Abstract
Background: One of the objectives of the Millennium Development Goals is to improve child health. We describe the burden of pediatric surgical disease at a tertiary hospital in Malawi.
Methods: We conducted a retrospective analysis of a pediatric surgery database at Kamuzu Central Hospital in Malawi for the calendar year 2012. Variables included patient demographics, admission diagnosis, primary surgery and outcome.
Results: A total of 1170 pediatric patients aged 0–17 years were admitted to the surgical service during the study period. The mean age was 6.9 years, and 62% were male. Trauma was the most common indication for admission (51%, n = 596), and 67% (n = 779) of all patients were managed non-operatively. Neonates and patients managed non-operatively had a significantly increased risk of mortality.
Conclusion: Only a third of patients admitted to the pediatric surgery service underwent surgery. More than half of patients with congenital anomalies did not undergo surgical intervention. Importantly, patients who underwent surgery had a survival advantage.
Keywords: sub-Saharan Africa, low- and middle-income country, training, neonatal surgery
Introduction
The Millennium Development Goals are eight international development goals that were originally established in 2000 by the United Nations to encourage development by improving social and economic conditions in the world's poorest countries. The fourth Millennium Development Goal includes improving health and reducing pediatric mortality [1]. Although nutrition, sanitation and vaccination campaigns are being implemented aggressively, pediatric surgical disease is overlooked [2]. It has been demonstrated that the burden of morbidity and mortality averted by surgical intervention may equal the burden of disease averted by vaccination programs [3]; a greater emphasis on pediatric surgical care may have a significant impact on reducing childhood mortality. A recent survey of households in Sierra Leone demonstrated that 25% of respondents had a condition that required surgical management. Furthermore, 25% of household deaths within the past year may have been averted if surgical interventions were available [4].
Limited data show that injury, congenital abnormalities and infection are the most common surgical diseases in children in sub-Saharan Africa [5]. Injury is the leading cause of morbidity and mortality in African children from age 4 to 45 years [6]. In Uganda, 12% of households reported a disability related to injury. This burden of disease can be ameliorated by timely access to quality surgical care [7]. Among congenital anomalies, commonly encountered problems include inguinal hernias, genitourinary anomalies, anorectal malformations, myelomeningoceles and cleft lip and palate [8]. Common infections requiring surgical intervention include typhoid intestinal perforation, appendicitis, primary peritonitis, pyomyositis, necrotizing fasciitis and osteomyelitis [9]. In high-income countries, improved clinical and nutritional care of pediatric surgical patients has improved survival. In low- and middle-income countries, there is a need for clear health policies that supports and embraces surgical training and improvements in surgical infrastructure [10].
Epidemiologic data on the burden of pediatric surgical disease in sub-Saharan Africa are limited. In Malawi, only one pediatric surgeon provides care for >6 million children under the age of 15 years [11, 12]. In this study, we aim to describe the burden of pediatric surgical disease at a tertiary general hospital with a pediatric ward in Lilongwe, Malawi, using a hospital-based admission database. We describe demographics, admission diagnoses, procedures and outcomes.
Methods
We conducted a retrospective analysis of a pediatric surgery database at Kamuzu Central Hospital (KCH) in Lilongwe, Malawi. KCH is a 600-bed hospital and a referral center for the central region of the country, with a population of about 9 million persons. There are dedicated children’s wards, including a surgical ward, neonatal intensive care unit, pediatric intensive care unit and intermediate care ward. During the study period, there were four full-time (greater than 6-month commitment) general surgeons, three full-time orthopedic surgeons and multiple clinical officers and a 10-resident general surgery residency training program. Plain and contrast radiography, abdominal ultrasound and echocardiography were consistently available. Weights, heart rate, urine output and capillary refill were consistently recorded. Pulse oximetry was frequently available, whereas pediatric blood pressure measurements were infrequently recorded. There was no pediatric surgeon, whereas one pediatric critical care specialist was available during the study period and a pediatric oncologist was intermittently available. The majority of hospital-based deliveries in the region take place at a different facility. Data on whether patients were inborn or transferred were not available.
The pediatric surgery database included all admitted patients under the age of 18 years with a pediatric surgical diagnosis (defined as being admitted to the pediatric surgical ward or having the required pediatric surgical consultation). We analyzed data collected during the calendar year 2012 (pediatric surgery database, N = 1170). The institutional review board at the University of North Carolina and the National Health Science and Research Council of Malawi approved this study.
Data obtained included patient demographics (age, gender), admission diagnosis and vital signs, primary surgery (if performed) and outcome (discharge, death or abscond). Mortality was defined as in-hospital death. Data analysis was performed using STATA v11 software. Distribution for age, gender, diagnosis, surgical intervention and outcome is described. Chi-square and logistic regression analyses were used to determine the association between outcome and the variables of admission diagnosis, age, gender and surgical management.
Results
There were 1170 pediatric patients between the ages of 0–17 years admitted to the surgical service during the study period. This represented 18% of all surgical admissions. Males represented 62% of the patients (n = 705). Mean age was 6.9 years (SD ± 5.6), with a preponderance of children aged under 5 years (48%, n = 563). Median age was 6 years.
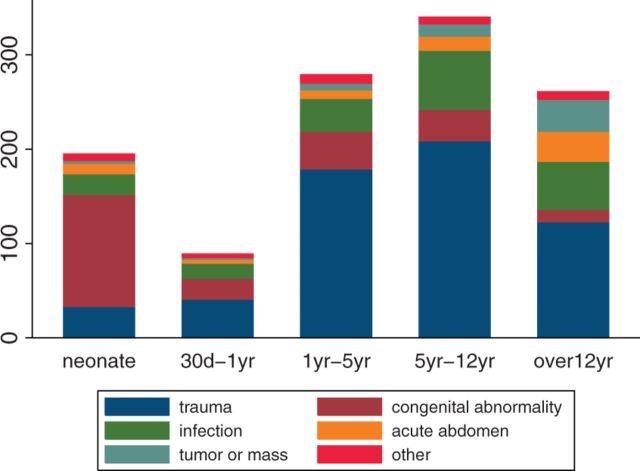
Traumatic injury was the most common indication for admission (Table 1), and in all age-groups with the exception of neonates, in whom congenital abnormalities were the most common indication for admission (Fig. 1).
Table 1.
Surgical management and outcome by admission diagnosis among pediatric surgical patients at KCH in 2012
| Diagnosis | n (%) | Operated n (%)a | Died during admission n (%)a |
|---|---|---|---|
| Trauma | 596 (51) | 143 (16) | 28 (5) |
| Burns | 195 (17) | 49 (28) | 24 (14) |
| Other | 401 (34) | 87 (25) | 4 (1) |
| Congenital | 204 (17) | 96 (43) | 35 (18) |
| Hernia/hydrocele | 74 (6) | 55 (77) | 0 (0) |
| Gastoschisis/omphalocele | 34 (3) | 10 (29) | 21 (62) |
| Spina bifida | 25 (2) | 2 (9) | 2 (8) |
| Imperforate anus | 16 (1) | 14 (88) | 5 (31) |
| Hypospadias/urethral anomaly | 12 (1) | 6 (50) | 0 (0) |
| Undescended testis | 10 (1) | 3 (30) | 0 (0) |
| Cleft lip/palate | 9 (1) | 8 (13) | 1 (11) |
| Hemangioma/ lymphangioma | 6 (1) | 3 (50) | 1 (17) |
| Hirschsprung’s disease | 4 (0) | 2 (50) | 1 (25) |
| Pyloric stenosis | 4 (0) | 1 (25) | 1 (25) |
| Congenital diaphragmatic hernia | 3 (0) | 0 (0) | 0 (0) |
| Tracheoesophageal fistula/esophageal atresia | 2 (0) | 0 (0) | 0 (0) |
| Intestinal atresia | 2 (0) | 1 (50) | 2 (100) |
| Congenital fistula | 1 (0) | 0 (0) | 0 (0) |
| Craniostenosis | 1 (0) | 0 (0) | 0 (0) |
| Orthopedic anomaly | 1 (0) | 0 (0) | 0 (0) |
| Infection | 198 (17) | 72 (40) | 6 (3) |
| Soft tissue | 122 (10) | 48 (41) | 1 (1) |
| Bone/joint | 50 (4) | 19 (34) | 1 (2) |
| Brain/cerebrospinal fluid | 20 (2) | 3 (15) | 4 (22) |
| Urinary system | 6 (1) | 4 (67) | 0 (0) |
| Abdominal | 71 (6) | 37 (59) | 2 (3) |
| Obstructive | 26 (2) | 17 (74) | 1 (4) |
| Non-obstructive | 45 (4) | 24 (67) | 1 (2) |
| Tumor/mass | 59 (5) | 31 (56) | 2 (4) |
| Benign | 53 (5) | 28 (56) | 1 (2) |
| Malignant | 6 (1) | 3 (60) | 1 (17) |
| Other | 42 (4) | 12 (38) | 7 (19) |
| Total | 1170 (100) | 392 (35) | 80 (7) |
aPercentages based on patients with recorded management and outcomes, not on total of all patients in the group.
Fig. 1.
Diagnosis by age-group among pediatric surgical patients at KCH in 2012.
The majority of patients were managed non-operatively, with only 35% (n = 392) of patients undergoing surgery (Table 2). Of the 392 patients who underwent operations, 87 (22%) were operated emergently. Thirty-four (23%) trauma cases, 23 (24%) congenital cases, 7 (10%) infectious cases and 23 (66%) acute abdominal cases required emergent surgeries. No tumor or unclassified case was emergent, and three cases were not classified as emergent or elective. There was no statistically significant difference in rates of surgical intervention by age (p = 0.08) or gender (p = 0.91). There was no significant difference in the rate of surgical intervention for obstructive and non-obstructive abdominal conditions [odds ratio (OR): 1.65, p = 0.32)]. Differences in rates of surgical intervention by admission diagnosis varied significantly (p < 0.001) (Table 3).
Table 2.
Odds of pediatric surgical patients at KCH undergoing surgical management in 2012a
| n operated (%) | n non-operated (%) | Adjusted odds ratio (95% CI)b | |
|---|---|---|---|
| Admission Diagnosis | |||
| Trauma | 143 (26) | 405 (74) | 1.00 |
| Congenital anomaly | 96 (43) | 129 (57) | 2.44 (1.71–3.50) |
| Acute abdomen | 37 (59) | 26 (41) | 3.97 (2.30–6.87) |
| Infection | 72 (40) | 107 (60) | 1.90 (1.33–2.72) |
| Mass or tumor | 31 (56) | 24 (44) | 3.41 (1.92–6.05) |
| Other | 12 (38) | 20 (62) | 1.76 (0.84–3.71) |
| Gender | |||
| Male | 238 (36) | 431 (64) | 1.00 |
| Female | 141 (35) | 238 (65) | 1.00 (0.77–1.29)c |
aNumbers may not correspond with the numbers given in the first table because operative vs. non-operative management was not recorded for all patients.
bAdjusted for age and gender unless otherwise specified.
cAdjusted for age.
Table 3.
Operations performed among pediatric surgical patients at KCH in 2012
| Procedure | n (%) |
|---|---|
| Skin and soft tissue | 96 (25) |
| Incision and drainage | 43 (11) |
| Excision of mass/tumor | 19 (5) |
| Biopsy | 14 (4) |
| Laceration repair | 11 (3) |
| Debridement | 9 (2) |
| Orthopedic | 68 (17) |
| Manipulation under anesthesia | 51 (13) |
| Open reduction and internal fixation | 5 (1) |
| External fixation | 4 (1) |
| Other | 8 (2) |
| Abdominal | 67 (17) |
| Colostomy | 15 (4) |
| Appendectomy | 14 (4) |
| Omphalocele repair | 7 (2) |
| Small bowel repair | 6 (2) |
| Other exploratory laparotomy | 6 (2) |
| Splenectomy | 4 (1) |
| Small bowel resection | 3 (1) |
| Primary peritonitis | 3 (1) |
| Large bowel resection | 2 (1) |
| Gastroschisis repair | 2 (1) |
| Adhesiolysis | 1 (0) |
| Colostomy reversal | 1 (0) |
| Gastrostomy | 1 (0) |
| Ileostomy | 1 (0) |
| Pyloromyotomy | 1 (0) |
| Burns | 51 (13) |
| Excision of burn | 29 (7) |
| Skin graft | 19 (5) |
| Fasciotomy | 2 (1) |
| Contracture release | 1 (0) |
| Hernia repair | 40 (10) |
| Inguinal | 32 (8) |
| Femoral | 5 (1) |
| Umbilical | 3 (1) |
| Urology | 30 (8) |
| Hydrocele repair | 11 (3) |
| Circumcision | 8 (2) |
| Orchidectomy/orchidopexy | 6 (2) |
| Cystoscopy | 4 (4) |
| Urethral dilation | 1 (0) |
| Endoscopy | 17 (4) |
| Foreign body removal | 11 (3) |
| Other | 6 (2) |
| Other | 22 (6) |
| Neurosurgery | 6 (2) |
| Ear, nose, throat | 3 (1) |
| Chest | 1 (0) |
| Other | 12 (3) |
| Total | 391 (100) |
Overall, 7% (n = 80) of patients died (Table 4). Females, neonates and patients who did not undergo surgical management were significantly more likely to die. Patients who underwent surgery had 2.94 adjusted odds of survival compared with those who did not undergo surgery [confidence interval (CI): 1.51–5.76, p < 0.001)]. Specifically among patients with congenital abnormalities, those who underwent surgery had 3.72 adjusted odds of survival compared with those who did not undergo surgery (CI: 1.27–10.87, p < 0.001). Patient selection for operative procedure was based on clinical finding and standard indication for operative management. Consultant surgeons’ comfort level with procedure and perceived increased chances for survival may also influence operative decision making. Patients thought to need more complex surgeries or with other comorbidities that place them at an increased risk of death were referred to the only other central hospital in the country with a pediatric surgeon or were sent home.
Table 4.
Bivariable associations between patient characteristics and mortality (n = 80) among pediatric surgical patients at KCH in 2012
| Characteristic | n | Deaths n (%)a | p-value |
|---|---|---|---|
| Age | |||
| 0–30 days | 195 | 50 (27) | <0.001 |
| 31 days–1 year | 89 | 3 (4) | |
| 1–5 years | 279 | 13 (5) | |
| 5–12 years | 341 | 9 (3) | |
| >12 years | 265 | 5 (2) | |
| Patient gender | |||
| Male | 705 | 31 (5) | 0.008 |
| Female | 427 | 35 (9) | |
| Admission diagnosis | |||
| Trauma | 596 | 28 (5) | <0.001 |
| Infection | 198 | 6 (3) | |
| Congenital abnormality | 204 | 35 (18) | |
| Acute abdomen | 71 | 2 (3) | |
| Tumor or mass | 59 | 2 (4) | |
| Other | 42 | 7 (19) | |
| Surgical management | |||
| No | 714 | 66 (10) | <0.001 |
| Yes | 392 | 14 (4) |
aPercentages based on patients with recorded outcomes, not on total of all patients in the group.
Discussion
Only a third of patients admitted to the pediatric surgery service underwent surgery. Among patients with congenital anomalies, which are unlikely to resolve without surgical intervention, less than half underwent surgery. Importantly, pediatric surgical patients who underwent surgery had significantly higher odds of survival.
There is a paucity of data describing the burden of pediatric surgical disease in sub-Saharan Africa; however, the pattern of admission diagnoses in our population is similar to that mentioned in other studies [4, 6, 13]. Trauma was the most common admission diagnosis overall and was the second most common admission diagnosis among neonates. Although we currently cannot describe the burden of accidental vs. non-accidental trauma in pediatric patients in our setting, this is an important area for future research.
The majority of patients with congenital anomalies did not undergo surgery. Importantly, one quarter of patients with congenital abnormalities who underwent surgery were operated under emergent circumstances. In our setting, less than 10% of patients with congenital anomalies who underwent surgery died, whereas 27% of those who did not undergo surgery died. One study in Nigeria found that among patients who underwent emergency surgery for management of congenital anomalies, postoperative mortality was 31%. This study did not describe mortality in patients who did not undergo surgery [14]. The differences in mortality likely reflect significant selection bias for healthier patients for surgery. In this resource-poor setting with no dedicated pediatric surgeon, the tendency is to operate on less complex patients, whereas more complex patients are managed conservatively or sent home. Lack of training in pediatric surgical procedures, lack of adequate pediatric anesthesia and postoperative critical care and lack of follow-up services generate reluctance to operate on this high-mortality population.
Surprisingly, similar proportions of patients with obstructive vs. non-obstructive abdominal processes underwent surgery. The decision to operate is based on clinical signs in the absence of great diagnostic adjuncts. Surgery is sometimes seen as both a diagnostic and a therapeutic maneuver, particularly in resource-poor settings. Furthermore, patients with mild non-obstructive abdominal symptoms may have been managed without surgical consultation, and therefore the non-obstructive abdominal cases referred for surgical consultation could have been more severe.
Among congenital abnormalities, other sub-Saharan African studies have reported a preponderance of anorectal malformations [15, 16]. Our study cohort shows that hernias, hydrocele and abdominal wall defects are more common. The differences in abnormalities likely reflect a difference in referral patterns rather than a difference in the prevalence of congenital anomalies in our setting, as patients with complex abnormalities may be referred to Queen Elizabeth Central Hospital in Blantyre where a pediatric surgeon is available.
There was only one pediatric surgeon serving all of Malawi at the time of this study, or 0.06 pediatric surgeons per million children [17] and no pediatric surgeon at the facility. Even in Nigeria [18] and South Africa [18], which are comparatively more developed sub-Saharan African countries [19], there were only 0.45 and 0.52 pediatric surgeons per million children, respectively. In the USA, there are 10.6 general pediatric surgeons and 24.3 pediatric specialty surgeons [20] per million children [21]. A survey of training opportunities for pediatric surgery in eight sub-Saharan African countries showed that programs varied from 2 to 4 years of general surgery followed by 0–4 years of pediatric surgery training [7]. Although out-migration of medical professionals from sub-Saharan Africa to high-income countries is well described [22], more research is needed related to pediatric surgeons in particular.
This study is limited by only capturing patients on a single admission and lacks follow-up data. Our data do not distinguish patients who are healthy at discharge from patients who are discharged on palliative care with a poor prognosis. Palliative discharge frequently occurs in our setting when patients have severe congenital anomalies or cancer, for example, which may lead to underestimation of mortality. Our data overestimate survival as suggested by our survival results in patients with esophageal atresias. As described in previous studies of pediatric surgical patients in sub-Saharan Africa, postoperative follow-up was limited by challenges in transport to KCH and health literacy of our population [17]. Although previous studies show increased rates of postoperative infections in pediatric surgical patients in sub-Saharan Africa [23, 24], our data do not consistently capture postoperative complications, and we cannot compare these findings with our population. Additionally, although our data show a lack of chest procedures, this does not necessarily indicate a lack of chest pathology, but rather the limitations of surgical infrastructure and a lack of pediatric ventilators in our setting. Further, although it is significant that patients who underwent surgery had lower inpatient mortality, this likely reflects a selection bias for surgically desirable patients.
In conclusion, although surgery is associated with better outcomes in this population, nearly two-thirds of patients are managed non-operatively. Although non-operative management of pediatric trauma is appropriate, congenital anomalies warranting inpatient management are unlikely to resolve without surgical intervention. Improved outcome from pediatric surgical disease requires prompt identification and referral, but in our case, more than half of patients with congenital anomalies who were referred to this tertiary surgical facility did not undergo surgical intervention. Improved pediatric surgical care delivery, particularly for those with congenital anomalies, requires prompt recognition and referral. Given the pediatric surgical manpower shortage in Malawi, the training of general surgeons in quality pediatric surgical care is imperative. Furthermore, the concept of task shifting to address the health care workforce in sub-Saharan Africa by training non-physician clinicians, as has been duplicated successfully in other specialties such as anesthesiology, orthopedic surgery and obstetrics, should be actively pursued. Within the limitations of our pediatric surgery practice environment, selection of pediatric patients for surgical intervention when indicated is associated with a survival benefit.
Funding
C.K. is supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Heart, Blood and Lung Institute and the NIH Office of Research for Women’s Health through the Fogarty Global Health Fellows Program Consortium comprising the University of North Carolina, John Hopkins, Morehouse and Tulane (1R25TW009340-01) and the American Recovery and Reinvestment Act. This work was also supported by the University of North Carolina Center for AIDS Research, an NIH-funded program (P30 AI50410).
References
- 1. Statistics Division, Department of Economic and Social Affairs, United Nations. Millennium Development Goals: 2012 Progress Chart. United Nations, 2012.
- 2.van den Ent MM, Brown DW, Hoekstra EJ, et al. Measles mortality reduction contributes substantially to reduction of all cause mortality among children less than five years of age, 1990–2008. J Infect Dis. 2011;204:S18–23. doi: 10.1093/infdis/jir081. [DOI] [PubMed] [Google Scholar]
- 3.Ozgediz D, Jamison D, Cherian M, et al. The burden of surgical conditions and access to surgical care in low- and middle-income countries. Bull World Health Organ. 2008;86:646–7. doi: 10.2471/BLT.07.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groen RS, Samai M, Stewart KA, et al. Untreated surgical conditions in Sierra Leone: a cluster randomised, cross-sectional, countrywide survey. Lancet. 2012;380:1082–7. doi: 10.1016/S0140-6736(12)61081-2. [DOI] [PubMed] [Google Scholar]
- 5.Bowley DM, Rogers TN, Meyers T, et al. Surgeons are failing to recognize children with HIV infection. J Pediatr Surg. 2007;42:431–4. doi: 10.1016/j.jpedsurg.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Bickler SW, Rode H. Surgical services for children in developing countries. Bull World Health Organ. 2002;80:829–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Kobusingye O, Guwatudde D, Lett R. Injury patterns in rural and urban Uganda. Inj Prev. 2001;7:46–50. doi: 10.1136/ip.7.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirdan LB, Ameh EA, Abantanga FA, et al. Challenges of training and delivery of pediatric surgical services in Africa. J Pediatr Surg. 2010;45:610–8. doi: 10.1016/j.jpedsurg.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Ameh EA, Abantanga FA, Birabwa-Male D. Surgical aspects of bacterial infection in African children. Semin Pediatr Surg. 2012;21:116–24. doi: 10.1053/j.sempedsurg.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Chirdan LB, Ngiloi PJ, Elhalaby EA. Neonatal surgery in Africa. Semin Pediatr Surg. 2012;21:151–9. doi: 10.1053/j.sempedsurg.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 11. Population Reference Bureau, Malawi Department of Population, Malawi Ministry of Finance and Development Planning. Malawi Population Data Sheet 2012. Population Reference Bureau; 2012.
- 12. Population under age 15 [Internet]. Henry J. Kaiser Family Foundation, Menlo Park, CA, 2012 [cited March 29, 2013]. http://www.globalhealthfacts.org/data/topic/map.aspx?ind=82#table.
- 13.Kiser MM, Samuel JC, Mclean SE, et al. Epidemiology of pediatric injury in Malawi: burden of disease and implications for prevention. Int J Surg. 2012;10:611–7. doi: 10.1016/j.ijsu.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Ameh EA, Dogo PM, Nmadu PT. Emergency neonatal surgery in a developing country. Pediatr Surg Int. 2001;17:448–51. doi: 10.1007/s003830000551. [DOI] [PubMed] [Google Scholar]
- 15.Moore SW, Sidler D, Hadley GP. Anorectal malformations in Africa. S Afr J Surg. 2005;43:174–5. [PubMed] [Google Scholar]
- 16.Calisti A, Belay K, Mazzoni G, et al. Promoting major pediatric surgical care in a low-income country: a 4-year experience in Eritrea. World J Surg. 2011;35:760–6. doi: 10.1007/s00268-011-0992-z. [DOI] [PubMed] [Google Scholar]
- 17.Elhalaby EA, Millar AJ. Challenges of pediatric surgical practice in Africa. Semin Pediatr Surg. 2012;21:101–2. doi: 10.1053/j.sempedsurg.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Ameh EA, Adejuyigbe O, Nmadu PT. Pediatric surgery in Nigeria. J Pediatr Surg. 2006;41:542–6. doi: 10.1016/j.jpedsurg.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Human Development Report 2013 Team. The rise of the south: human progress in a diverse world. United Nations Development Programme (UNDP), 2013. [Google Scholar]
- 20.American College of Surgeons Health Policy Research Institute. The Surgical Workforce in the United States: Profile and Recent Trends [cited February 2, 2013]. http://www.acshpri.org/documents/ACSHPRI_Surgical_Workforce_in_US_apr2010.pdf. [Google Scholar]
- 21. Child population: Number of children (in millions) ages 0–17 in the united states by age, 1950–2011 and projected 2012–2050 [Internet]. Forum on Child and Family Statistics. Washington, DC: ChildStats.gov, 2012 [cited March 29, 2013]. http://www.childstats.gov/americaschildren/tables/pop1.asp.
- 22.Hagopian A, Thompson MJ, Fordyce M, et al. The migration of physicians from sub-Saharan Africa to the United States of America: measures of the African brain drain. Hum Resour Health. 2004;2:17. doi: 10.1186/1478-4491-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameh EA, Mshelbwala PM, Nasir AA, et al. Surgical site infection in children: prospective analysis of the burden and risk factors in a sub-Saharan African setting. Surg Infect (Larchmt) 2009;10:105–9. doi: 10.1089/sur.2007.082. [DOI] [PubMed] [Google Scholar]
- 24.Wood JH, Nthumba PM, Stepita-Poenaru E, et al. Pediatric surgical site infection in the developing world: a Kenyan experience. Pediatr Surg Int. 2012;28:523–7. doi: 10.1007/s00383-012-3058-x. [DOI] [PubMed] [Google Scholar]