Abstract
Bromoacetyl-phenylalanyl-tRNAphe bound to 70S E. coli ribosomes reacts covalently with proteins of the 50S subunit. The major reactions are with proteins L2 and L27. In the presence of poly(U), 70S-bound bromoacetyl-phenylalanyl-tRNAphe can participate in peptidebond formation with phenylalanyl-tRNAphe or puromycin. Most of the products of these reactions are also found covalently attached to L2 and L27. Chloramphenicol and sparsomycin markedly inhibit the peptide-bond formation. These results strongly suggest that bromoacetylphenylalanyl-tRNAphe can function as a normal peptidyl-tRNA and that the 50S proteins, L2 and L27, are located in the peptidyl-tRNA binding site. The side reactions of bromoacetyl-phenylalanyl-tRNAphe are with one or more 50S proteins from the set L14-17, L6 and/or L11, and L26. These occur to a much less extent than the reactions with L2 and L27. Any functional significance of the side reactions is unknown.
Keywords: protein L2 and L27, affinity labeling, protein synthesis, two-dimensional electrophoresis, antibiotics
Full text
PDF
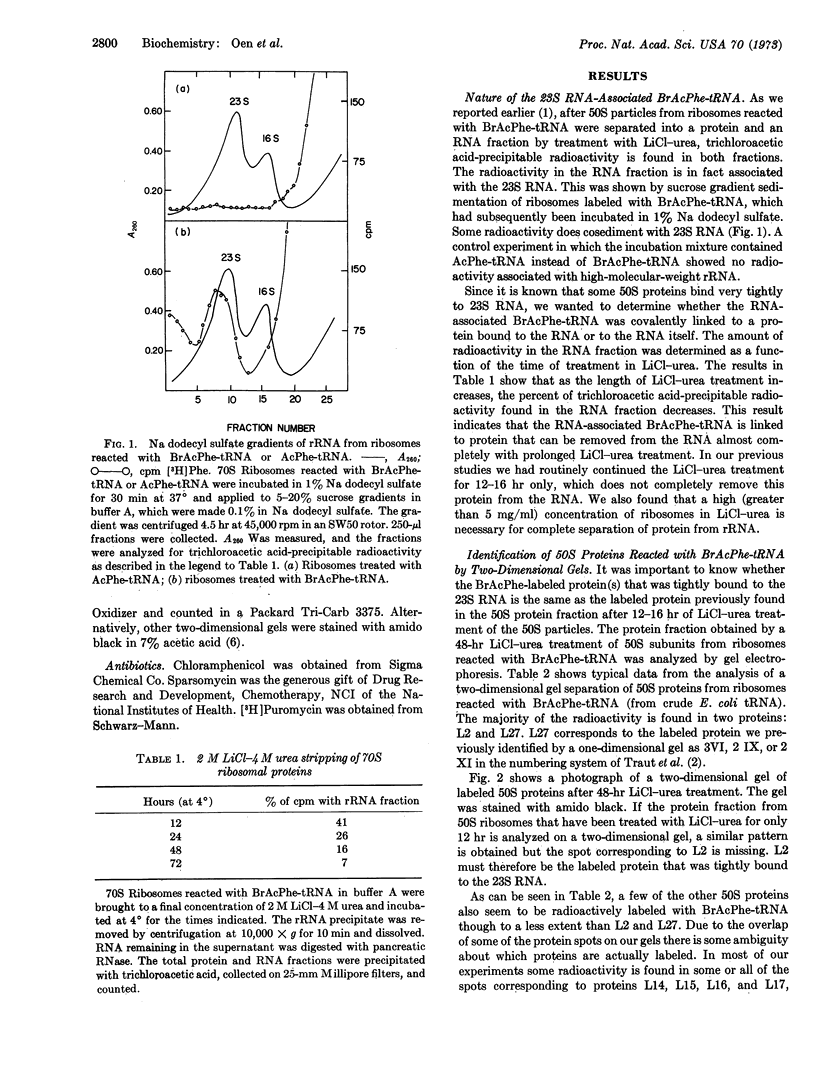

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
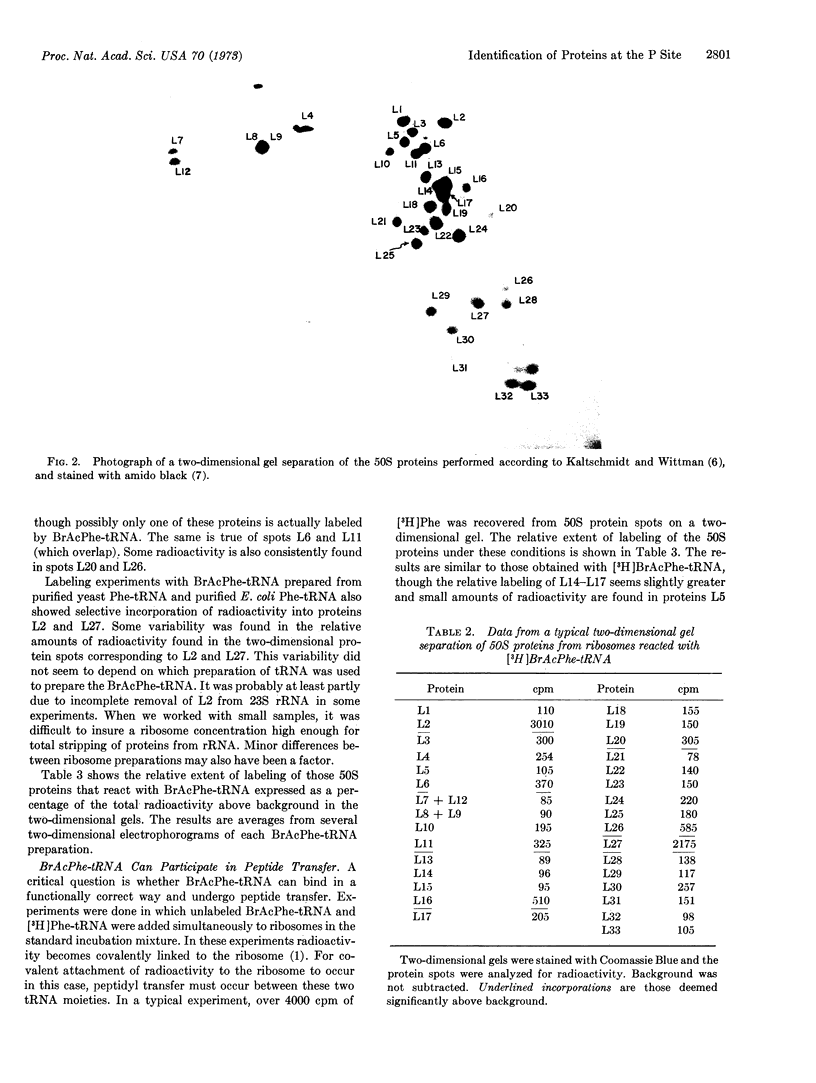
- Crichton R. R., Wittmann H. G. Ribosomal proteins. XXIV. Trypsin digestion as a possible probe of the conformation of Escherichia coli ribosomes. Mol Gen Genet. 1972;114(2):95–105. doi: 10.1007/BF00332780. [DOI] [PubMed] [Google Scholar]
- Dzionara M., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. 8. Molecular weights of isolated ribosomal proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1909–1913. doi: 10.1073/pnas.67.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S., Erdmann V., Nomura M. Reconstitution of 50S ribosomal subunits from protein-free ribonucleic acid. Biochemistry. 1973 Jan 16;12(2):220–224. doi: 10.1021/bi00726a007. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. Isolation of proteins from 50S ribosomal subunits of Escherichia coli. Eur J Biochem. 1971 Nov 11;23(1):12–16. doi: 10.1111/j.1432-1033.1971.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Hsiung N., Cantor C. R. Reaction of celite-bound fluorescein isothiocyanate with the 50S E. coli ribosomal subunit. Arch Biochem Biophys. 1973 Jul;157(1):125–132. doi: 10.1016/0003-9861(73)90397-4. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
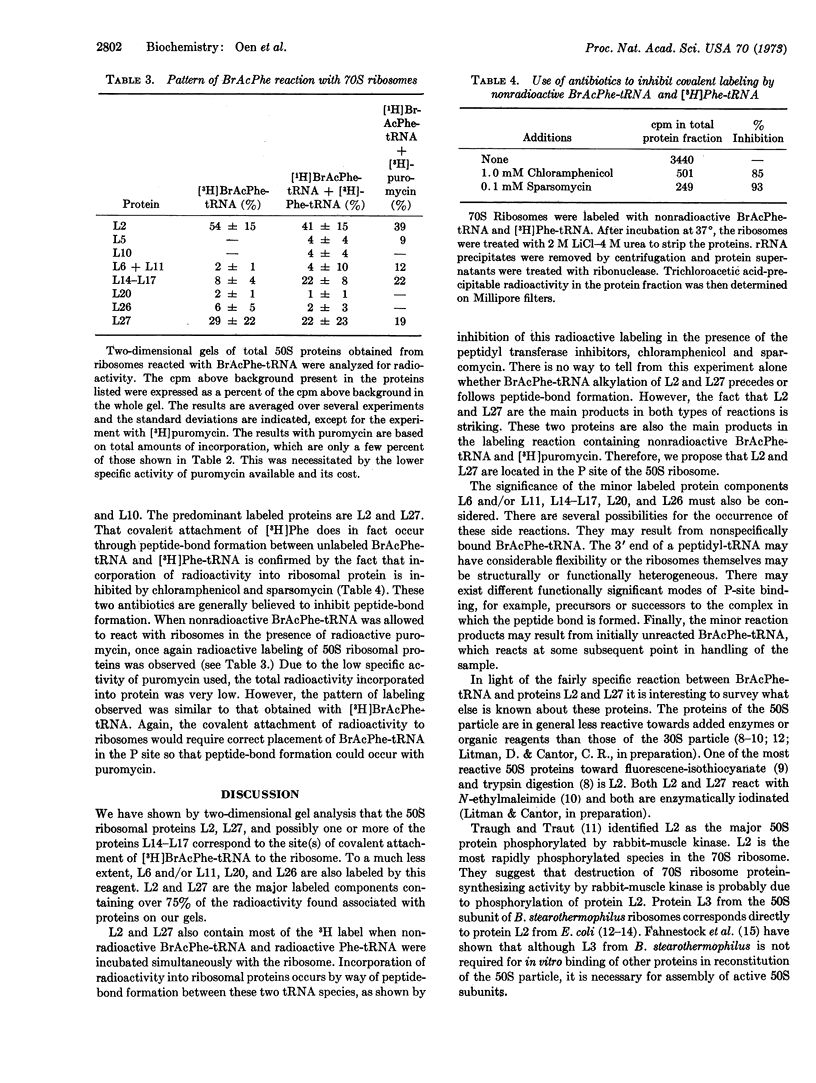
- Lanks K. W., Sciscenti J., Weinstein I. B., Cantor C. R. Studies on rat liver phenylalanyl transfer ribonucleic acid synthetase. I. Purification, stabilization, and complex formation. J Biol Chem. 1971 Jun 10;246(11):3494–3499. [PubMed] [Google Scholar]
- Moore P. B. Reaction of N-ethyl maleimide with the ribosomes of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):169–184. doi: 10.1016/0022-2836(71)90456-6. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Oen H., Cantor C. R. Covalent attachment of a peptidyl-transfer RNA analog to the 50S subunit of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1972 Apr;69(4):837–841. doi: 10.1073/pnas.69.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Wilchek M., Zamir A. Mapping of Escherichia coli ribosomal components involved in peptidyl transferase activity. Proc Natl Acad Sci U S A. 1973 May;70(5):1423–1426. doi: 10.1073/pnas.70.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Daya L., Rak K. H., Garrett R. A. Ribosomal proteins. XXX. Specific protein binding sites on 23S RNA of Escherichia coli. Mol Gen Genet. 1972;114(2):125–133. doi: 10.1007/BF00332783. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Traut R. R. Phosphorylation of ribosomal proteins of Escherichia coli by protein kinase from rabbit skeletal muscle. Biochemistry. 1972 Jun 20;11(13):2503–2509. doi: 10.1021/bi00763a019. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Delius H., Ahmad-Zadeh C., Bickle T. A., Pearson P., Tissières A. Ribosomal proteins of E. Coli: stoichiometry and implications for ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:25–38. doi: 10.1101/sqb.1969.034.01.007. [DOI] [PubMed] [Google Scholar]
- Weber H. J. Stoichiometric measurements of 30S and 50S ribosomal proteins from Escherichia coli. Mol Gen Genet. 1972;119(3):233–248. doi: 10.1007/BF00333861. [DOI] [PubMed] [Google Scholar]