Abstract
Aging is a major risk factor for cerebrovascular disease. Growth hormone (GH) and its anabolic mediator, insulin-like growth factor (IGF)-1, decrease with advancing age and this decline has been shown to promote vascular dysfunction. In addition, lower GH/IGF-1 levels are associated with higher stroke mortality in humans. These results suggest that decreased GH/IGF-1 level is an important factor in increased risk of cerebrovascular diseases. This study was designed to assess whether GH/IGF-1-deficiency influences the outcome of cerebral ischemia. We found that endothelin-1-induced middle cerebral artery occlusion resulted in a modest but nonsignificant decrease in cerebral infarct size in GH/IGF-1 deficient dw/dw rats compared with control heterozygous littermates and dw/dw rats with early-life GH treatment. Expression of endothelin receptors and endothelin-1-induced constriction of the middle cerebral arteries were similar in the three experimental groups. Interestingly, dw/dw rats exhibited reduced brain edema and less astrocytic infiltration compared with their heterozygous littermates and this effect was reversed by GH-treatment. Because reactive astrocytes are critical for the regulation of poststroke inflammatory processes, maintenance of the blood–brain barrier and neural repair, further studies are warranted to determine the long-term functional consequences of decreased astrocytic activation in GH/IGF-1 deficient animals after cerebral ischemia.
Key Words: Insulin-like growth factor-1, Cerebral ischemia, Vascular aging, Endothelin-1.
The risk of ischemic stroke and transient ischemic attack increases with age (1). Aging is not only the single most important independent risk factor for the incidence of stroke, but also is a significant predictor of stroke severity (2,3). Older patients with ischemic stroke differ in clinical characteristics and experience higher mortality than younger patients (4). Although studies in experimental animals confirm that severity of cerebral injury following acute ischemia is increased by aging (5–7), the underlying mechanisms remain elusive.
There is an increasing evidence that the age-related decline in circulating growth hormone (GH) and insulin-like growth factor (IGF)-1 levels has a key role in functional and structural impairment of the cerebral vasculature in the elderly (reviewed recently in Ref. (8)), which could contribute to increased incidence of stroke and poor recovery after stroke. The cardiovascular system is an important target organ for GH and IGF-1 and it is well-documented that in human patients GH deficiency and low circulating levels of IGF-1 significantly increase the risk for cerebrovascular disease (9–16). There are strong experimental data to suggest that GH/IGF-1 deficiency promotes the development of atherosclerosis (recently reviewed in Ref. (17)), alters hemostasis (18), and compromises cerebrovascular function (recently reviewed in Ref. (8)). It is generally accepted that in humans the lack of cerebrovascular protective effects conferred by the GH/IGF-1 axis is responsible for the association between short stature and increased incidence of stroke (19,20). Studies using laboratory animal models support the conclusions of clinical and epidemiological studies (21,22) that treatment with GH or IGF-1 confers protection after stroke (23,24).
The Lewis dwarf rat (dw/dw) is a novel model of age-related GH/IGF-1 deficiency. Lewis dwarf rats have normal pituitary function except for a selective genetic GH deficiency (25,26) and they mimic many of the pathophysiological alterations present in human patients with age-related GH/IGF-1 deficiency (including mild cognitive impairment (27) and vascular dysfunction (28,29)). Importantly, GH/IGF-1 deficiency in Lewis dwarf rats significantly increases the incidence of late-life cerebral hemorrhages (30), similar to the effects of aging in humans. Despite these advances, the consequences of GH/IGF-1 deficiency on neuronal damage after ischemic insult have not been fully investigated.
The current study was designed to determine the effects of GH/IGF-1 deficiency on several outcomes of cerebral ischemia using the dw/dw rat as the model system because it closely mimics the age-related decline in GH and IGF-1. In this study, cerebral ischemia was induced using a potent vasoconstrictor (endothelin-1) injected proximal to the middle cerebral artery (MCA)to cause a transient but profound reduction of local blood flow to the cortex and striatum (31). This method provides rapid occlusion of the MCA and a gradual reperfusion that lasts for 16–22 hours (31), mimicking the temporal events of an embolic stroke. As primary endpoints infarct size, neuronal death, cerebral edema formation, and astrocyte activation were investigated.
Materials and Methods
Animals
Homozygous dwarf (dw/dw) rats of the Lewis origin were purchased from Harlan Industries (Indianapolis, IN). Previous studies indicated that dw/dw rats have a recessive mutation in the transcription factor necessary for the maturation of the somatotroph and therefore, pituitary GH production and plasma GH levels are reduced with no changes in other anterior pituitary hormones (26,30,32,33). Homozygous dwarf (dw/dw) males were bred with females of the Lewis strain to create heterozygous offspring of normal size. Heterozygous females were bred with dw/dw males to produce both dw/dw (dwarf) and heterozygote (normal size) animals used in this study. Identification of dw/dw from their HZ littermates was based on body weight, body growth rate, and serum IGF-1 levels as described previously (34). On postnatal day 40, 11 male dwarf rats were randomly divided into dw/dw+saline and dw/dw+GH groups and injected with saline or 300µg recombinant porcine GH (Alpharma, distributed by OzBioPharm, Knoxfield, VIC, Australia), respectively, twice per day (9–10 am and 3–4 pm) for 8wk. Twelve HZ rats received saline injections. Injections were continued until sacrifice 3 days postischemia. All animal protocols were in accordance with Guidelines for the Care and Use of Experimental Animals and approved by the Institutional Animal Care and Use Committee of OUHSC.
Assessment of the Effects of GH/IGF-1 Deficiency on Endothelin-1-Induced Constriction of Isolated Middle Cerebral Arteries
Both GH/IGF-1 deficiency and aging (28,35–45) are associated with complex phenotypic changes in the vasculature. To confirm that GH/IGF-1 deficiency does not alter the vascular responses to endothelin-1, we assessed endothelin-1-induced constriction of isolated MCAs in vitro. Male dw/dw or HZ rats (3 months, n = 5/each) were euthanized and segments of the MCAs were isolated for ex vivo assessment of vasomotor function as described previously (46–48). In brief, isolated segments of MCAs were mounted onto two glass micropipettes in an organ chamber and pressurized to 60 mmHg by a pressure servo-control system (Living Systems Instrumentation, Burlington, VE). Inner vascular diameter was measured with a custom-built videomicroscope system and continuously recorded using a computerized data acquisition system. All vessels were allowed to stabilize for 60 minutes in oxygenated (21% O2, 5% CO2, 75% N2) Krebs’ buffer (at 37°C). To assess the effects of GH/IGF-1 deficiency on vascular sensitivity to endothelin-1, changes in diameter of MCAs in response to increasing concentrations of endothelin-1 (from 0.1 to 100 nM) were obtained. With 100 nM of endothelin-1, both groups reached maximal constriction with complete lumen occlusion. Therefore, the average percentage of vessel diameter changes at this concentration was calculated and set as 100%; thereafter, all the results were normalized to this average value.
Western Blotting
To analyze the protein expression levels of endothelin-1 receptors, protein extracts of cerebral cortices (34) were used for western blotting. Tissue samples were homogenized on ice in freshly prepared lysis buffer consisting of RIPA (radio-immunoprecipitation assay) buffer, 1% volume of protease inhibitor cocktail, 2 mM sodium orthovanadate (Na3VO4), and 0.1 mg/mL phenylmethylsulfonyl fluoride. Tissue homogenates were mixed 1:1 by volume with 2 × SDS sample loading buffer [100 mM Tris–HCl pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, and 200 mM dithiothreitol] and reduced by heating at 95°C for 10 minutes. Proteins were separated by polyacrylamide gel electrophoresis using a 4%–20% gradient Tris–HCl gels (Bio-Rad, Hercules, CA) and transferred onto polyvinylidene fluoride membranes (Bio-Rad) using a semidry transfer cell (Bio-Rad). Membranes were immersed in blocking buffer (LI-COR Biosciences, Lincoln, NE) at room temperature for 1 hour and then incubated at 4°C overnight with polyclonal rabbit antiendothelin receptor A (ETAR), rabbit antiendothelin receptor B (ETBR), or monoclonal mouse anti-α-tubulin (Abcam, Cambridge, MA) according to the manufacturer’s instructions. Thereafter, membranes were washed with PBS containing 0.5% Tween-20 for 10 minutes (three times) and incubated at room temperature for 1 hour with fluorescent-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc, West Grove, PA). After another three washes, the membranes were scanned using an Odyssey infrared imaging system (LI-COR Biosciences). Density analysis was performed using Odyssey V3.0 software and normalized to α-tubulin.
Induction of Ischemic Stroke
For endothelin-induced stroke, animals were anesthetized with isoflurane and mounted on a stereotaxic frame (Kopf Instruments, Tujunga, CA). An incision was made to expose the skull. A hole with a diameter of 1 mm was made (A-P: +0.2 mm, L-VL: +4.0 mm) using a dental burr. Endothelin-1 (120 pmol, Calbiochem, Billerica, MA) in 3 µL saline was injected with a 30 G needle attached to a Hamilton syringe (Reno, NV) at a depth of 9.2 mm. The coordinates for the injection were determined based on the rat brain atlas (49). Empirical adjustments were made for variations in the Lewis strain and verified visually in pilot studies by an injection of 3 µL 0.1% Evans blue solution. The endothelin-1 solution was injected at 0.1 µL/15 seconds and the needle remained in situ for 3 minutes before retraction. The bone was filled with sterile bone wax and the wound was closed with 4-0 silk suture. After receiving a topical application of bupivacaine (Vedco Inc., St Joseph, MO) and a subcutaneous injection of 2.5 mg enrofloxacin (Sigma-Aldrich, St Loius, MO [other chemicals, if not specified, are from the same source]), the animals were allowed to recover from anesthesia and returned to their home cage. Body temperature was monitored throughout the procedure and maintained at 37 ± 0.5°C with a heating pad. Animals were monitored postoperatively for signs of stress.
Evaluation of Brain Swelling and Infarct Size
On postischemia day 3, animals were anesthetized using a mixture of ketamine and xylazine (80 and 12 mg/kg, respectively). Approximately 0.2 mL blood was collected from the left ventricle of the heart. All blood samples were collected before 12 pm. The animal was transcardially perfused with PBS containing 5200 units/L heparin followed by 4% paraformaldehyde-0.1 M PBS. Brains were removed from the skull and postfixed in 4% paraformaldehyde-0.1 M PBS at 4°C overnight followed by 10%, 20%, and 30% sucrose solution in PBS, each at 4°C overnight. Brains were embedded in CryoGel (Electron Microscopy Sciences, Hatfield, PA), frozen on dry ice, and sectioned coronally on a cryostat (Leica Microsystems Inc, Buffalo Grove, IL). Sections (40 µm) were free floated in cryopreservative (25% glycerol, 25% ethylene glycol, 50% 0.1 M PBS) and stored at −20°C until stained.
Serial sections throughout the brain with an interval of 760 µm were stained with 0.5% cresyl violet and imaged with a ScanScope CS scanner (Aperio, Vista, CA) at 20× magnification. Evaluation of infarct size was performed with StereoInvestigator software (MBF Bioscience, Williston, VT). The infarct area was defined as reduced Nissl staining and necrosis with pyknotic nuclei. The area of contralateral hemisphere and the undamaged ipsilateral hemisphere in each section were measured with the Cavalieri Estimator method using a grid spacing of 100 µm. The volume was calculated as the section interval (760 µm) × sum of the areas measured in the sampled sections. To calculate the infarct size, an indirect method (50) was used to correct for the confounding effect of brain edema. In brief, the infarct volume is calculated as [V contralateral hemisphere − V undamaged ipsilateral hemisphere]. Brain swelling was defined as an increased volume of the infarcted hemisphere (51,52) and expressed as a percentage of the contralateral hemisphere: (V ipsilateral hemisphere − V contralateral hemisphere)/V contralateral hemisphere ×100%.
Immunohistochemistry
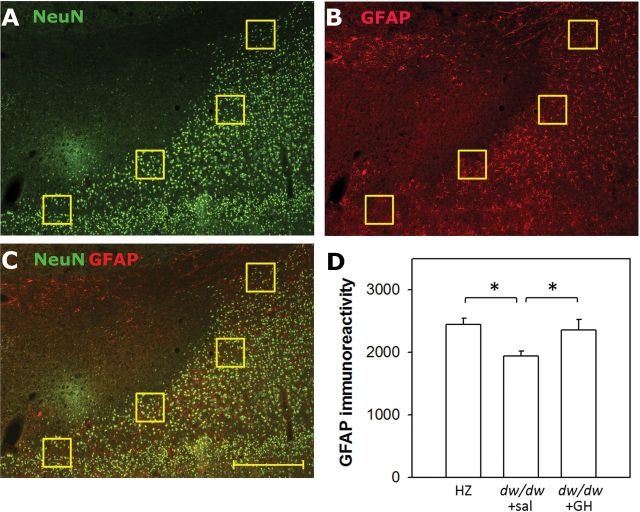
Brain sections containing the infarct areas were used for immunohistochemistry as described previously (53). The primary antibodies were a combination of a monoclonal mouse anti-NeuN antibody (EMD Millipore, Billerica, Massachusetts) and a polyclonal rabbit antiglial fibrillary acidic protein (GFAP, Abcam) antibody. To assess the extent of astrocytic infiltration, four identical regions of interest (ROIs) were draw at the cortical penumbra area based on the NeuN staining (Figure 4A). Each ROI was a 200 × 200 µm square. In the same image, mean density of GFAP staining from four ROIs was averaged for the section and then data from multiple sections were averaged for each animal.
Figure 4.
Early-life growth hormone (GH)/insulin-like growth factor-1 deficiency leads to attenuated astrocytic infiltration after ischemia. Sections were costained with NeuN (green, A) antibody for viable neurons and with glial fibrillary acidic protein (GFAP) (red, B) antibody for activated astrocytes. Their colocalization is presented in the merged image (C). Four evenly spaced, square identical regions of interest (ROIs) were drawn to outline the cortical penumbra according to NeuN staining, and the mean intensity of GFAP staining in 4 ROIs were averaged for each section, and further averaged among sections for each animal. In the dw/dw+sal group, GFAP staining was restricted in a smaller area than the NeuN staining and thus has a lower GFAP intensity in the ROIs; in contrast, in the other two groups, GFAP+ staining was either aligned with NeuN+ staining or invaded towards the infarct core (NeuN− area) and therefore has higher intensity in the ROIs (D). Scale bar = 500 µm. *p < .05 for the indicated comparisons. n = 6, 12, 5 for the dw/dw+sal, HZ, and dw/dw+GH groups, respectively. Data were analyzed by one-way analysis of variance and presented as mean ± SEM.
Enzyme-Linked Immunosorbent Assay
The whole blood collected at sacrifice was allowed to clot at room temperature for ~30 minutes, and serum was collected by centrifugation at 2,500g for 20 minutes at 4°C. Sera were stored at −80°C until used. Serum levels of IGF-1 were determined by enzyme-linked immunosorbent assay (R&D) according to the manufacturers’ instructions.
Statistics
Data were analyzed using Sigma-Stat 3.5 software (Systat Software, Chicago, Illinois). Western blotting results were analyzed by one-way analysis of variance (ANOVA). The vascular responses to endothelin were analyzed by two-way repeated measures ANOVA (using group and endothelin-1 concentration as independent variables) followed by the Student–Newman–Keuls test. Because normality tests on infarct volume failed, nonparametric tests were used. To assess the effect of genotype on infarct size, a rank sum test was used to compare the dw/dw+sal to the HZ group. To determine the effect of GH treatment, the same test was performed between the dw/dw+sal and dw/dw+GH groups. Brain edema was analyzed using ANOVA on ranks followed by the Dunn’s method. The GFAP density was analyzed by one-way ANOVA followed by the Student–Newman–Keuls method. All results are presented as mean ± SEM.
Results
Serum IGF-1 Levels Are Reduced in Lewis Dwarf Rats and Restored by GH Replacement
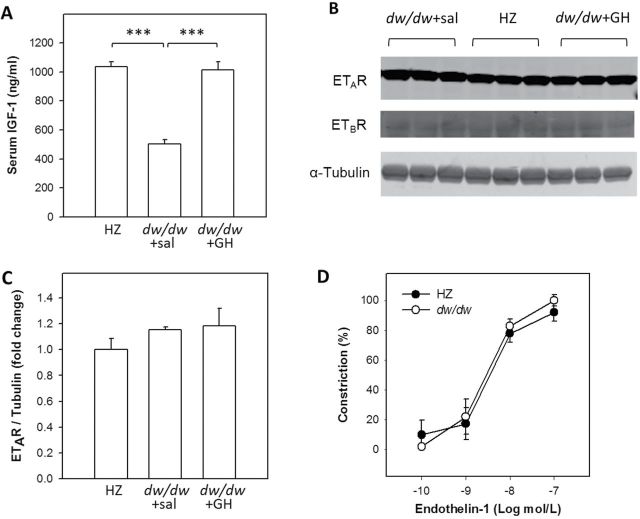
Consistent with our previous results, peri-pubertal serum IGF-1 levels in the dw/dw rats were 51.5% lower than in the heterozygous littermates (502.3 ± 30.9 vs 1035.6 ± 34.8ng/mL, respectively, p < .001). Treatment with GH restored IGF-1 levels to those found in normal animals (1012.7 ± 58.0ng/mL, p < .001, Figure 1A).
Figure 1.
Validation of the insulin-like growth factor (IGF)-1 deficiency in the Lewis dwarf rats and characterization of the vascular responses to endothelin-1. (A) Serum IGF-1 levels were determined by enzyme-linked immunosorbent assay. One-way analysis of variance (ANOVA) revealed a significant difference among groups (p < .001) and pairwise comparisons demonstrate that the serum IGF-1 level in the dw/dw+sal group is significantly reduced compared to the HZ group (p < .001) and growth hormone–treated group (p < .001). Homogenates of cerebral cortex from a separate cohort was used for Western blotting to semiquantify the expression of A-type (ETAR) and B-type (ETBR) receptors. Representative images are shown in (B) and quantitation of ETAR expression is shown in (C). One-way ANOVA demonstrated that ETAR expression is similar among three groups (p = .389). Expression of ETBR was too low to be quantitated but appeared not to be different among groups. n = 3/group. (D) Constriction of cannulated middle cerebral arteries isolated from dw/dw and HZ rats in response to increasing concentrations of endothelin-1. Constriction was calculated as a percentage of changes in diameter from the baseline and normalized to the maximum constriction which was reached at 100nM. There is no significant difference between the endothelin-1 -induced constriction of middle cerebral arteries isolated from dw/dw and HZ rats. Data are mean ± SEM (n = 5/group).
GH/IGF-1 Deficiency Does Not Alter Expression of Endothelin Receptors
Western blots demonstrated that ETAR protein levels were similar among the three treatment groups (Figure 1B and C, p = .389). The expression of ETBR was too low to be reliably quantitated but appeared to be similar among groups.
GH/IGF-1 Deficiency Does Not Alter Endothelin-1-Induced Constriction of Isolated Middle Cerebral Arteries
Endothelin-1 elicited robust dose-dependent constriction in isolated MCAs. Maximal constriction with complete lumen occlusion was reached at 100 nM in both groups. No differences in endothelin-induced MCA constriction were evident between the dw/dw and HZ groups (Figure 1D), supporting the validity of our experimental model.
Effect of GH/IGF-1 Deficiency on Ischemic Infarct Size in Response to by Endothelin-1
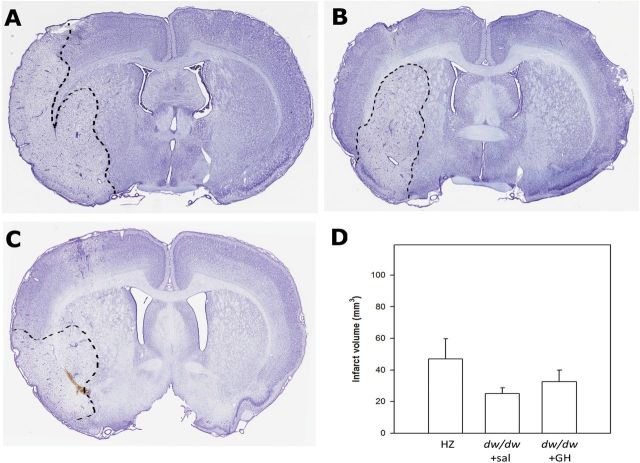
Perivascular endothelin injection produced substantial infarcts in all three groups. We chose to calculate infarct size using the indirect method by subtracting the volume of noninfarct region in the ipsilateral hemisphere from the total volume of the contralateral hemisphere. Using this method, the values of infarct volume were substantially smaller than that calculated by directly measuring the volume of infarct (data not shown), indicating that the direct method overestimated infarct size due to ischemia-caused edema. As shown in Figure 2, the infarct volume was not statistically different among the three groups (p = .571). Nevertheless, the HZ group demonstrated a trend towards increased infarct size as compared to the dw/dw+sal group (p = .253). Importantly, replacement of GH at levels that induced complete restoration of circulating IGF-1 levels did not modify infarct size.
Figure 2.
Growth hormone (GH)/insulin-like growth factor-1 deficiency does not affect the severity of endothelin-1-induced cerebral ischemic infarct. (A–C) Representative images of coronal sections stained with cresyl violet, the border of infarct area is outlined with a dashed line. The HZ (A) and the dw/dw+GH group (C) exhibited more pronounced asymmetry between hemispheres compared to the dw/dw+sal group (B). (D) Summary data for infarct size calculated by the indirect method which excludes error introduced by brain swelling. There is no statistic difference between the three groups (p = .571, one-way analysis of variance on ranks). Data are shown as mean ± SEM. n = 6, 12, 5 for the dw/dw+sal, HZ, and dw/dw+GH group, respectively.
Effect of GH/IGF-1 Deficiency on Brain Edema After Ischemia
Endothelin-induced ischemia resulted in increased volume of the ipsilateral hemisphere in the HZ animals (8.0% ± 2.4%) and dw/dw+GH groups (7.0% ± 2.1%). Images at higher magnifications (Figure 3A) demonstrate reduced cell density in the ipsilateral compared with the contralateral hemisphere. Based on the morphological changes of increased tissue volume and reduced cell density, we conclude that the change in brain volume is a result of and a surrogate for brain edema. One-way ANOVA on ranks indicated that significant differences in brain edema occurred among the three groups (p = .004). Pairwise multiple comparisons indicated that the dw/dw+sal group (0.6% ± 0.4%) had significantly reduced edema compared with the other two groups (Figure 3B, p < .05 for both dw/dw+sal vs dw/dw+GH and dw/dw+sal vs HZ).
Figure 3.
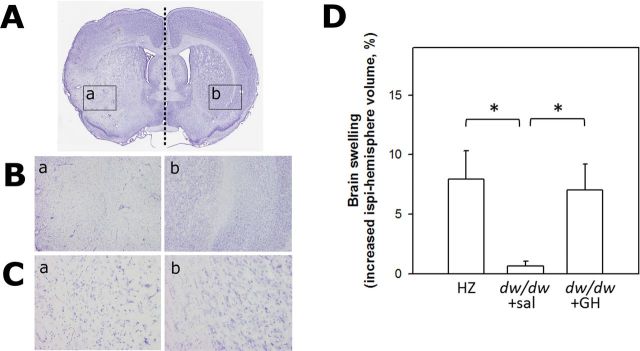
Animals with early-life growth hormone (GH)/insulin-like growth factor-1 deficiency exhibit the lowest level of brain edema after ischemia. Edema is calculated as increased volume of the ipsilateral hemisphere and data are shown as percentage of the contralateral hemisphere. (A) A representative image of a coronal section stained with cresyl violet, in which a straight dashed line is drawn to show the midline of the brain and the border of infarct area is outlined with arrows and two rectangles corresponding to the ischemic area (left) and contralateral intact tissue (right), respectively (shown in detail at 4× (B) and 20× (C) magnification). (D) Results of the quantitative volumetric analysis. Data were analyzed by one-way analysis of variance on ranks followed by Dunn’s pairwise comparisons and presented as mean ± SEM. *p < .05 for the indicated comparisons. n = 6, 12, 5 for the dw/dw+sal, HZ, and dw/dw+GH groups, respectively.
Effect of GH/IGF-1 Deficiency on Astrocytic Activation After Ischemia
Three days after endothelin-induced ischemia, all three treatment groups exhibited profound GFAP staining indicating astrocytic activation. NeuN staining revealed a clear delineation between the infarcted and viable tissue. By costaining with GFAP, the glia scar, composed of activated astrocytes, was visible in the areas surrounding the infarct (Figure 4C). The dw/dw+sal group showed reduced expansion/infiltration of activated astrocytes into the NeuN+ area in the cortex as compared to the other two groups. Intensity of GFAP staining at the cortical penumbra region was significantly lower in the dw/dw+sal group as compared to the other two groups (p < .05, Figure 4D).
Discussion
Ischemic stroke, which accounts for 87% of all strokes, is a major cause of mortality and the leading cause of long-term disability in the elderly in the United States (1). Population-based studies show that age is the most important independent risk factor for stroke, with stroke rates doubling every decade after the age of 55 years (1,3). Aging not only increases the incidence of stroke, but also increases severity of stroke and impairs recovery. It is well established that aging is associated with reductions in circulating GH and IGF-1 levels. Our results demonstrate that a peripubertal-onset of circulating GH and IGF-1 deficiency is associated with complex effects on stroke volume and the cellular changes in the brain that occur in response to stroke. Deficiency of GH/IGF-1 does not significantly affect infarct size but substantially reduces ischemia-induced brain edema and astrocytic infiltration.
In our study, we elected to use the Lewis dwarf (dw/dw) rats, which exhibit deficiencies in circulating GH/IGF-1 early in life, thereby mimicking the GH/IGF-1 reductions that occur during aging without having to age the animals and introduce additional confounding age-related factors. Ischemia was induced by microinjection of a potent vasoconstrictor into the proximity of the MCA. Hence, it was critical to ensure that the vasoconstriction produced by endothelin-1 was equivalent in the treatment groups. Analysis of the protein expression of ETAR and ETBR in the cortex and striatum demonstrated that changes in circulating IGF-1 levels did not modify the expression of these receptors. Ex vivo assessment of vessel constriction verified that MCAs derived from dw/dw and HZ rats respond to endothelin-1 similarly. These data validated the animal model and ischemic model used in this study.
It is generally accepted that the increased cerebrovascular risk that occurs with lower levels of GH/IGF-1 is responsible for the association between short stature and increased incidence of stroke (19,20). In addition, numerous studies support the concept that age-related decreases in levels of circulating GH and IGF-1 have an impact on the pathophysiology of cerebral vasculature. Circulating GH/IGF-1 levels dramatically decrease after stroke (54–56), lower circulating GH/IGF-1 is associated with poor recovery (56–59), and GH/IGF-1 treatment has been reported to be neuroprotective (23,60). However, the majority of the studies regarding the effects of GH/IGF-1 on stroke are focused on poststroke treatment for improved recovery. Only a few studies have concentrated on the association of stroke with endogenous GH/IGF-1 levels and the data that are available are controversial. Earlier studies indicate that Lewis dwarf rats exhibit increased incidence of late-life cerebral hemorrhage and peripubertal GH replacement reduces the incidence of hemorrhagic stroke and extends life span (30). Neuronal knockout of the IGF-1 receptor exacerbates hypoxia/ischemia-induced brain injury in neonatal mice (61). Recent studies on mice with adult-onset circulating IGF-1 deficiency also show an increased prevalence of hypertension-induced cerebral hemorrhages (Z. Ungvari and P. Toth, unpublished data, 2014). In contrast, mice with neonatal-onset IGF-1 deficiency were protected from embolic ischemia compared to their wildtype littermates, and adult normal 129/SV mice with chronic IGF-1 treatment exhibited increased brain infarct size after MCA occlusion (62). In the current study, infarct size was not statistically different among groups even though the dw/dw+sal group showed a tendency of reduced infarct volume compared with the HZ control. This is potentially the results of the relatively small sample size especially in the dw/dw+sal and dw/dw+GH groups, given the fact that brain ischemic damage often exhibits substantial variance in animal models. Nevertheless, our data revealed that deficiency of circulating GH/IGF-1 levels in Lewis dwarf rats reduces ischemia-induced brain edema and astrocytic infiltration. The mechanisms by which circulating levels of GH and IGF-1 influence the development and clinical outcome of stroke are likely multifaceted (55,63–65), and involve vasoprotective effects, effects on clot formation, neuronal protection, oxidative stress, as well as effects on astrocytes.
Abundant in vitro (66) and in vivo evidence suggest that GH and IGF-1 have neuroprotective properties, promoting cell growth and proliferation while suppressing apoptosis, oxidative stress, and inflammation (see Refs. (67–70)). Because of the pleiotropic actions of GH and IGF-1, these hormones are likely to have effects on vasculature, astrocytes, and neurons, which independently or collectively influence stroke outcomes. This was apparent in this study because effects were observed on diverse endpoints.
Based on the current literature and the results of our studies, GH/IGF-1 appears to have differential effects on hemorrhagic versus acute ischemic stroke. These hormones protect the cerebrovasculature and prevent hypertension-induced vascular injury, whereas deficiency of these hormones exacerbates vascular damage contributing to hemorrhagic stroke (Ungvari and Toth, unpublished data, 2014). However, in ischemic stroke, higher levels of GH/IGF-1 appear to have pleiotropic actions. There is increasing evidence that in humans lower IGF-1 levels are significantly related to risk of acute ischemic stroke (71). Moreover, GH and IGF-1 treatments were shown to protect neurons against hypoxia-induced damage (60,72–75). Nevertheless, the effect of GH/IGF-1 deficiency on infarct volume in the Lewis dwarf rat model used was not significant. The specific mechanisms underlying these differential effects are presently unclear. The direct actions of GH/IGF-1 on neuronal cells may be related to both increased cellular metabolism and antiapoptotic effects, which are well-known actions of both GH and IGF-1. Interestingly, the effects of GH/IGF-1 deficiency on edema and astrocytic infiltration were highly significant and suggest that these processes are important contributing factors in the clinical sequelae after ischemic stroke.
Cerebral edema is an important complication that forms in the early hours of ischemic stroke by processes involving increased transport of water from the blood into brain. It is followed by vasogenic edema caused by breakdown of the blood–brain barrier ~4 hours after the ischemia. So far, there have been a few studies related to the effects of IGF-1 on ischemia-induced brain edema. Blunting IGF-1 receptor action in neuronal precursors and their progeny was reported to exacerbate hypoxic/ischemic-induced brain swelling/edema in mice(61), suggesting that the IGF-1 axis might be protective on cerebral edema. In the Lewis model, circulating GH and IGF-1 are present during before and after the stroke in HZ and dw/dw rats replaced with GH. In contrast, we found that circulating GH and IGF-1 deficiencies in Lewis dwarf rats were associated with decreased cerebral edema on postischemic day 3. Even though GH treatment is known to stimulate water retention in the body, its effect on water/salt balance has rarely been studied, especially in the context of cerebral ischemic injury. Yamamura and colleagues (76) reported that GH treatment on Wistar rats did not exacerbate brain edema caused by freeze brain injury. However, whether GH deficiency could attenuate edema in the same situation was not assessed. Our findings that GH deficiency in the dwarf rats is associated with reduced brain edema after ischemic stroke add important information to our knowledge on the role of GH/IGF-1 in ischemic brain injury. .
Reactive astrogliosis is a universal response of astrocytes to brain injury induced by ischemia, which involves rapid proliferation and changes in the cellular phenotype (77). Here, we report that postischemic reactive astrocytosis is reduced in Lewis dwarf rats and increased by GH replacement. This finding can be expected on the basis of earlier findings that IGF-1 regulates astrocyte proliferation both in vitro (78) and in vivo (79). Furthermore, mice overexpressing GH have increased GFAP levels in the brain (80). Because reactive astrocytes are critical for the regulation of inflammatory processes, maintenance of the blood–brain barrier and neural repair, further studies are warranted to determine the long-term functional consequences of decreased astrocytosis in GH/IGF-1 deficiency.
In conclusion, GH/IGF-1 deficiency does not significantly alter infarct size after endothelin-1-induced focal cerebral ischemia but significantly attenuates other ischemic parameters including edema and astrocytic infiltration. Because GH treatment initiated after endothelin-1-induced focal cerebral ischemia in rats was reported to accelerate recovery of motor function and significantly improves spatial memory on the Morris water maze test (23), further studies are warranted to determine the specific mechanisms for the actions of GH/IGF-1 that contribute to functional recovery. Finally, because the vasculature appears to be a primary target of the GH/IGF-1 axis and there are data suggesting that GH/IGF-1 deficiency may affect vascular fragility (81), the effects of GH/IGF-1 deficiency on the pathogenesis of hemorrhagic stroke should be elucidated.
Funding
American Heart Association (to P.T., A.C., and Z.U.); National Center for Complementary and Alternative Medicine (R01-AT006526 to Z.U.); National Institute on Aging (AG031085 to A.C.; AG038747 to W.E.S.); Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.); Ellison Medical Foundation (to W.E.S.).
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 3. Rojas JI, Zurrú MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very oldrisk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur J Neurol. 2007;14:895–899. [DOI] [PubMed] [Google Scholar]
- 4. Fonarow GC, Reeves MJ, Zhao X, et al. ; Get With the Guidelines-Stroke Steering Committee and Investigators. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. [DOI] [PubMed] [Google Scholar]
- 5. Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667; discussion 1668. [DOI] [PubMed] [Google Scholar]
- 6. DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood–brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonntag WE, Deak F, Ashpole N, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosén T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. [DOI] [PubMed] [Google Scholar]
- 10. Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81:1169–1172. [DOI] [PubMed] [Google Scholar]
- 11. Bülow B, Hagmar L, Mikoczy Z, Nordström CH, Erfurth EM. Increased cerebrovascular mortality in patients with hypopituitarism. Clin Endocrinol (Oxf). 1997;46:75–81. [DOI] [PubMed] [Google Scholar]
- 12. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431. [DOI] [PubMed] [Google Scholar]
- 13. Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. [DOI] [PubMed] [Google Scholar]
- 14. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. [DOI] [PubMed] [Google Scholar]
- 15. Webb SM, Mo D, Lamberts SW, et al. ; International HypoCCS Advisory Board. Metabolic, cardiovascular, and cerebrovascular outcomes in growth hormone-deficient subjects with previous cushing’s disease or non-functioning pituitary adenoma. J Clin Endocrinol Metab. 2010;95:630–638. [DOI] [PubMed] [Google Scholar]
- 16. Graham MR, Evans P, Davies B, Baker JS. Arterial pulse wave velocity, inflammatory markers, pathological GH and IGF states, cardiovascular and cerebrovascular disease. Vasc Health Risk Manag. 2008;4:1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sartorio A, Cattaneo M, Bucciarelli P, et al. Alterations of haemostatic and fibrinolytic markers in adult patients with growth hormone deficiency and with acromegaly. Exp Clin Endocrinol Diabetes. 2000;108:486–492. [DOI] [PubMed] [Google Scholar]
- 19. Goldbourt U, Tanne D. Body height is associated with decreased long-term stroke but not coronary heart disease mortality? Stroke. 2002;33:743–748. [DOI] [PubMed] [Google Scholar]
- 20. Parker DR, Lapane KL, Lasater TM, Carleton RA. Short stature and cardiovascular disease among men and women from two southeastern New England communities. Int J Epidemiol. 1998;27:970–975. [DOI] [PubMed] [Google Scholar]
- 21. Aberg ND, Olsson S, Aberg D, et al. Genetic variation at the IGF1 locus shows association with post-stroke outcome and to circulating IGF1. Eur J Endocrinol. 2013;169:759–765. [DOI] [PubMed] [Google Scholar]
- 22. Bondanelli M, Ambrosio MR, Onofri A, et al. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. J Clin Endocrinol Metab. 2006;91:3928–3934. [DOI] [PubMed] [Google Scholar]
- 23. Pathipati P, Surus A, Williams CE, Scheepens A. Delayed and chronic treatment with growth hormone after endothelin-induced stroke in the adult rat. Behav Brain Res. 2009;204:93–101. [DOI] [PubMed] [Google Scholar]
- 24. Zhu W, Fan Y, Hao Q, et al. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carter CS, Ramsey MM, Ingram RL, et al. Models of growth hormone and IGF-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57:B177–B188. [DOI] [PubMed] [Google Scholar]
- 26. Charlton HM, Clark RG, Robinson IC, et al. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58. [DOI] [PubMed] [Google Scholar]
- 27. Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. [DOI] [PubMed] [Google Scholar]
- 31. Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1993;13:865–871. [DOI] [PubMed] [Google Scholar]
- 32. Nogami H, Takeuchi T. Increased population of nonhormone-producing cells suggests the presence of dysfunctional growth hormone cells in the anterior pituitary gland of the spontaneous dwarf rat. Neuroendocrinology. 1993;57:374–380. [DOI] [PubMed] [Google Scholar]
- 33. Tierney T, Robinson IC. Increased lactotrophs despite decreased somatotrophs in the dwarf (dw/dw) rat: a defect in the regulation of lactotroph/somatotroph cell fate? J Endocrinol. 2002;175:435–446. [DOI] [PubMed] [Google Scholar]
- 34. Yan H, Mitschelen M, Bixler GV, et al. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence. J Endocrinol. 2011;211:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tucsek Z, Toth P, Sosnowska D, et al. Obesity in aging exacerbates blood–brain barrier disruption, neuroinflammation, and oxidative stress in the mouse Hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2013. November 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Csiszar A, Sosnowska D, Tucsek Z, et al. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungvari Z, Tucsek Z, Sosnowska D, et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol Biol Med Sci. 2011;66:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toth P, Csiszar A, Sosnowska D, et al. Treatment with the cytochrome P450 omega-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br J Pharmacol. 2013;168:1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toth P, Csiszar A, Tucsek Z, et al. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toth P, Tucsek Z, Sosnowska D, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London, UK: Academic Press; 2006. [Google Scholar]
- 50. Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. [DOI] [PubMed] [Google Scholar]
- 51. Paulson JR, Yang T, Selvaraj PK, et al. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther. 2010;332:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:1160–1167. [DOI] [PubMed] [Google Scholar]
- 53. Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience. 2011;185:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dimopoulou I, Kouyialis AT, Orfanos S, et al. Endocrine alterations in critically ill patients with stroke during the early recovery period. Neurocrit Care. 2005;3:224–229. [DOI] [PubMed] [Google Scholar]
- 55. Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. [DOI] [PubMed] [Google Scholar]
- 56. Denti L, Annoni V, Cattadori E, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. [DOI] [PubMed] [Google Scholar]
- 57. De Smedt A, Brouns R, Uyttenboogaart M, et al. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–2185. [DOI] [PubMed] [Google Scholar]
- 58. Bendel S, Koivisto T, Ryynanen OP, et al. Insulin like growth factor-I in acute subarachnoid hemorrhage: a prospective cohort study. Crit Care. 2010;14:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aberg D, Jood K, Blomstrand C, et al. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab. 2011;96:E1055–E1064. [DOI] [PubMed] [Google Scholar]
- 60. Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–462. [DOI] [PubMed] [Google Scholar]
- 61. Liu W, D’Ercole JA, Ye P. Blunting type 1 insulin-like growth factor receptor expression exacerbates neuronal apoptosis following hypoxic/ischemic injury. BMC Neurosci. 2011;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Endres M, Piriz J, Gertz K, et al. Serum insulin-like growth factor I and ischemic brain injury. Brain Res. 2007;1185:328–335. [DOI] [PubMed] [Google Scholar]
- 63. Fletcher L, Kohli S, Sprague SM, et al. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. [DOI] [PubMed] [Google Scholar]
- 64. Kooijman R, Sarre S, Michotte Y, De Keyser J. Insulin-like growth factor I: a potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke. 2009;40:e83–e88. [DOI] [PubMed] [Google Scholar]
- 65. Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Tang Y, Zhang W, et al. Insulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemia. FEBS J. 2013;280:3658–3668. [DOI] [PubMed] [Google Scholar]
- 67. Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70:384–396. [DOI] [PubMed] [Google Scholar]
- 68. Torres Aleman I. Insulin-like growth factor-1 and central neurodegenerative diseases. Endocrinol Metab Clin North Am. 2012;41:395–408, vii. [DOI] [PubMed] [Google Scholar]
- 69. Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48:1–9. [DOI] [PubMed] [Google Scholar]
- 70. Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology. 2012;79:2148–2153. [DOI] [PubMed] [Google Scholar]
- 71. Dong X, Chang G, Ji XF, Tao DB, Wang YX. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9:e94845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72. Gluckman P, Klempt N, Guan J, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. [DOI] [PubMed] [Google Scholar]
- 73. Guan J, Gunn AJ, Sirimanne ES, et al. The window of opportunity for neuronal rescue with insulin-like growth factor-1 after hypoxia-ischemia in rats is critically modulated by cerebral temperature during recovery. J Cereb Blood Flow Metab. 2000;20:513–519. [DOI] [PubMed] [Google Scholar]
- 74. Guan J, Williams C, Gunning M, Mallard C, Gluckman P. The effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult rats. J Cereb Blood Flow Metab. 1993;13:609–616. [DOI] [PubMed] [Google Scholar]
- 75. Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yamamura H, Hiraide A, Matsuoka T, Takaoka M, Shimazu T, Sugimoto H. Does growth hormone augment brain edema caused by brain injury? A study with a freeze brain injury model in the rat. J Trauma. 1999;46:292–296. [DOI] [PubMed] [Google Scholar]
- 77. Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tranque PA, Calle R, Naftolin F, Robbins R. Involvement of protein kinase-C in the mitogenic effect of insulin-like growth factor-I on rat astrocytes. Endocrinology. 1992;131:1948–1954. [DOI] [PubMed] [Google Scholar]
- 79. Ni W, Rajkumar K, Nagy JI, Murphy LJ. Impaired brain development and reduced astrocyte response to injury in transgenic mice expressing IGF binding protein-1. Brain Res. 1997;769:97–107. [DOI] [PubMed] [Google Scholar]
- 80. Miller DB, Bartke A, O’Callaghan JP. Increased glial fibrillary acidic protein (GFAP) levels in the brains of transgenic mice expressing the bovine growth hormone (bGH) gene. Exp Gerontol. 1995;30:383–400. [DOI] [PubMed] [Google Scholar]
- 81. Yeap BB, Chubb SA, McCaul KA, et al. Associations of IGF1 and its binding proteins with abdominal aortic aneurysm and aortic diameter in older men. Eur J Endocrinol. 2012;166:191–197. [DOI] [PubMed] [Google Scholar]