Abstract
Background
African American (AA) women have a disproportionately high incidence of estrogen receptor–negative (ER-) breast cancer, a subtype with a largely unexplained etiology. Because childbearing patterns also differ by race/ethnicity, with higher parity and a lower prevalence of lactation in AA women, we investigated the relation of parity and lactation to risk of specific breast cancer subtypes.
Methods
Questionnaire data from two cohort and two case-control studies of breast cancer in AA women were combined and harmonized. Case patients were classified as ER+ (n = 2446), ER- (n = 1252), or triple negative (ER-, PR-, HER2-; n = 567) based on pathology data; there were 14180 control patients. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated in polytomous logistic regression analysis with adjustment for study, age, reproductive and other risk factors.
Results
ORs for parity relative to nulliparity was 0.92 (95% CI = 0.81 to 1.03) for ER+, 1.33 (95% CI = 1.11 to 1.59) for ER-, and 1.37 (95% CI = 1.06 to 1.70) for triple-negative breast cancer. Lactation was associated with a reduced risk of ER- (OR = 0.81, 95% CI = 0.69 to 0.95) but not ER+ cancer. ER- cancer risk increased with each additional birth in women who had not breastfed, with an OR of 1.68 (95% CI = 1.15 to 2.44) for 4 or more births relative to one birth with lactation.
Conclusions
The findings suggest that parous women who have not breastfed are at increased risk of ER- and triple-negative breast cancer. Promotion of lactation may be an effective tool for reducing occurrence of the subtypes that contribute disproportionately to breast cancer mortality.
Breast tumors characterized by a lack of estrogen receptors (ERs) are associated with an aggressive pathology and poor prognosis (1). They occur more frequently in African American (AA) than other US women at every age (1,2). The causal etiology of ER- breast cancer is largely unexplained. It has long been thought that parous women have a reduced risk of breast cancer (3), but recent research indicates that this applies primarily to ER+ breast cancer (4,5). In fact, preliminary evidence suggests that parous women may actually have an increased risk of ER- breast cancer, and that the increase may be ameliorated by breastfeeding (6–12). If so, the higher parity (13,14) and lower prevalence of lactation (15,16) in AA women relative to white women could be a contributor to the racial disparity in ER- cancer. Lactation uptake has improved in the United States in the past 30 years, but increases in AA women have been the smallest; in the most recent prevalence report from the National Immunization Survey (2008 data), the percentage of infants who had ever breastfed had increased to 58.9% among black infants as compared with 75.2% among white infants (17). In a collaborative project that pools data and samples from four large ongoing studies, we investigated the relation of parity and lactation to specific subtypes of breast cancer in AA women.
Methods
Study Population
The present analysis is based on data from the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium, a collaboration of four epidemiologic studies with large numbers of AA participants. The individual studies—Black Women’s Health Study (BWHS) (18), Multiethnic Cohort Study (MEC) (19), Carolina Breast Cancer Study (CBCS) (20), and Women’s Circle of Health Study (WCHS) (21,22)—and the AMBER consortium (23) have been described in detail previously. Institutional Review Board approval was granted for each individual study and for the AMBER consortium, and informed consent was provided by all study participants.
Briefly, the BWHS is a prospective cohort study of 59000 African American women who were enrolled by mail questionnaire in 1995 and have been followed by biennial questionnaire since then. Median age at baseline was 38 years, range 21 to 69 years. Breast cancer cases are identified through self-report and are confirmed by medical records or through linkage with state cancer registries and the National Death Index. The MEC is also a prospective cohort study, begun in 1993 and including participants aged 45 to 69 years from Hawaii and Southern California, with AAs primarily from California. Data collection is by mail questionnaire, with follow-up questionnaires mailed at five-year intervals, and linkage with the California and Hawaii state cancer registries and the National Death Index. For both BWHS and MEC, AMBER includes all incident breast cancer case patients and four control patients per case selected from the cohort, frequency matched to cases on five-year age category and questionnaire completed most recently (prior to case diagnosis).
The CBCS, a population-based case-control study, was conducted in North Carolina from 1993 through 2001 and included women aged 20 to 74 years. Cases were identified through the North Carolina Central Cancer Registry and control patients from Division of Motor Vehicle lists (age <65 years) and Health Care Financing Administration lists (age ≥ 65 years). Data were collected by in-person interview. The WCHS is an ongoing case-control study that was initiated in 2002 in New York City hospitals and later expanded to several counties in New Jersey. Breast cancer case patients, aged 20 to 75 years, are identified through the New Jersey Cancer Registry and control patients through random digit dialing of residential telephone and cell phone numbers and through churches and community organizations, with data collection by in-person interview.
Eligible case patients for the present analysis were 5087 women with a first diagnosis of invasive breast cancer or ductal carcinoma in situ. Pathology data from hospital records or cancer registry records were used to classify cancers by subtype based on ER, progesterone receptor (PR), and human epidermal receptor 2 (HER2). Case patients were classified as ER+ or PR+ if expression of the marker was greater than zero. Case patients were classified as triple negative (ER-, PR-, HER2-) if HER2 results were reported as zero or 1+, or were 2+ by immunohistochemistry but negative by fluorescence in situ hybridization. Because HER2 testing was uncommon before 2005, HER2 data were missing for over 50% of case patients. Case patients with missing data on ER status (n = 1371) were excluded, as were case patients with missing data on parity (n = 18), leaving a total of 3698 case patients for analysis. Among those case patients, 63% had HER2 data.
Statistical Analysis
Each study had collected data on age at first birth and number of births, and all except MEC had data on year of last birth and breastfeeding (ever or never) for most participants. Polytomous logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the relation of parity and lactation to risk of ER+ and ER- breast cancer. Separate analyses were carried out for triple-negative breast cancer using conventional logistic regression. Reduced models controlled for age in five-year categories, study, geographic region, and period (1993–1998, 1999–2005, and 2006–2013). Multivariable models controlled for those factors plus family history of breast cancer, age at menarche, oral contraceptive use, body mass index, years of education, alcohol consumption, and cigarette smoking. Analyses restricted to parous women additionally controlled for age at first birth, years since last birth, and number of births. A meta-analysis of study-specific results was performed under a random-effects model. All statistical tests were two-sided, with P less than .05 used as the cutpoint for statistical significance. To test for trend across number of births, an ordinal variable was treated as a continuous variable in the regression models. Interaction on the multiplicative scale was tested by the likelihood ratio test, comparing models with and without multiplicative interaction terms. Analyses were performed using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC).
To assess whether pregnancy-associated breast cancers (24) (PABC)—breast cancer within 10 years of a birth—differ by subtype, we compared the relative proportions of ER+ and ER- tumors among PABC and non-PABC within strata of age at diagnosis: younger than age 40 years, 40–44, and 45 years and older. Stratifying by age at diagnosis is critical because the relative proportion of ER- tumors decreases with age, and time since last birth is also related to age.
Results
Table 1 gives characteristics of the study participants. There were 14180 control patients, 2446 ER+ breast cancer case patients, and 1252 ER- breast cancer case patients; of the ER- case patients, 567 could be classified as triple negative. The CBCS had the highest proportion of ER- case patients and the MEC the lowest, largely reflecting the age distributions of the various studies. Distributions of parity and age at first birth differed by study, with higher parity and earlier age at first birth in the CBCS and MEC, studies that disproportionately included earlier birth cohorts and women of low socioeconomic status. Overall, 43% of parous control patients had breastfed at least one baby, with similar proportions across studies.
Table 1.
Characteristics of breast cancer case patients and control patients by study
| Characteristics | BWHS* N (%) | CBCS* N (%) | MEC* N (%) | WCHS* N (%) | Total N (%) |
|---|---|---|---|---|---|
| Breast cancer case patients | |||||
| Total | 1290 | 804 | 826 | 778 | 3698 |
| ER+ | 846 (65.6) | 436 (54.2) | 611 (74.0) | 553 (71.1) | 2446 (66.1) |
| ER- | 444 (34.4) | 368 (45.8) | 215 (26.0) | 225 (28.9) | 1252 (33.9) |
| ER-, PR-, HER2- | 153 | 210 | 74 | 130 | 567 |
| Age at diagnosis, y | |||||
| <40 | 97 (7.5) | 129 (16.0) | 0 (0.0) | 97 (12.5) | 323 (8.7) |
| 40–49 | 380 (29.5) | 262 (32.6) | 10 (1.2) | 209 (26.9) | 861 (23.3) |
| 50–59 | 447 (34.7) | 190 (23.9) | 124 (15.0) | 278 (35.7) | 1039 (28.1) |
| ≥60 | 366 (28.4) | 223 (27.7) | 692 (83.8) | 194 (24.9) | 1475 (39.9) |
| Childbearing factors among control patients | |||||
| Number of births | |||||
| 0 | 1907 (23.0) | 86 (10.9) | 560 (13.6) | 156 (16.1) | 2709 (19.1) |
| 1 | 1888 (22.8) | 136 (17.3) | 701 (17.0) | 223 (22.9) | 2948 (20.8) |
| 2 | 2249 (27.1) | 196 (24.9) | 825 (20.0) | 271 (27.9) | 3541 (25.0) |
| 3 | 1199 (14.5) | 139 (17.6) | 636 (15.4) | 166 (17.1) | 2140 (15.1) |
| ≥4 | 1044 (12.6) | 231 (29.3) | 1411 (34.1) | 156 (16.1) | 2842 (20.0) |
| Lactation, among parous women | |||||
| Never | 3576 (57.1) | 424 (60.4) | —† | 422 (51.7) | 4422 (56.8) |
| Ever | 2692 (42.9) | 278 (39.6) | —† | 394 (48.3) | 3364 (43.2) |
| Age at first birth, y | |||||
| <20 | 1989 (31.5) | 348 (49.8) | 1894 (55.4) | 314 (38.6) | 4545 (40.4) |
| 20–24 | 2203 (34.9) | 216 (30.9) | 997 (29.2) | 246 (30.2) | 3662 (32.6) |
| 25–29 | 1246 (19.7) | 82 (11.7) | 341 (10.0) | 120 (14.7) | 1789 (15.9) |
| ≥30 | 871 (13.8) | 53 (7.6) | 184 (5.4) | 134 (16.5) | 1242 (11.1) |
* BWHS = Black Women’s Health Study; CBCS = Carolina Breast Cancer Study; MEC = Multiethnic Cohort; WCHS = Women’s Circle of Health Study.
† Lactation data not available for most MEC participants.
The multivariable OR for ever-parous vs nulliparous in relation to ER+ breast cancer was 0.92 (95% CI = 0.81 to 1.03), and there was not a statistically significant trend with increasing number of births (Table 2). Lactation was not associated with a reduced risk. By contrast, parity was associated with an increased risk of ER- breast cancer (multivariable OR = 1.33, 95% CI = 1.11 to 1.59), and risk increased with increasing number of births (P trend = .04). Among parous women, ever lactation was associated with a reduced risk of ER- breast cancer (OR = 0.81, 95% CI = 0.69 to 0.95). For triple-negative breast cancer, ORs were 1.37 (95% CI = 1.06 to 1.79) for ever vs never parous and 0.81 (0.65 to 1.02) for ever vs never lactation. A meta-analysis of study-specific results yielded ORs very similar to results from pooling of individual data: ORs for parous vs nulliparous were 0.86 (95% CI = 0.71 to 1.06) for ER+ cancer and 1.27 (95% CI = 1.06 to 1.53) for ER- cancer, and ORs for ever vs never lactation were 1.01 (95% CI = 0.79 to 1.31) and 0.79 (0.64 to 0.98) for ER+ and ER- cancer, respectively.
Table 2.
Parity and lactation in relation to ER+, ER-, and triple-negative breast cancer
| ER+ | ER- | ER-, PR-, HER2- | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reproductive factors | Control patients N | Case patients N | OR (95% CI)* | OR (95% CI)† | Case patients N | OR (95% CI)* | OR (95% CI)† | Case patients N | OR (95% CI)* | OR (95% CI)† |
| Nulliparous | 2709 | 443 | 1.00 (referent) | 1.00 (referent) | 169 | 1.00 (referent) | 1.00 (referent) | 73 | 1.00 (referent) | 1.00 (referent) |
| Parous | 11471 | 2003 | 0.89 (0.79 to 1.00) | 0.92 (0.81 to 1.03) | 1083 | 1.35 (1.13 to 1.60) | 1.33 (1.11 to 1.59) | 494 | 1.39 (1.08 to 1.81) | 1.37 (1.06 to 1.79) |
| Among parous women only | ||||||||||
| Number of births | ||||||||||
| 1 | 2948 | 508 | 1.00 (referent) | 1.00 (referent) | 238 | 1.00 (referent) | 1.00 (referent) | 98 | 1.00 (referent) | 1.00 (referent) |
| 2 | 3541 | 604 | 0.94 (0.83 to 1.08) | 0.98 (0.85 to 1.12) | 342 | 1.16 (0.97 to 1.39) | 1.17 (0.97 to 1.40) | 167 | 1.37 (1.05 to 1.78) | 1.35 (1.03 to 1.77) |
| 3 | 2140 | 397 | 0.97 (0.84 to 1.13) | 1.05 (0.90 to 1.22) | 224 | 1.21 (0.99 to 1.48) | 1.24 (1.01 to 1.53) | 102 | 1.32 (0.98 to 1.76) | 1.27 (0.93 to 1.73) |
| ≥4 | 2842 | 494 | 0.81 (0.70 to 0.93) | 0.91 (0.78 to 1.07) | 279 | 1.16 (0.95 to 1.41) | 1.26 (1.01 to 1.56) | 127 | 1.25 (0.94 to 1.67) | 1.27 (0.93 to 1.75) |
| P trend = .01 | P trend = .40 | P trend = .13 | P trend = .04 | P trend = .22 | P trend = .25 | |||||
| Lactation‡ | ||||||||||
| Never | 4422 | 822 | 1.00 (referent) | 1.00 (referent) | 556 | 1.00 (referent) | 1.00 (referent) | 269 | 1.00 (referent) | 1.00 (referent) |
| Ever | 3364 | 644 | 1.04 (0.92 to 1.17) | 1.04 (0.91 to 1.18) | 329 | 0.83 (0.71 to 0.97) | 0.81 (0.69 to 0.95) | 160 | 0.83 (0.67 to 1.02) | 0.81 (0.65 to 1.02) |
| Lactation | ||||||||||
| Ever | ||||||||||
| Number of births | ||||||||||
| 1 | 792 | 142 | 1.00 (referent) | 1.00 (referent) | 64 | 1.00 (referent) | 1.00 (referent) | 31 | 1.00 (referent) | 1.00 (referent) |
| 2 | 1161 | 213 | 1.00 (0.78 to 1.27) | 1.03 (0.81 to 1.32) | 106 | 1.16 (0.83 to 1.62) | 1.18 (0.84 to 1.65) | 52 | 1.17 (0.73 to 1.86) | 1.13 (0.70 to 1.81) |
| 3 | 679 | 128 | 0.90 (0.69 to 1.19) | 0.99 (0.75 to 1.32) | 60 | 1.03 (0.70 to 1.50) | 1.08 (0.73 to 1.59) | 31 | 1.07 (0.63 to 1.81) | 1.00 (0.58 to 1.71) |
| ≥4 | 732 | 161 | 0.75 (0.57 to 0.99) | 0.85 (0.64 to 1.14) | 99 | 1.22 (0.86 to 1.74) | 1.33 (0.91 to 1.95) | 46 | 1.08 (0.65 to 1.77) | 1.01 (0.59 to 1.73) |
| Never | ||||||||||
| Number of births | ||||||||||
| 1 | 1414 | 258 | 0.98 (0.77 to 1.23) | 1.02 (0.80 to 1.30) | 143 | 1.19 (0.87 to 1.64) | 1.22 (0.88 to 1.69) | 61 | 1.06 (0.67 to 1.67) | 1.03 (0.64 to 1.63) |
| 2 | 1527 | 274 | 0.87 (0.69 to 1.10) | 0.94 (0.74 to 1.20) | 186 | 1.34 (0.99 to 1.83) | 1.38 (1.00 to 1.91) | 98 | 1.46 (0.95 to 2.25) | 1.39 (0.89 to 2.18) |
| 3 | 808 | 149 | 0.82 (0.63 to 1.07) | 0.91 (0.69 to 1.21) | 116 | 1.41 (1.00 to 1.97) | 1.46 (1.02 to 2.09) | 51 | 1.21 (0.75 to 1.95) | 1.12 (0.67 to 1.86) |
| ≥4 | 673 | 141 | 0.78 (0.59 to 1.03) | 0.88 (0.65 to 1.18) | 111 | 1.55 (1.10 to 2.19) | 1.68 (1.15 to 2.44) | 59 | 1.61 (1.00 to 2.58) | 1.51 (0.90 to 2.52) |
* Adjusted for age, study, time period and geographic region.
† Adjusted for age, study, time period, geographic region, education, age at menarche, alcohol consumption, smoking, body mass index, first degree family history of breast cancer, and oral contraceptive use. Analyses restricted to parous women additionally adjusted for age at first birth, years since last birth, and number of births.
‡ Multiethnic Cohort (MEC) case patients and control patients excluded from lactation analyses because of lack of data on lactation.
The joint effects of parity and lactation were assessed in analyses restricted to parous women, with a reference category of women who had only one birth and had breastfed (Table 2 and Figure 1). In the fully adjusted model, ORs for ER+ cancer were below 1.0 but not statistically significant for the highest parity category, regardless of lactation status. Risk of ER- breast cancer increased markedly by increasing parity among women who had never breastfed, with ORs ranging from 1.22 (95% CI = 0.88 to 1.69) for one birth to 1.68 (95% CI = 1.15 to 2.44) for four or more births. The ORs for similar comparisons among women who had breastfed were considerably lower, below 1.2 in all categories except four or more births (1.33, 95% CI = 0.91 to 1.95). For triple-negative breast cancer, there was no evidence of an association among women who had breastfed, whereas the OR for four or more births among women who had not breastfed was 1.51 (95% CI = 0.90 to 2.52).
Figure 1.
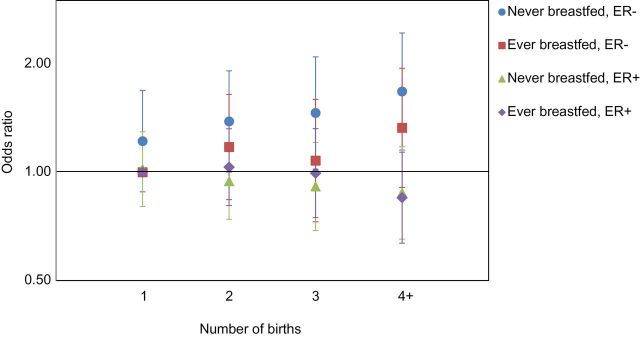
Odds ratios for number of births in relation to ER- and ER+ breast cancer, according to lactation history. Reference is women who had only one birth and had breastfed.
As shown in Table 3, results were similar across age strata (all P interaction values > .05). For ER+ breast cancer, ORs for parity were below or at 1.0 in each age group, and there was no evidence of an association with lactation in any age group. For ER- breast cancer, ORs for parity were above 1.0 in every age group, with the highest OR observed for patients younger than age 40 years; ORs for lactation relative to never lactation were below 1.0 in every age group.
Table 3.
Parity and lactation in relation to ER+ and ER- breast cancer, stratified by age*
| Reproductive factors | <40 years | 40–49 years | 50–59 years | 60+ years | P interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Case patient/ control patient | OR (95% CI) | Case patient/ control patient | OR (95% CI) | Case patient/ control patient | OR (95% CI) | Case patient/ control patient | OR (95% CI) | ||
| Parity | |||||||||
| ER+ | |||||||||
| Nulliparous | 45/451 | 1.00 (referent) | 119/754 | 1.00 (referent) | 124/685 | 1.00 (referent) | 156/817 | 1.00 (referent) | .26 |
| Parous | 121/695 | 1.00 (0.64 to 1.55) | 401/2393 | 0.88 (0.69 to 1.12) | 536/3203 | 0.81 (0.64 to 1.02) | 944/5178 | 0.99 (0.82 to 1.21) | |
| ER- | |||||||||
| Nulliparous | 33/451 | 1.00 (referent) | 54/754 | 1.00 (referent) | 41/685 | 1.00 (referent) | 42/817 | 1.00 (referent) | .68 |
| Parous | 124/695 | 1.60 (1.00 to 2.57) | 287/2393 | 1.15 (0.83 to 1.60) | 339/3203 | 1.43 (1.01 to 2.04) | 333/5178 | 1.25 (0.89 to 1.75) | |
| Lactation | |||||||||
| ER+ | |||||||||
| Never breastfed | 64/328 | 1.00 (referent) | 213/1,243 | 1.00 (referent) | 274/1532 | 1.00 (referent) | 271/1319 | 1.00 (referent) | .57 |
| Ever breastfed | 56/356 | 0.99 (0.60 to 1.63) | 181/1060 | 1.08 (0.84 to 1.39) | 192/1041 | 1.03 (0.81 to 1.29) | 215/907 | 0.98 (0.79 to 1.23) | |
| ER- | |||||||||
| Never breastfed | 76/328 | 1.00 (referent) | 195/1243 | 1.00 (referent) | 180/1532 | 1.00 (referent) | 105/1319 | 1.00 (referent) | .34 |
| Ever breastfed | 48/356 | 0.86 (0.52 to 1.41) | 86/1060 | 0.62 (0.46 to 0.85) | 117/1041 | 0.99 (0.75 to 1.31) | 78/907 | 0.80 (0.57 to 1.12) | |
* Adjusted for age, study, time period, geographic region, education, age at menarche, alcohol consumption, smoking, body mass index, first-degree family history of breast cancer and oral contraceptive use. Lactation analyses additionally adjusted for age at first birth, years since last birth, and number of births.
Multiethnic Cohort (MEC) case patients and control patients excluded from lactation analyses because of lack of data on lactation.
Figure 2 displays ORs for parity relative to nulliparity according to years since completion of childbearing. For ER+ breast cancer, all ORs were below 1.0, and the OR for 25 or more years since last birth was 0.82 (95% CI = 0.69 to 0.97). In contrast, for ER- cancer, all ORs were greater than 1.0, and the OR for 25 or more years since last birth was 1.27 (95% CI = 0.99 to 1.64).
Figure 2.
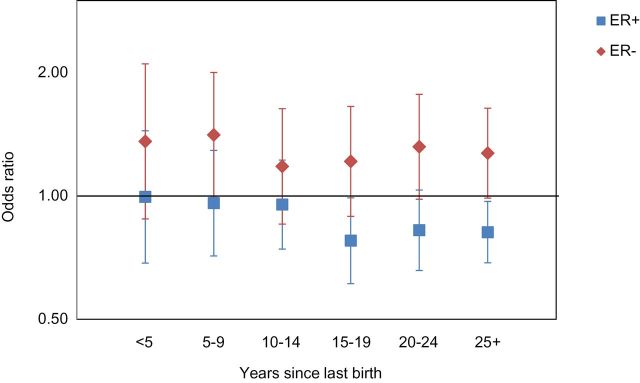
Odds ratios for parity in relation to breast cancer according to years since last birth. Reference is nulliparous women.
PABC and non-PABC were similar with respect to the proportion of ER- tumors in each age group: 52% among PABC and 49% among non-PABC before age 40 years; 43% and 44% at ages 40 to 44 years; and 34% and 35% at ages 45 years and older.
In analyses stratified on age at first birth (data not shown), parity was associated with reduced risk of ER+ cancer for women with first birth before age 25 years (OR = 0.83, 95% CI = 0.73 to 0.94) but not for women with a later first birth. For ER- cancer, parity was associated with increased risk both among women younger than age 25 years at first birth (OR = 1.33, 95% CI = 1.10 to 1.60) and aged 25 or older years at first birth (OR = 1.33, 95% CI = 1.07 to 1.65).
Results were similar when analyses were restricted to invasive breast cancers (87% of total). Results for tumors classified as ER+/PR+ were similar to results for all ER+ tumors and results for ER-/PR- tumors were similar to results for all ER- tumors (data not shown).
Discussion
In this collaborative study of breast cancer in AA women, parous women were estimated to have a 33% higher risk of ER- breast cancer and a 37% higher risk of triple-negative breast cancer, relative to nulliparous women. Breastfeeding modified the association with parity: At each level of parity, women who had breastfed had a lower risk of ER- and triple-negative breast cancer than did women who had never breastfed. The observed associations were not confined to early-onset breast cancer or to breast cancer that occurred relatively soon after a pregnancy.
Most previous studies have been underpowered to assess risk factors for ER- or triple-negative breast cancer. About half provide evidence for an association of parity with increased risk of ER- breast cancer (6–12,25–27), while the others indicate no association (28–38). Evidence regarding breastfeeding is more consistent, with most but not all studies indicating an inverse association of lactation with risk of ER- breast cancer (6,8,9,11,27–31,34,36,37,39). A notable exception is the recent case-case analysis of breast cancer in women of Mexican descent, which compared 159 triple-negative with 571 luminal A breast cancers (26). While parity was higher among triple-negative case patients, the prevalence of lactation was higher among triple-negative case patients than luminal A case patients, even taking into account the number of births.
With 1252 ER- breast cancer case patients, including 567 classified as triple negative, the present study provides strong evidence of an adverse effect of pregnancy and an ameliorating effect of lactation. Importantly, the study was conducted in AA women, the population group at highest risk of ER- cancer. There have been three previous studies, mainly of European ancestry (EA) women, that also had a large number of ER- cases (25,27,38), but only one examined the joint effects of parity and lactation (27). That study, from the Breast Cancer Family Registry, included 920 ER-/PR- case patients, of which 131 were in AA women (27). Results were very similar to the present findings: High parity without lactation was positively associated with ER-/PR- breast cancer, whereas there was no association with high parity in women with a history of lactation (27). The EPIC study, with 998 ER- case patients, found no association of parity with ER- breast cancer (38). Of note, 81% of the participants had breastfed, as compared with only 43% of AMBER participants, and data were not presented on the relation of parity in the absence of lactation. In pooled data from the Breast Cancer Association Consortium (3895 ER- case patients), parity was not associated with risk of ER- cancer, and no data were available on lactation (25).
Each of the studies in our consortium has published on this topic previously, but the CBCS (8) and MEC (34) did not present results for AA women separately; parity was not associated with ER- cancer in the overall MEC data (34) but was associated with increased risk in the CBCS (8).
Analyses conducted within strata of ages at diagnosis and within strata of years since last birth indicate that the positive association of parity with ER- breast cancer is not limited to early-onset breast cancer and persists for many years after the pregnancy. The mechanisms behind this long-term effect may be different from the mechanisms operating for PABC, which occurs within 10 years of a pregnancy, a window of risk that may allow progression of preclinical disease to clinical disease irrespective of biologic subtype. Schedin et al. have hypothesized that an adverse effect of pregnancy may stem from immune system/inflammatory processes that occur during postpartum involution (40). Involution involves an influx of immune cells, activation of fibroblasts, and extracellular matrix deposition, all hallmarks of a tumorigenic wound-healing environment (41). The mechanisms by which ER- breast cancer is selectively influenced by pregnancy and lactation are uncertain. Lactation may prevent disordered involution and thus, possibly, the tissue inflammation associated with postpartum breast involution (42). Involution in women who have breastfed typically occurs gradually over a period of weeks or months as the mother moves from exclusive to partial breastfeeding and then to less frequent feedings. Whether gradual weaning returns the lactating gland to its prepregnancy state in a more coordinated and less tumorigenic process of apoptosis and remodeling remains to be determined. In addition, lactation may induce differentiation of a population of cells susceptible to transformation, effectively depleting putative cancer-initiating cells (43). Here we provide evidence that ER- targets may be specifically reduced as a result of lactation. Further research is needed to understand the mechanisms by which ER- breast cancer could be selectively influenced by pregnancy and lactation.
Parity was associated with a small reduction in risk of ER+ cancer if the first birth occurred before age 25 years, but not if the first birth was later. Breastfeeding was not associated with reduced risk of ER+ cancer. As reviewed by Althuis (4) and Ma (5), and consistent with publications since then (6,8,9,25,35,38), the vast majority of studies that have assessed parity in relation to risk of ER+ breast cancer have found either a weak inverse association with increasing number of births or no association. Findings regarding the relation of breastfeeding to risk of ER+ breast cancer have been less consistent, with some studies suggesting an inverse association and others no association (44,45).
A limitation of the present study was a lack of data on HER2 for many case patients. Testing for HER2 expression did not become widespread until 2005, and additional testing by FISH for those with weak expression has been inconsistent and based on changing parameters. As a result, we were able to identify only 567 triple-negative case patients out of all ER- case patients. We did not have a sufficient number of case patients with HER2 overexpression (ER-, PR-, HER2+) for separate analysis. We also did not have the additional markers needed for classification of luminal breast cancer or basal-like breast cancer.
Another limitation was the quality of the data on breastfeeding. We did not have information on length of lactation for each birth, exclusive breastfeeding, or characterization of weaning. Thus we were unable to estimate the minimum duration required for a reduction in risk of ER- cancer.
Our findings suggest that parous women are at increased risk of ER- and triple-negative breast cancer, and that lactation may ameliorate the effects of pregnancy and childbirth. This may explain, in part, why AA women, who typically have more children (13,14) but a lower prevalence of lactation than US white women (15,16), are disproportionately affected by ER- breast cancer. This profile, other factors being equal, would predict a higher incidence of ER- and triple-negative breast cancer, as has been observed in national data (1,2). A possible beneficial effect of lactation on future risk of ER- breast cancer is important information for the individual woman, who can take this into account when weighing the pros and cons of breastfeeding her baby. It is also critical information for the institutions whose policies determine whether lactation will be feasible for women who work outside the home.
Funding
This work was supported by the National Institutes of Health (NIH) (grants P01 CA151135 to CBA, AFO, JRP; R01 CA058420 to LR; UM1 CA164974 to LR; R01 CA100598 to CBA, EVB; UM1 CA164973 to LNK; RO1 CA54281 to LNK; P50 CA58223 to Charles Perou at UNC) and the University Cancer Research Fund of North Carolina.
Supplementary Material
The sponsors did not have a role in the design or conduct of study, the interpretation of results, or the decision to submit the manuscript for publication.
We thank participants and staff of the contributing studies. We wish also to acknowledge the late Robert Millikan, DVM, MPH, PhD, who was instrumental in the creation of this consortium. Pathology data were obtained from numerous state cancer registries (Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Illinois, Indiana, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, New Jersey, New York, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia). The results reported do not necessarily represent their views or the views of the NIH.
References
- 1. Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat. 2002;74(3):199–211. [DOI] [PubMed] [Google Scholar]
- 2. Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104(14):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. [DOI] [PubMed] [Google Scholar]
- 4. Althuis MD, Fergenbaum JH, Garcia-Closas M, et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558–1568. [PubMed] [Google Scholar]
- 5. Ma H, Bernstein L, Pike MC, et al. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phipps AI, Chlebowski RT, Prentice R, et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103(6):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer JR, Boggs DA, Wise LA, et al. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010;116(21):4933–4943. [DOI] [PubMed] [Google Scholar]
- 11. Lord SJ, Bernstein L, Johnson KA, et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ambrosone CB, Zirpoli G, Ruszczyk M, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control. 2014;25(2):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 14. Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23. 2005(25):1–160. [PubMed] [Google Scholar]
- 15. McDowell MM, Wang CY, Kennedy-Stephenson J. Breastfeeding in the United States: findings from the national health and nutrition examination surveys, 1999–2006. NCHS Data Brief. 2008(5):1–8. [PubMed] [Google Scholar]
- 16. Racial and ethnic differences in breastfeeding initiation and duration, by state - National Immunization Survey, United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59(11):327–334. [PubMed] [Google Scholar]
- 17. Progress in increasing breastfeeding and reducing racial/ethnic differences - United States, 2000–2008 births. MMWR Morb Mortal Wkly Rep. 2013;62(5):77–80. [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50(2):56–58. [PubMed] [Google Scholar]
- 19. Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35(1):51–60. [DOI] [PubMed] [Google Scholar]
- 21. Ambrosone CB, Ciupak GL, Bandera EV, et al. Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol. 2009;2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bandera EV, Chandran U, Zirpoli G, et al. Rethinking sources of representative controls for the conduct of case-control studies in minority populations. BMC Med Res Methodol. 2013;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Callihan EB, Gao D, Jindal S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138(2):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez ME, Wertheim BC, Natarajan L, et al. Reproductive factors, heterogeneity, and breast tumor subtypes in women of mexican descent. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Work ME, John EM, Andrulis IL, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer. 2014;110(5):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao PP, Shu XO, Gao YT, et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol. 2011;174(6):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li CI, Beaber EF, Tang MT, et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137(2):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma H, Henderson KD, Sullivan-Halley J, et al. Pregnancy-related factors and the risk of breast carcinoma in situ and invasive breast cancer among postmenopausal women in the California Teachers Study cohort. Breast Cancer Res. 2010;12(3):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phipps AI, Buist DS, Malone KE, et al. Reproductive history and risk of three breast cancer subtypes defined by three biomarkers. Cancer Causes Control. 2011;22(3):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phipps AI, Malone KE, Porter PL, et al. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Setiawan VW, Monroe KR, Wilkens LR, et al. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamimi RM, Colditz GA, Hazra A, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ursin G, Bernstein L, Lord SJ, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93(3):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ritte R, Tikk K, Lukanova A, et al. Reproductive factors and risk of hormone receptor positive and negative breast cancer: a cohort study. BMC Cancer. 2013;13:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nichols HB, Trentham-Dietz A, Love RR, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):41–47. [PubMed] [Google Scholar]
- 40. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–291. [DOI] [PubMed] [Google Scholar]
- 41. Clarkson RW, Wayland MT, Lee J, et al. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6(2):R92–R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med. 2006;8(32):1–15. [DOI] [PubMed] [Google Scholar]
- 43. Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat. 1982;2(1):5–73. [DOI] [PubMed] [Google Scholar]
- 44. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–195. [DOI] [PubMed] [Google Scholar]
- 45. Michels KB, Willett WC, Rosner BA, et al. Prospective assessment of breastfeeding and breast cancer incidence among 89887 women. Lancet. 1996;347(8999):431–436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


