score were computed (28). The p value was calculated using Fisher’s Exact Test based on overlap between genes in the list and known genes pertaining to a function or targets of a transcriptional regulator. The activation z-score is used to infer likely activation states of a function or upstream regulator based on the direction of changes in the gene list and literature-derived functional or regulation directions. A z-score cutoff of >|2| was applied to limit lists to only those function and regulators with considerable activation (positive z-score) or inhibition (negative z-score). For pathway analyses, an overlap p value and ratio were computed. The p value was calculated using the same Fisher’s Exact Test approach as above. The ratio was determined by the number of the genes in the gene list divided by the total number of genes associated with the pathway. Venn diagrams were created using Venny software (29). For additional details on the bioinformatics methods, see the Supplementary Methods.
Quantitative Reverse Transcription–PCR
Confirmation of gene expression levels was performed as previously described (21,26). cDNA was synthesized from purified RNA (CA1: n = 6–7 Adult, n = 21–22 Aged; CA3: n = 6–7 Adult, n = 21–22 Aged; DG: n = 6–7 Adult, n = 21–22 Aged; Whole Hipp.: n = 8 Adult, n = 22 Aged) with the ABI High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) from 1 µg RNA. Quantitative PCR (qPCR) analysis of targets of interest was performed using TaqMan Assay-On-Demand (Applied Biosystems) gene-specific primers/probe assays (see Supplementary Table 1) and a 7900HT Sequence Detection System (Applied Biosystems). Relative gene expression was calculated with SDS 2.2.2 software using the 2−ΔΔCt analysis method with β-actin as an endogenous control. Statistical analysis of age-related (ie, Adult vs Aged) gene expression changes in microarray-confirmation qPCR experiments was performed by two-tailed t-testing with Benjamini–Hochberg multiple testing correction. For confirmation of cognition regulated genes (eg, transthyretin-Ttr) and analysis of variance with Student–Newman–Keuls (SNK) post hoc was used.
Results
Behavioral Stratification
Hippocampal-dependent cognitive performance in Adult and Aged rats was assessed in a spatial learning and memory paradigm (Morris water maze). Aged animals were segregated after behavioral testing into cognitively Intact or cognitively Impaired groups based on mean proximity to the escape platform during probe trials as described previously (17,21–23). Spatial learning and memory was superior in Adult and Aged cognitively Intact rats compared with Aged cognitively Impaired rats (p < .001, one-way analysis of variance, SNK post hoc) (Supplementary Figure 1) with Aged Impaired rats (59±0.9cm) performing significantly inferior to Adult (48±1.1cm) and Aged Intact (48±1.4cm) rats in probe trial mean proximity to platform distance. This cohort of animals has been described previously (21–23,30).
Transcriptome of Hippocampal Subregions
Microarray analysis was performed on Adult, Aged Intact, and Aged Impaired RNA samples from CA1, CA3, and DG. Using an expression similarity approach (31) to compare our data to public rat microarray datasets available in NCBI’s GEO as a quality check, we validated that our hippocampal gene expression profiles were most similar, experimentally, to other hippocampal samples (Supplementary Table 2). A principal component analysis of the subregions showed they clustered closer together in gene expression space to the brain-related samples than to other tissue types (Supplementary Figure 2). Gene expression data from hippocampal subregions was first compared to assess commonalities and differences in the genes expressed within each region (Figure 1). Genes were considered expressed in a subregion if a “present” or “marginal” detection call was found for all of the biological replicates in that subregion. This analysis was performed in both Adult (Figure 1A) and Aged (both Intact and Impaired) rats (Figure 1B). Greater than 90% of transcripts detected met this criterion in all three subregions. However, there were also genes detected in only one or two of the subregions. Using the expression levels for every gene that met the detection criteria, the relationships between samples and between regions were examined, samples from each region clustered together, as shown in the dendrogram and principal component analysis, demonstrating subregion specificity to the pattern of gene expression. The expression pattern of genes in pyramidal regions (CA1 and CA3) was also more similar compared to the DG in both Adult and Aged animals.
Figure 1.
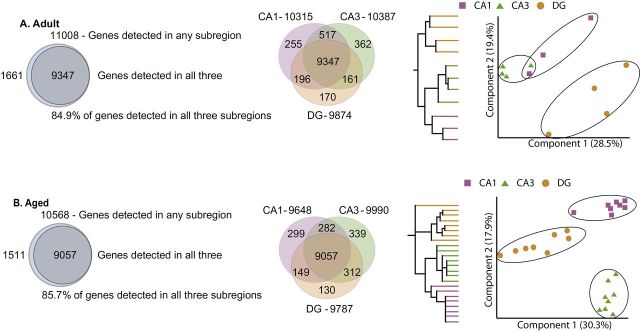
Relationship between hippocampal subregion transcriptomes with aging. (A) The majority of genes detected as expressed in Adult animals were detected in each subregion, but transcripts specific to each subregion were also observed. Additional transcripts were detected in two of the three subregions with CA1 and CA3 sharing more commonly expressed genes than the DG. When subjected to hierarchical clustering (each color-coded line represents an individual animal), the expression pattern of detected genes was more similar between CA1 and CA3. Similarly, when visualized in a principal component analysis, each subregion separated uniquely, with the pyramidal regions (CA1 and CA3) demonstrating a more similar overall pattern of gene expression than with the DG. (B) A similar pattern of commonly expressed and subregion-specific gene expression was observed in Aged animals.
Previously, we have reported the accuracy of the subregion dissections with known reported subregion-specific/enhanced transcripts (Tiam1 and Dsp in the DG, Bok and Spok1 in CA3, and Nov in the CA1) from this cohort of animals (30). To confirm additional potential subregion markers, qPCR was performed on selected targets in the full sample set (Supplementary Figure 3). All of the genes examined were confirmed and demonstrated significant preferential expression, ranging from 2- to 20-fold, in one of the three subregions examined. These confirmed genes were then examined in the Allen Mouse Brain Atlas (32,33). Similar patterns of subregion-restricted/enhanced gene expression were observed in the mouse brain (Supplementary Figure 4). These results confirm the accuracy of the dissections and provide new potential mRNA markers for each subregion.
Age-Related Changes in Gene Expression
To identify age-related differences in gene expression, Adult rats were compared to Aged (combined Intact and Impaired groups) (t-test, p < .05, >|1.2| fold change). Age-related differences in gene expression were compared across hippocampal subregions and the majority of changes were restricted to one subregion, with some commonalities between subregions (Figure 2A). Taking sets of genes comprising the union of the aging changes across all of the subregions, the pattern of differential expression was highest between CA1 and CA3 (r = .743, p < .05E–318). A smaller degree of correlation was also observed between DG and CA1 (r = .153, p < .05E–11) and between DG and CA3 (r = .295, p = .05E–38) (Supplementary Figure 5).
Figure 2.
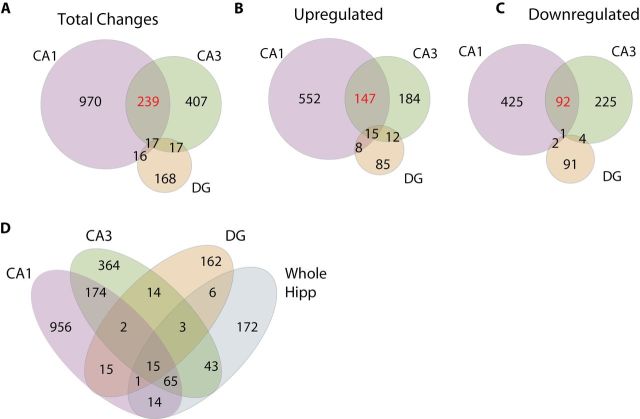
Aging-related changes in gene expression. (A) Differences in gene expression with aging were determined for each. The most changes were identified in CA1, followed by CA3 and DG (circle areas are proportional). Both when examined as total changes (A) and when separated into upregulated (B) and downregulated (C) changes, more commonalities were evident between CA1 and CA3 than with the DG. In every case, expression changes common to CA1 and CA3 (highlighted in red) occurred in the same direction. (D) When age-related changes in each subregion were compared to changes observed in the second cohort from a whole hippocampus dissection, only minority of the subregion changes could be observed.
When a gene was differentially expressed in multiple regions, 97% of the time it is was regulated in the same manner (increased or decreased) with aging across subregions (Figure 2B and C). Genes commonly regulated across all three subregions were overrepresented for inflammatory processes (antigen processing and presentation, Gene Ontology Analysis corrected p < .001) and upregulation by interferon gamma (Ifng, Ingenuity z-score: 2.595, overlap p < .001). The CA1 and CA3 demonstrated the largest number of common differentially expressed genes (239) and every shared gene was either upregulated or downregulated in both subregions. For these shared responses, the genes were over represented for inflammatory (antigen processing and presentation, p < .001) and lysosomal processes (p < .02, Gene Ontology Analysis). To assess the effect of using whole hippocampus for identifying age-regulated genes, differential expression in Cohort 2 rats between Adult and Aged was compared to the subregion-specific microarray data. Most of the changes observed in the subregion analyses were not detected when whole hippocampal tissue was examined (Figure 2D). For full lists of differentially expressed genes, see Supplementary Table 3.
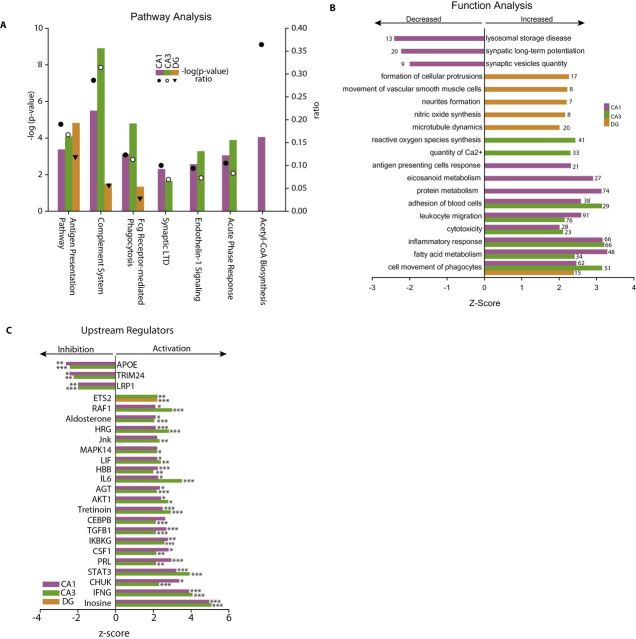
To identify pathways and biological functions that may be altered by differential gene expression, Ingenuity Pathway Analysis was performed using the differentially expressed gene lists. Commonly regulated pathways across all subregions included upregulation of inflammatory processes; including antigen presentation, complement and Fcγ phagocytosis (Figure 3A). A number of pathways were significantly regulated in both CA1 and CA3 but not DG with a range of inflammatory, neuronal, and vascular pathways identified (Supplementary Table 4). As pathway analysis is limited to fully described signaling cascades, biological functions were also queried and returned a number of functions, which were overrepresented in age-related gene expression changes and coordinately expressed in a manner suggesting a concerted effect on function (Fisher’s Exact Test p < .05 and z-score > |2.0|) (Figure 3B). Again, more commonly effected processes where observed between CA1 and CA3 than with DG and a number of functions were significantly regulated in only one subregion (Supplementary Table 5). To assess if sets of differentially expressed genes shared common upstream regulators that could be driving age-related gene expression changes, a similar statistical approach of both overrepresentation and coregulation in a manner consistent with activation or inhibition (z-score > |2.0|) was used (Supplementary Table 6). CA1 and CA3 shared a large number of common endocrine, cytokine, and signal transduction regulators with little overlap to those regulators observed in the DG (Figure 3C).
Figure 3.
Pathway, function, and regulatory analysis of transcriptomic changes. Age-related gene expression changes were analyzed with Ingenuity Knowledge Base for differentially regulated pathways (A), functions (B), and regulators (C). The significance of the association between the data set and canonical pathways was measured in two ways: (1) a ratio of the number of molecules from the data set that map to the pathway divided by the total number of molecules that map to the canonical pathway is displayed and (2) Fisher’s Exact Test was used to calculate a p value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone. A subset of commonly regulated functions and pathways is shown. For regulation analysis, the manner of change was included to determine where sets of genes where coordinately regulated. The likelihood of the association given the data set was assessed by Fisher’s Exact Test, ***p < .001, **p < .01, *p < .05. Z-scores are based on prior knowledge of known regulatory functions and direction of changes in the current dataset. Z-scores >2 indicate significant activation with aging and <−2 indicate significant inhibition with aging. Examples of common regulators in CA1, CA3, and DG are shown.
Finally, we again turned to examining the overall similarity of our experimental expression profiles in comparison with the public databases, but this time identified public samples, which were similar to Adult samples but distant from Aged samples, or vice versa, in expression space. Interestingly, we found that, relative to Adult samples, the Aged samples were closer to samples obtained from injured neural tissue in this multidimensional expression space (Supplementary Table 7). The Adult samples, by contrast, were closest to neural samples from control mice. This suggests that some of the hippocampal changes associated with aging are similar, expression-wise, to injured tissue.
Validation of Age-Related Gene Expression Changes
To confirm selected age-related changes in gene expression, qPCR was performed using the full sample set from Cohort 1. qPCR confirmation not only provides an orthogonal technique to increase confidence in the results but also allows for higher statistical power through a larger sample size and rigorous multiple testing correction. See Supplementary Figure 6 for cross-referencing of confirmed targets with microarray data. Selected genes demonstrating common regulation across two subregions (Arhgap9, Emp3, Fxyd1, Fxyd3, Ncf1) or all three subregions (Bmp7, Gipr, Glra2) were confirmed (Figure 4A) as significantly regulated with aging. A selection of genes were also confirmed that demonstrated region specific changes in gene expression (Fgf12, Ms4a6, Cobl1, Prg4) or with alternate regulation across subregions (Hapln2) (Figure 4B). To empirically test whether subregion-common or subregion-specific changes could be observed in whole hippocampal preparations, two common (Gipr and Bmp7) and two specific changes (Fgf12 and Cobl) were analyzed by qPCR in whole hippocampal samples from Cohort 2. Significant upregulation of Gipr and Bmp7 with aging was observed in whole hippocampus, but no changes were detectable in Fgf12 or Cobl when analyzed in whole hippocampal samples (Supplementary Figure 7). A number of inflammation-related targets (C1s, C3, Cd74, Ctse, Fcgr2b, Hspb1, Lgals3, RT1-Ba, RT1-Db1, S100a6, and Serping1) identified in the microarray analysis have already been demonstrated in our previous work to be upregulated with aging in the hippocampus of the same animal model in a directed study of neuroinflammation (21). All of these confirmations suggest a high confidence in the microarray results and the utility of this database as a resource for investigations of hippocampal gene expression changes with aging.
Figure 4.
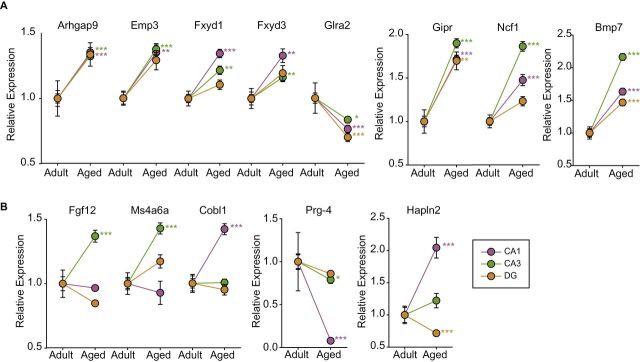
Confirmation of selected target genes by qPCR. Genes identified as regulated with age in the microarray analysis in multiple hippocampal subregions (A) or in only one subregion (B) were confirmed by gene-specific qPCR. ***p < .001, **p < .01, *p < .05, t-test by region with Benjamini–Hochberg multiple testing correction. Arhgap9 = Rho GTPase activating protein 9; Bmp7 = bone morphogenetic protein 7; Cobl = cordon-bleu protein; EMP3 = epithelial membrane protein 3; Fgf12 = fibroblast growth factor 12; Fxyd1 = FXYD domain containing ion transport regulator 1; Fxyd3 = FXYD domain containing ion transport regulator 3; Glra2 = glycine receptor, alpha 2; Gipr = gastric inhibitory polypeptide receptor; Hapln2 = hyaluronan and proteoglycan link protein 2; Ms4a6a = membrane-spanning 4-domains, subfamily A, member 6A; Ncf1 = neutrophil cytosolic factor 1; Prg4 = proteoglycan 4.
Cognition-Related Changes in Gene Expression
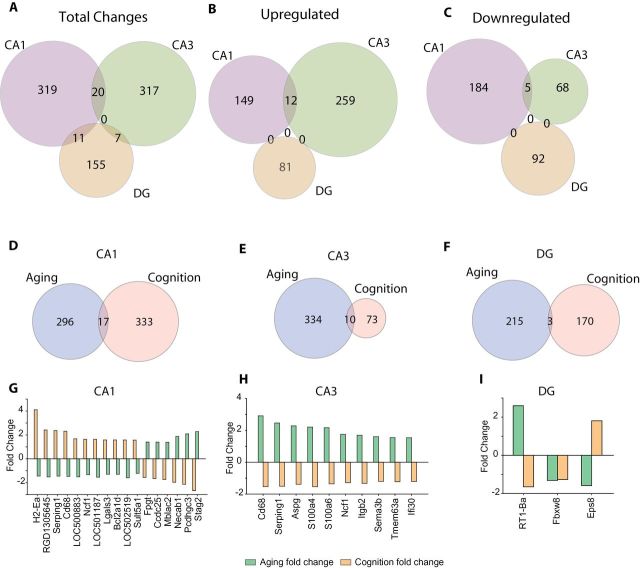
To identify differentially regulated genes related specifically to cognitive decline, gene expression differences between Aged Intact and Aged Impaired groups were determined for each subregion. Smaller numbers of changes were observed than in the age-related comparison, with few commonalities between regions, and no genes differentially expressed in all three subregions (Figure 5A and Supplementary Table 8). When split to identify genes regulated in the same manner, differences were primarily restricted to one subregion, though there were several genes shared by CA1 and CA3 (Figure 5B and C). Cognition- and age-related changes were then compared by subregion to assess whether age-related genes were also regulated with cognitive decline. In all three subregions, only a limited number of genes were observed to be both regulated with age and cognitive status (Figure 5D–F and Supplementary Table 9). Taking these genes in the intersection of age- and cognition-related changes, a clear inverse relationship was evident (Figure 5G–I).
Figure 5.
Cognitive impairment-related changes in gene expression. (A) Differences in gene expression between Aged Intact and Aged Impaired were determined for each subregion. A similar number of changes were observed in CA1 and CA3 with limited coregulation overlap between CA1 and CA3 (B and C) and none with DG. Age-regulated and cognition-regulated genes within each brain region were compared (D–F) and only a small number of genes were identified as regulated with both aging and cognition. These common genes in each subregion (G–I) in all cases, but one demonstrated an opposing direction of change with aging and cognitive impairment.
As with the age-related changes in gene expression, the genes differentially expressed with cognitive decline were assessed in their biological context. From pathway analysis, some of the same pathways observed in the aging analysis (eg, complement) were observed with cognitive impairment, while a number of new pathways were also evident as overrepresented in cognition-related changes (Figure 6A and Supplementary Table 10). Pathway analysis, however, only utilizes overrepresentation and does not provide a directionality of the potential outcomes. In the biological function analysis, this was evident as some of the same functions were overrepresented in cognition-related changes, including cell movement of phagocytes, leukocyte migration, and quantity of Ca2+ (Figure 6B and Supplementary Table 11). However, the z-scores for these functions were the inverse of those seen with aging, suggesting a potential opposing effect on function. Additionally, other functional effects were observed with significant z-scores such as vascularization and necrosis. Further extending this analysis, upstream regulators of the cognition-related gene expression changes were analyzed. Like the function analysis, a number of the same regulators were observed as the aging comparison, but in each case, those were oppositely regulated (Figure 6C and Supplementary Table 12).
Figure 6.
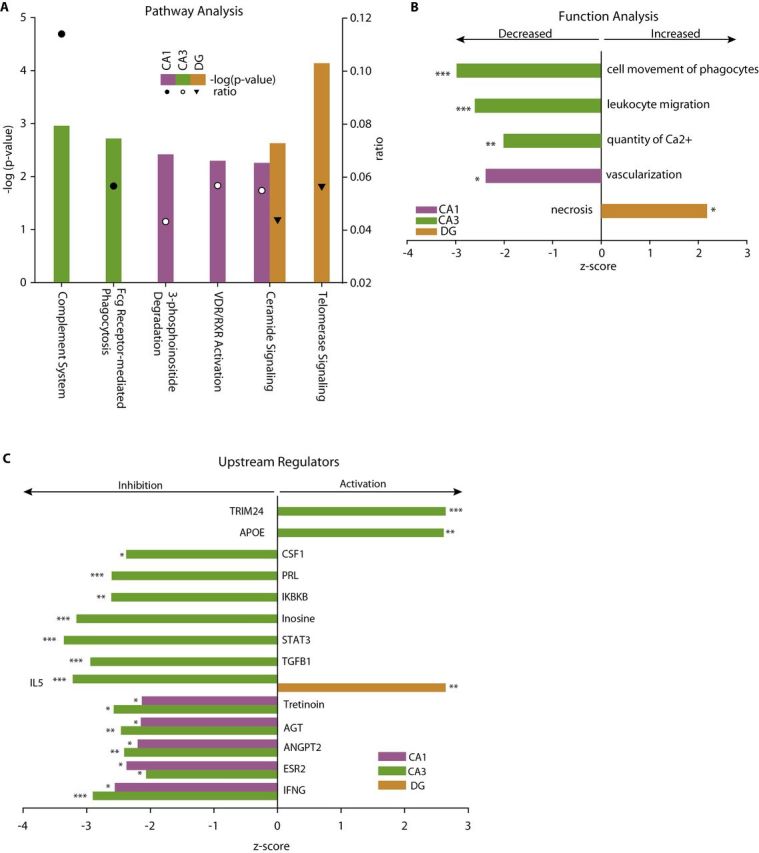
Pathway, function, and regulatory analysis of cognition-related changes. A moderate number of pathways, functions, and upstream regulators were identified from the cognition-related gene expression changes. Among the pathways (A) with a significant overrepresentation in cognition-related changes, some of the same pathways (eg, Complement and Fcγ phagocytosis) as the age-related analysis were evident, with additional novel pathways not observed in the aging analysis. In the functional analysis (B), a similar pattern of functions observed with aging and novel functions were observed. Importantly, functions observed with aging were predicted to be inversely affected with cognition than was observed with aging (eg, leukocyte migration and quantity of Ca2+). Analogously, upstream regulators identified (C) were oppositely affected in cognitively Impaired and Intact rats (eg, INFG and TRIM24). The likelihood of the association given the data set was assessed by Fisher’s Exact Test, ***p < .001, **p < .01, *p < .05. Z-scores are based on prior knowledge of known regulatory functions and direction of changes in the current dataset. Z-scores >2 indicate activation with cognitive impairment and <−2 indicate inhibition with cognitive impairment.
One of the largest magnitude cognition-related changes observed across subregions was transthyretin (Ttr), which was confirmed by qPCR (Figure 7A). Significantly lower levels of Ttr were observed in the CA3 and DG of Aged Impaired animals as compared to Aged Intact. To extend these findings, and treat the behavioral performance as a continuum rather than as two categories, Ttr expression was correlated to mean proximity to platform values in the Aged animals. In both the CA3 and DG, lower Ttr expression was significantly correlated with higher mean proximity to platform values. In other words, lower Ttr expression in Aged animals was associated with worse cognitive performance (Figure 7B and C). Similar trends were also observed for different expression and correlation in the CA1, but these did not reach statistical significance.
Figure 7.
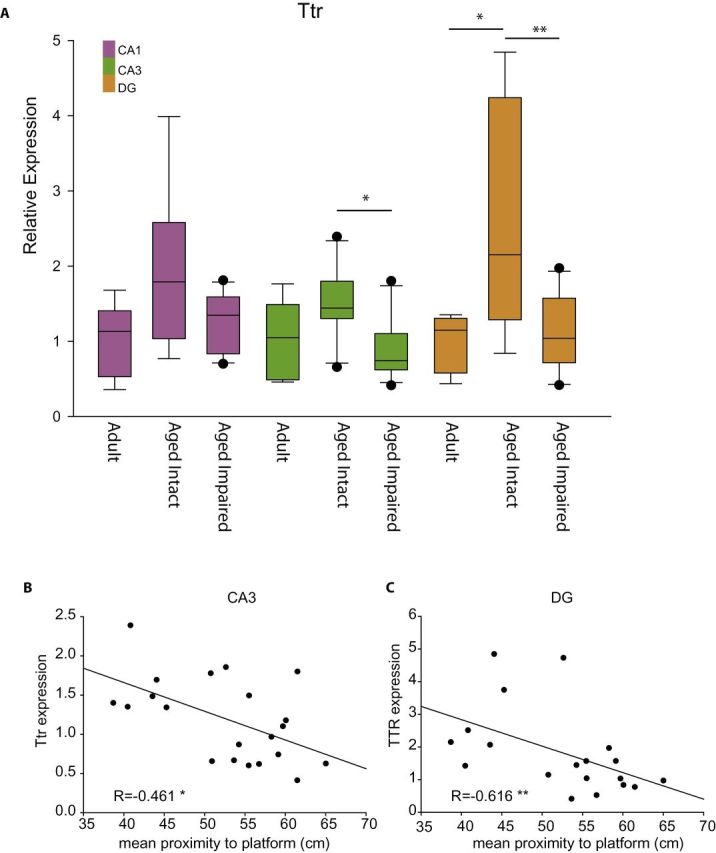
Orthoginal confirmation of cognition-related suppression of transthyretin. Transthyretin (Ttr) expression (A) was confirmed across all three groups and brain regions. In both the CA3 and DG, a significantly lower level of Ttr expression was observed, with a similar trend in CA1 (analysis of variance, SNK post hoc **p < .01, *p < .05, n = 5–7 Aged, n = 8–9 Aged Intact, n = 11–12 Aged Impaired). To treat the behavioral performance as a continuum rather than as two populations, Ttr expression was correlated to behavioral performance in Aged animals. In both the CA3 (B) and DG (C), Ttr expression negatively correlated to mean proximity to platform distance in the probe trial, that is, lower Ttr expression was associated with worse behavioral performance. Pearson correlation, *p < .05, **p < .01, n = 21 CA3, n = 19 DG.
Discussion
In this work, we demonstrate that gene expression with aging and cognitive decline is distinct in each subregion of the hippocampus with the majority of transcriptomic changes observed in only one subregion, with some commonalities across multiple regions. At the gene and the pathway levels, CA1 and CA3 shared more commonalities than either did with the DG. Orthogonal confirmation of novel gene expression changes by qPCR demonstrated the analytical validity of the findings and suggests novel processes that may be regulated with aging. When gene expression findings were compared to whole hippocampal microarray and qPCR data, common changes could often be identified, but subregion-restricted changes were no longer evident. Lastly, cognition-related changes demonstrated distinct patterns across the subregions, and in cases where genes did overlap with age-related changes, the regulation was inversed. These data suggest that at the transcript level, Aged Impaired rats have a distinct phenotype rather than an exacerbation of a common aging response.
Using the Adult data from each subregion, we began by identifying genes whose expression was restricted or highly enriched in individual subregions. These markers can be used to both validate the subregion dissections and gain insight into the specific functions of each hippocampal subregion. Enrichment of Tiam1 and Dsp in the DG, Bok and Spok1 in CA3, and Nov in the CA1 has previously been reported in mice by Gage and colleagues (34,35). More recently, Dingledine and colleagues assessed gene expression in laser capture microdissected cell bodies from rat CA1, CA3, and DG; observing enriched Tiam1 in DG, Lphn2 and Nov in CA1 (36). Examination of the supplemental detail from this report, which used Sprague Dawley rats, reveals that most of the regional differences confirmed in this study are also evident in this report. These genes could be used as controls for hippocampal subregion dissections in future studies.
Only a limited number of previous reports have sought to examine age- and cognition-related gene expression across hippocampal subregions (19,20) and most comparisons between hippocampal subregions have been between different studies; for a comprehensive review, see (13). Previously, Foster and colleagues examined the same strain of rats at slightly older ages (18 and 28 months) and found regionally specific aging effects on gene expression and observed that some of these changes are modulated by caloric restriction (19). While the focus of this report was not a direct comparison across subregions and with whole hippocampal data, these findings generally agree with those reported here. Gallagher and colleagues have also previously reported a comparison of transcriptomic changes in CA1, CA3, and DG of 6 months and 24–26 months old Long-Evans rats behaviorally characterized for spatial learning and memory (20). While the cross region comparison was not the focus of their work, they also report a greater correlation between CA1 and CA3 age-related changes than with the DG and limited shared cognition-related gene expression changes across subregions.
We chose a number of genes of special interest to confirm by qPCR for their potential functional roles and to confirm the microarray data by an orthogonal method. This approach provides a higher confidence in the gene expression data and allows application of more rigorous multiple testing comparisons. Microarray targets were confirmed by qPCR and demonstrated similar levels of differences to the microarray data. Changes confirmed by qPCR that were observed across multiple subregions included Arhgap9, Bmp7, Emp3, Fxyd1, Fxyd3, Gipr, Glra2, and Ncf1. Arhgap9 (Rho GTPase Activating Protein 9) inactivates Rho-type GTPases by converting them to a GDP-bound state. While Arhgap9 is primarily active against CDC42 and RAC1, it has activity for Rhoa (37). We have previously demonstrated a cognition specific upregulation of the myelin associated inhibitor pathway, including Rhoa (30). Activation of Arhgap9 with aging could suppress Rhoa activity. Bone morphogenic proteins are critical to brain development and in particular neurogenesis in the dentate (38,39), raising the potential for the induction of BMP7 in all three subregions to be a protective mechanism with aging. Emp3 (epithelial membrane protein 3) is induced with injury in axons (40) and is regulated by retinoic acid (41), supporting studies that have found the induction of the retinoic acid cascade with aging. Fxyd1 and Fxyd3 (FXYD domain-containing ion transport regulator) are regulators of Na/K ATPases (42). While the central nervous system functions of Fxyd3 are unknown, Fxyd1, also known as phospholemman, is a MeCP target gene that is upregulated with Rett syndrome (43). Overexpression of Fxyd1 is associated with reduced dendritic arborization and cognitive impairments can be rescued by deletion of Fxyd1 (44). As such, an upregulation with aging may contribute to reduced dendritic complexity and cognitive impairments with aging. Gipr (gastric inhibitory polypeptide receptor) was found to be upregulated with aging in all three subregions. While the function of Gipr in the hippocampus has not been directly examined, Gipr knockout has impaired learning and memory, potentially suggesting that Gipr induction is protective (45).
A selection of novel age-regulated genes observed in only one subregion and a gene regulated with age across subregions were confirmed by qPCR. These genes were chosen and are of interest because their changes within hippocampal subregions, in terms of function, are similar to what has been described in the literature (13). FGF12 (fibroblast growth factor 12), or FHF1 (fibroblast growth factor homologous factor 1), is a known regulator of membrane depolarization and signal propagation of action potentials through blockade of voltage-gated sodium channels (46,47). Additionally, FGF12 was shown to associate with MAPK signaling in the brain by interaction with islet-brain 2 (IB2), leading to the recruitment of tissue-specific protein kinase signaling (48). More recently, it has been shown that FGF12 has a role in regulating neuronal morphology and neurite outgrowth by negatively regulating NFκB signaling through NEMO (49). Inhibiting signal propagation and neurite outgrowth with FGF12 expression could contribute to decreased synaptic transmission with aging in CA3 (13).
MS4A6A (membrane-spanning 4-domains, subfamily A, member 6A) is an integral membrane protein that has been found to be associated with late-onset Alzheimer’s disease. In addition, expression levels are positively associated with brain pathology (50). This gene locus is not only involved in Alzheimer’s disease, but expression levels are also associated with mild cognitive impairment. MS4A6A is also known to be involved in immune response pathways, where it might function as a component of immune cell activation (51). Increased expression in the CA3 with aging may have similarities to what has been previously shown where immune function is increased in the CA3 with aging (13).
Cobl (Cordon-bleu) is a highly conserved gene initially found to be an important regulator of neural tube formation, closure and neurulation (52). Further investigation has led to identifying Cobl as a nucleator for actin assembly in the brain where expression leads to neurite branching; as a result, Cobl is thought to be a regulator of cellular morphology and cytoskeleton dynamics in the brain (53). Additionally, it was found that Cobl expression is highly enriched in purkinje cells of the cerebellum, and the interactions with F-actin assembly contributes to purkinje cells’ unique and broad arborization patterns (54). With aging, it has been shown that cytoskeletal structure is increased in CA1, fitting the expression pattern of Cobl in this study. However, both neurite outgrowth factors and neuronal development are found to be decreased with aging in CA1 (13). The cytoskeletal rearrangements induced by Cobl are a possible outcome of expression with age, as opposed to neurite outgrowth associated with Cobl in development.
Prg4 (proteoglycan 4) and Hapln2 (hyaluronan and proteoglycan link protein 2) are important components of the extracellular matrix. Little is known about the expression of these genes in the central nervous system. Prg4 expression increases in a mouse beta 2 adrenergic receptor knockout model of middle cerebral arterial stroke (55). Hapln2 is one of four HA-linked proteins expressed in the brain and loss of expression occurs with malignant gliomas (56). With aging, extracellular matrix function has been shown to decrease in CA1 (13). In CA1, loss of Prg4 signaling could contribute to extracellular matrix dysfunction with aging, while Hapln2 induction with age in the CA1 may act as a compensatory mechanism for loss of Prg4 expression. Future studies will need to examine protein and expression localization of these age-related changes.
Multiple upstream regulators of gene expression with aging and cognition were identified. Interestingly, the majority of regulators activated with aging were detected as inhibited with cognitive impairment. Tretinoin, or all-trans retinoic acid, has been identified as a potential therapeutic or supplement to treat aging-related phenotypes. In mice, aging decreases the expression and actions of thyroid receptor and retinoic acid receptor in the whole brain and hippocampus (57). Supplementation with retinoic acid restores the expression of thyroid receptor and retinoic acid receptor in 22 and 24-month-old mice (58). Target genes of retinoic acid receptor and thyroid receptor ultimately lead to expression of factors relating to dendrite spine density and synaptic plasticity, leading to the conclusion that maintaining the expression of these factors with aging through retinoic acid supplementation could mitigate the plasticity-related deficits in learning and memory seen in hippocampal aging (59–61).
Inosine, a metabolite of adenosine, is a compound of interest for treating patients with traumatic brain injuries and spinal cord injuries. In experimental models of spinal cord injuries, inosine treatment significantly increased the level of axon sprouting and synaptic connections, as well as increasing motor control (62). Inosine also improves functional recovery in experimental traumatic brain injuries by increasing synaptic plasticity (63). Additionally, inosine treatment increases BDNF transcription and cellular proliferation in neuronal cell culture, ultimately increasing cell viability (64). The induction of inosine-regulated genes with aging and their suppression with cognitive decline is another example of the inverse relationship between aging and cognition responses. Additional interventional studies are needed to further interrogate these regulators and their opposing activation with aging and cognitive impairment.
Cognitively impaired animals had decreased expression of Ttr (transthyretin). Previously Brouillette and Quirion described a hippocampal downregulation of Ttr in cognitively impaired 24M old Long-Evans rats as compared to age-matched cognitively intact rats and a restoration of spatial memory with retinoic acid treatment (65). Our findings confirm the downregulation of Ttr with cognitive impairment in CA3 and DG and a trend towards downregulation in CA1. The expression pattern, including that in Adult rats, in our study is an “inverted U,” suggesting a failure of the aging response. Further studies will need to examine Ttr protein expression and function as well as manipulation of Ttr function and its effect on learning and memory.
In summary, these findings demonstrate that each subregion of the hippocampus has a specific aging and cognition-related gene expression response and that these subregion-restricted changes are not observed when the whole hippocampus is examined. These results suggest that, when possible, hippocampal subregions rather than whole hippocampal preparations should be used in discovery analyses. We also demonstrate that the pyramidal regions of the hippocampus (CA1 and CA3) are more similar in gene expression patterns than either is with granule cell regions (DG). Strikingly, the gene expression changes related to cognitive decline were distinct from those observed with aging or the opposite of age-related changes. This agrees with our previous hippocampal proteomic and transcriptomic findings (17,21,22,30). Rather than cognitive decline representing an exaggerated phenotype, cognitive decline is associated in distinct pathways and a failure of the normal aging response. These results also serve as a resource for the brain aging and cognitive decline research communities for bioinformatics studies comparing different models systems and for data mining to identify targets to be tested in interventional studies.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by National Institute on Aging (NIA) (R01AG026607 and P01AG11370) and generous support from the Donald W. Reynolds Foundation.
Supplementary Material
Acknowledgments
We thank Julie Farley for invaluable assistance with animal studies, Heather VanGuilder for technical assistance, and Raymond K. Hessel for illustration assistance.
References
- 1. Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. :10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- 2. Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging. 2009;24:154–162. :10.1037/a0014283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Umegaki H, Hayashi T, Nomura H, et al. Cognitive dysfunction: an emerging concept of a new diabetic complication in the elderly. Geriatr Gerontol Int. 2013;13:28–34. :10.1111/j.1447-0594.2012.00922.x [DOI] [PubMed] [Google Scholar]
- 4. Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- 5. Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–173. [DOI] [PubMed] [Google Scholar]
- 8. Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen T, Schliemann T, Sørensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–147. [DOI] [PubMed] [Google Scholar]
- 10. Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. :10.1016/j.tins.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 11. Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:8. :10.3389/fnagi.2011.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. :10.1007/s11920-009-0082-1 [DOI] [PubMed] [Google Scholar]
- 13. Burger C. Region-specific genetic alterations in the aging hippocampus: implications for cognitive aging. Front Aging Neurosci. 2010;2:140. :10.3389/fnagi.2010.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: Mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012;67:754–759. :10.1093/gerona/gls112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kadish I, Thibault O, Blalock EM, et al. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. :10.1523/JNEUROSCI.4599-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rowe WB, Blalock EM, Chen KC, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. :10.1523/JNEUROSCI.4163-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman WM, VanGuilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. :10.1016/j.neuroscience.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanGuilder HD, Farley JA, Yan H, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–212. :10.1016/j.nbd.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeier Z, Madorsky I, Xu Y, Ogle WO, Notterpek L, Foster TC. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mech Ageing Dev. 2011;132:8–19. :10.1016/j.mad.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, Gallagher M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol Aging. 2011;32:1678–1692. :10.1016/j.neurobiolaging.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. VanGuilder HD, Bixler GV, Brucklacher RM, et al. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation. 2011;8:138. :10.1186/1742-2094-8-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vanguilder HD, Bixler GV, Sonntag WE, Freeman WM. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J Neurochem. 2012;121:77–98. :10.1111/j.1471-4159.2012.07671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanGuilder Starkey HD, Bixler GV, Sonntag WE, Freeman WM. Expression of NgR1-antagonizing proteins decreases with aging and cognitive decline in rat hippocampus. Cell Mol Neurobiol. 2013;33:483–488. :10.1007/s10571-013-9929-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging. 2005;26:683–688. [DOI] [PubMed] [Google Scholar]
- 25. VanGuilder Starkey HD, Van Kirk CA, Bixler GV, et al. Neuroglial expression of the MHCI pathway and PirB receptor is upregulated in the hippocampus with advanced aging. J Mol Neurosci. 2012;48:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bixler GV, Vanguilder HD, Brucklacher RM, Kimball SR, Bronson SK, Freeman WM. Chronic insulin treatment of diabetes does not fully normalize alterations in the retinal transcriptome. BMC Med Genomics. 2011;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. VanGuilder HD, Bixler GV, Kutzler L, et al. Multi-modal proteomic analysis of retinal protein expression alterations in a rat model of diabetic retinopathy. PLoS ONE. 2011;6:e16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krämer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliveros JC. VENNY: An Interactive Tool for Comparing Lists With Venn diagrams. 2007. [Google Scholar]
- 30. VanGuilder Starkey HD, Sonntag WE, Freeman WM. Increased hippocampal NgR1 signaling machinery in aged rats with deficits of spatial cognition. Eur J Neurosci. 2013;37:1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinman GE, Naiman S, Kanfi Y, Cohen H, Bar-Joseph Z. ExpressionBlast: mining large, unstructured expression databases. Nat Methods. 2013;10:925–926. :10.1038/nmeth.2630 [DOI] [PubMed] [Google Scholar]
- 32. Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. [DOI] [PubMed] [Google Scholar]
- 33. Allen Institute for Brain Science. Allen Mouse Brain Atlas http://mouse.brain-map.org/ Accessed December 3, 2014.
- 34. Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. :10.1523/JNEUROSCI.4710-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. [DOI] [PubMed] [Google Scholar]
- 36. Greene JG, Borges K, Dingledine R. Quantitative transcriptional neuroanatomy of the rat hippocampus: evidence for wide-ranging, pathway-specific heterogeneity among three principal cell layers. Hippocampus. 2009;19:253–264. :10.1002/hipo.20502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furukawa Y, Kawasoe T, Daigo Y, et al. Isolation of a novel human gene, ARHGAP9, encoding a rho-GTPase activating protein. Biochem Biophys Res Commun. 2001;284:643–649. :10.1006/bbrc.2001.5022 [DOI] [PubMed] [Google Scholar]
- 38. Caronia G, Wilcoxon J, Feldman P, Grove EA. Bone morphogenetic protein signaling in the developing telencephalon controls formation of the hippocampal dentate gyrus and modifies fear-related behavior. J Neurosci. 2010;30:6291–6301. :10.1523/JNEUROSCI.0550-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choe Y, Kozlova A, Graf D, Pleasure SJ. Bone morphogenic protein signaling is a major determinant of dentate development. J Neurosci. 2013;33:6766–6775. :10.1523/JNEUROSCI.0128-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bolin LM, McNeil T, Lucian LA, et al. HNMP-1: a novel hematopoietic and neural membrane protein differentially regulated in neural development and injury. J Neurosci. 1997;17:5493–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su D, Gudas LJ. Gene expression profiling elucidates a specific role for RARgamma in the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Biochem Pharmacol. 2008;75:1129–1160. :10.1016/j.bcp.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crambert G, Geering K. FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci STKE. 2003;2003:RE1. :10.1126/stke.2003.166.re1 [DOI] [PubMed] [Google Scholar]
- 43. Deng V, Matagne V, Banine F, et al. FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2-null mice. Hum Mol Genet. 2007;16:640–650. :10.1093/hmg/ddm007 [DOI] [PubMed] [Google Scholar]
- 44. Matagne V, Budden S, Ojeda SR, Raber J. Correcting deregulated Fxyd1 expression ameliorates a behavioral impairment in a mouse model of Rett syndrome. Brain Res. 2013;1496:104–114. :10.1016/j.brainres.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Faivre E, Gault VA, Thorens B, Hölscher C. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol. 2011;105:1574–1580. :10.1152/jn.00866.2010 [DOI] [PubMed] [Google Scholar]
- 46. Goldfarb M, Schoorlemmer J, Williams A, et al. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. :10.1016/j.neuron.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dover K, Solinas S, D’Angelo E, Goldfarb M. Long-term inactivation particle for voltage-gated sodium channels. J Physiol (Lond). 2010;588(Pt 19):3695–3711. :10.1113/jphysiol.2010.192559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schoorlemmer J, Goldfarb M. Fibroblast growth factor homologous factors are intracellular signaling proteins. Curr Biol. 2001;11:793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. König HG, Fenner BJ, Byrne JC, et al. Fibroblast growth factor homologous factor 1 interacts with NEMO to regulate NF-?B signaling in neurons. J Cell Sci. 2012;125(Pt 24):6058–6070. :10.1242/jcs.111880 [DOI] [PubMed] [Google Scholar]
- 50. Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS ONE. 2012;7:e50976. :10.1371/journal.pone.0050976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Proitsi P, Lee SH, Lunnon K, et al. ; AddNeuroMed Consortium. Alzheimer’s disease susceptibility variants in the MS4A6A gene are associated with altered levels of MS4A6A expression in blood. Neurobiol Aging. 2014;35:279–290. :10.1016/j.neurobiolaging.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 52. Carroll EA, Gerrelli D, Gasca S, et al. Cordon-bleu is a conserved gene involved in neural tube formation. Dev Biol. 2003;262:16–31. [DOI] [PubMed] [Google Scholar]
- 53. Ahuja R, Pinyol R, Reichenbach N, et al. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007;131:337–350. :10.1016/j.cell.2007.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haag N, Schwintzer L, Ahuja R, et al. The actin nucleator Cobl is crucial for Purkinje cell development and works in close conjunction with the F-actin binding protein Abp1. J Neurosci. 2012;32:17842–17856. :10.1523/JNEUROSCI.0843-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. White RE, Palm C, Xu L, et al. Mice lacking the ß2 adrenergic receptor have a unique genetic profile before and after focal brain ischaemia. ASN Neuro. 2012;4AN20110020. :10.1042/AN20110020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sim H, Hu B, Viapiano MS. Reduced expression of the hyaluronan and proteoglycan link proteins in malignant gliomas. J Biol Chem. 2009;284:26547–26556. :10.1074/jbc.M109.013185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Enderlin V, Pallet V, Alfos S, et al. Age-related decreases in mRNA for brain nuclear receptors and target genes are reversed by retinoic acid treatment. Neurosci Lett. 1997;229:125–129. [DOI] [PubMed] [Google Scholar]
- 58. Enderlin V, Alfos S, Pallet V, et al. Aging decreases the abundance of retinoic acid (RAR) and triiodothyronine (TR) nuclear receptor mRNA in rat brain: effect of the administration of retinoids. FEBS Lett. 1997;412:629–632. [DOI] [PubMed] [Google Scholar]
- 59. Mingaud F, Mormede C, Etchamendy N, et al. Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci. 2008;28:279–291. :10.1523/JNEUROSCI.4065-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54:489–495. :10.1002/mnfr.200900246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brossaud J, Roumes H, Moisan MP, Pallet V, Redonnet A, Corcuff JB. Retinoids and glucocorticoids target common genes in hippocampal HT22 cells. J Neurochem. 2013;125:518–531. :10.1111/jnc.12192 [DOI] [PubMed] [Google Scholar]
- 62. Kim D, Zai L, Liang P, Schaffling C, Ahlborn D, Benowitz LI. Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS ONE. 2013;8:e81948. :10.1371/journal.pone.0081948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555:78–88. :10.1016/j.brainres.2014.01.044 [DOI] [PubMed] [Google Scholar]
- 64. Muto J, Lee H, Lee H, et al. Oral administration of inosine produces antidepressant-like effects in mice. Sci Rep. 2014;4:4199. :10.1038/srep04199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brouillette J, Quirion R. Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging. 2008;29:1721–1732. :10.1016/j.neurobiolaging.2007.04.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.