Abstract
In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) causes ventral prostate agenesis in C57BL/6J mice by preventing ventral prostatic budding in the embryonic urogenital sinus (UGS). TCDD (5 μg/kg, po) administered to pregnant dams on embryonic day 15.5 (E15.5) activates the aryl hydrocarbon receptor in the UGS mesenchyme, disrupting the mesenchymally derived paracrine signaling that instructs epithelial prostatic budding. How TCDD alters the mesenchymal milieu is not well understood. We previously showed that TCDD disrupts some aspects of Wnt signaling in UGSs grown in vitro. Here we provide the first comprehensive, in vivo characterization of Wnt signaling in male E16.5 UGSs during normal development, and after in utero TCDD exposure. Vehicle- and TCDD-exposed UGSs were probed by in situ hybridization to assess relative abundance and localization of RNA from 46 genes that regulate Wnt signaling. TCDD altered the staining pattern of five genes, increasing staining for Wnt10a and Wnt16 and decreasing staining for Ror2, Rspo2, and Wif1. We also used immunohistochemistry to show, for the first time, activation of β-catenin (CTNNB1) signaling in ventral basal epithelium of control UGSs at E16.5. This onset of CTNNB1 signaling occurred immediately prior to the initiation of ventral prostatic budding and is characterized by a pronounced increase in CTNNB1 nuclear localization and subsequent expression of the CTNNB1 signaling target gene, Lef1. In utero TCDD exposure prevented the onset of CTNNB1 signaling and LEF1 expression in the ventral basal epithelium, thereby elucidating a likely mechanism by which TCDD contributes to failed prostatic budding in the ventral UGS.
Keywords: TCDD, Wnt, CTNNB1, mouse, prostate, development
Mouse prostate develops from the fetal urogenital sinus (UGS). Testicular androgens trigger production of paracrine signals in UGS mesenchyme that stimulate the formation of prostate duct progenitors (prostatic buds) from UGS epithelium (reviewed in Cunha, 2008; Thomson, 2008). Prostatic buds are specified in a precise pattern about the caudocranial and dorsoventral axes of the UGS creating anterior, dorsal, lateral, and ventral budding regions. Bud initiation and elongation occurs first in the anterior and dorsal regions beginning around embryonic day 16.5 (E16.5) and last in the lateral and ventral regions beginning around E17.5 (Lin et al., 2003).
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the prototypical aryl hydrocarbon receptor (AHR) agonist, is a persistent, global environmental contaminant that has been shown in toxicity studies to be a carcinogen, immunomodulator, endocrine disruptor, epigenetic modifier, and teratogen (Birnbaum and Fenton, 2003; Couture et al., 1990; Craig et al., 2011; Kerkvliet, 2009; King-Heiden et al., 2012; Knerr and Schrenk, 2006; Leng et al., 2014; Manikkam et al., 2012; Martinez et al., 2003; Sulentic and Kaminski, 2011; Tian, 2009). In utero exposure to TCDD can adversely affect the development of multiple organs and tissues in rodents, including prostate development (reviewed in Vezina et al., 2009). A single dose of TCDD (5 μg/kg) given to pregnant (E13.5–E15.5) C57BL/6J mice inhibits prostatic budding initiation, causing reduced numbers of dorsolateral prostatic buds and a complete lack of ventral prostatic buds (Lin et al., 2003; Vezina et al., 2008). TCDD inhibits budding by activating AHR in the UGS mesenchyme (Ko et al., 2004a). Though UGS mesenchyme is where androgens and TCDD act to promote or inhibit prostatic budding, respectively, TCDD does not seem to inhibit budding by directly downregulating androgen signaling (Ko et al., 2004b). Therefore, we hypothesized that TCDD alters mesenchymally derived paracrine signaling such that either the epithelium does not receive appropriate signals to initiate budding, or it receives signals that actively block budding. We previously studied the effects of TCDD exposure on selected members of the Egf, Fgf, Tgf, and Wnt paracrine signaling pathways (Abbott et al., 2003; Allgeier et al., 2008; Branam et al., 2013; Vezina et al., 2010). Multiple components of the Wnt signaling pathway were dysregulated in UGSs cultured in vitro with TCDD. Therefore, we aimed to confirm these findings in vivo and assess additional Wnt signaling components to identify TCDD-affected genes that could inhibit prostatic budding.
Wnt signaling is comprised of multiple intracellular signaling cascades that affect diverse cellular responses including transcription, proliferation, differentiation, and migration (Kharaishvili et al., 2011; Nishita et al., 2010; Simons et al., 2012). The Wnt/β-catenin signaling cascade is a major component of Wnt signaling. When activated, β-catenin (CTNNB1), which is degraded under resting conditions, accumulates within the cell, translocates into the nucleus, and activates transcription of target genes. Inhibitors of the Wnt/CTNNB1 signaling cascade, or conditional Ctnnb1 knockout, impair prostatic budding (Branam et al., 2013; Francis et al., 2013; Mehta et al., 2013; Simons et al., 2012). UGSs cultured in vitro with TCDD show reduced expression of CTNNB1 signaling target genes and fewer prostatic buds compared with control UGSs (Branam et al., 2013). Furthermore, AHR activation can downregulate CTNNB1 signaling (demonstrated by reduced CTNNB1 target gene expression) in other rodent tissues and cell types (Kawajiri et al., 2009; Procházková et al., 2011). Thus, CTNNB1 signaling is required for prostatic budding in mice, and AHR activation can downregulate CTNNB1 signaling.
Our previous in vitro study revealed that TCDD dysregulates Wnt signaling in the UGS; however, it focused on a limited number of genes. The present study is the first comprehensive in vivo evaluation of Wnt signaling activity in UGSs from E16.5 C57BL/6J male mice. At E16.5, some anterior and dorsal buds have formed, but lateral and ventral bud initiation has not yet occurred. Because TCDD exposure prevents ventral budding, we investigated how TCDD affects Wnt signaling in the ventral UGS prior to bud formation. We used in situ hybridization (ISH) to characterize the relative abundance and localization of RNAs from 46 Wnt pathway genes during both normal development and after exposure to TCDD. TCDD increased staining of wingless-related MMTV integration site 10a (Wnt10a) and Wnt16 and decreased staining of receptor tyrosine kinase-like orphan receptor 2 (Ror2), R-spondin2 (Rspo2), and Wnt inhibitory factor 1 (Wif1). In addition to this broad survey of Wnt signaling, we investigated CTNNB1 signaling in the UGS in detail. This is the first report to show activation of CTNNB1 signaling immediately prior to budding initiation in basal epithelial cells during normal prostate development. Immunohistochemistry (IHC) revealed that CTNNB1 accumulates intracellularly and translocates to the nucleus where it induces expression of known target genes Lymphoid enhancer binding factor 1 (Lef1) and Wif1, as well as putative target gene Ror2. In utero TCDD exposure blocks activation of CTNNB1 signaling in the ventral UGS. CTNNB1 fails to accumulate and, correspondingly, there is a pronounced reduction in target gene expression in ventral basal epithelium. This block in activation of CTNNB1 signaling is likely a key mechanism by which TCDD causes budding failure in the ventral UGS, culminating in ventral prostate agenesis.
MATERIALS AND METHODS
Mice and dosing regimen
Mice were housed in polysulfone cages containing corn cob bedding and maintained on 12-h light/dark cycles at 21 ± 1°C and 20–50% relative humidity. Feed (Harlan Teklad Rodent Diet 8604, Harlan Laboratories Inc., Madison, WI) and water were available ad libitum. All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals. C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) and used for all ISH and IHC experiments. To obtain timed-pregnant dams, females were paired overnight with males. The next morning was considered E0.5. On E15.5, mouse fetuses were exposed in utero to corn oil vehicle alone (5 ml/kg dam, po) or corn oil containing TCDD (5 μg/kg dam, po, 98% purity, Cambridge Isotopes Laboratories, Andover, MA). The 5 μg/kg dose of TCDD is the highest dose that can be given to pregnant (E13.5–E15.5) C57BL/6J female mice that won't affect embryo viability, but still completely block ventral prostatic bud formation (Lin et al., 2002). Dams were euthanized by CO2 asphyxiation at E16.0, E16.5, or E17.5.
In situ hybridization
UGSs were fixed overnight in 4% paraformaldehyde (PFA), dehydrated via graded incubations into 100% MeOH, and stored at −20°C until needed. On the day of sectioning, UGSs were rehydrated into PBS and cut with a vibrating microtome into 50 μm (sagittal) or 40 μm (transverse) sections. Riboprobe hybridization and staining procedures are described elsewhere (Abler et al., 2011; www.gudmap.org). Riboprobes were synthesized by PCR using primer sequences described previously (Mehta et al., 2011) that span sequences unique to each gene. The length of time required to stain sections after riboprobe hybridization varied from gene to gene depending on the relative abundance of RNA. Genes for which no RNA was detected were stained for up to 10 days before the procedure was stopped. Staining patterns in the UGS—which represent in vivo RNA transcription—were described for each riboprobe based on the analysis of sections from at least three litter-independent male embryos per treatment group. Descriptions of RNA transcription patterns were based on the anatomical ontology of the Edinburgh Mouse Atlas Project and modifications detailed by the GenitoUrinary Development Molecular Anatomy Project (GUDMAP, www.gudmap.org). Sections from vehicle- and TCDD-exposed UGSs were processed as a single unit so that RNA abundance and pattern could be compared between and within treatment groups. To ensure UGSs were processed in a manner that preserved high-quality RNA in all samples, we used a uroplakin 1b (Upk1b) riboprobe as a positive control and confirmed that Upk1b transcript was present in the expected abundance and pattern in at least one section from all vehicle- and TCDD-exposed UGSs used in this study.
Immunohistochemistry
UGS tissues were fixed overnight in 4% PFA, dehydrated into 100% ethanol, embedded in paraffin, and cut into 5 μm sagittal sections. Before staining, sections were rehydrated, treated with 3% hydrogen peroxide for 10 min, boiled in 10 mM sodium citrate for 20 min, and allowed to cool to room temperature to unmask epitopes. Sections were blocked for 2 h with blocking solution – 5% goat serum (Sigma-Aldrich, G9023) and 1% bovine serum albumin (EMD Millipore, 2910) in phosphate buffered saline containing 0.05% Tween-20 (PBST; Sigma-Aldrich, P3563). Primary antibodies against NP-CTNNB1 (Cell Signaling, 8814) or LEF1 (Cell Signaling, 2230) were diluted 1:2000 and 1:250, respectively, in blocking solution, applied to the section, and incubated overnight at 4°C. Sections were washed with PBST and subsequently incubated for 1 h with biotinylated goat anti-rabbit IgG secondary antibody (Vector Labs, BA-1000) diluted 1:500 in blocking solution. Sections were washed with PBST and incubated for 30 min with peroxidase-conjugated streptavidin (Vector Labs, PK-6100). After washing with PBST, staining was achieved by incubating sections with 3,3'-diaminobenzidine (DAB) solution (Life Technologies, 002020) for 2–5 min at room temperature. Sections were counterstained with hematoxylin to label nuclei.
IHC quantification and statistics
Immunohistochemical staining for LEF1 is nuclear and clearly present or absent within a cell. Within the UGS, epithelial LEF1 expression roughly defines the regions (anterior, dorsal, lateral, ventral) from which prostatic buds will emerge, and is restricted to the first 1–2 cell layers proximal to the mesenchyme. As such, at E16.5, LEF1 expression does not extend into the epithelium of the bladder or urethra so no artificial or arbitrary intraepithelial boundaries were created. All LEF1-positive cells in the ventral epithelium of the UGS were counted. One LEF1-stained section from each of the 10 vehicle-exposed and six TCDD-exposed UGSs was counted. Each UGS was from a different litter.
The Wilcoxon Rank Sum test was used to determine if there was a significant difference in the number of LEF1-positive epithelial cells in the ventral UGS between vehicle-exposed and TCDD-exposed UGSs. A p-value of ≤0.05 was considered significant.
RESULTS
TCDD Alters Abundance and In Situ Localization of Wnt10a and Wnt16 RNAs in the UGS
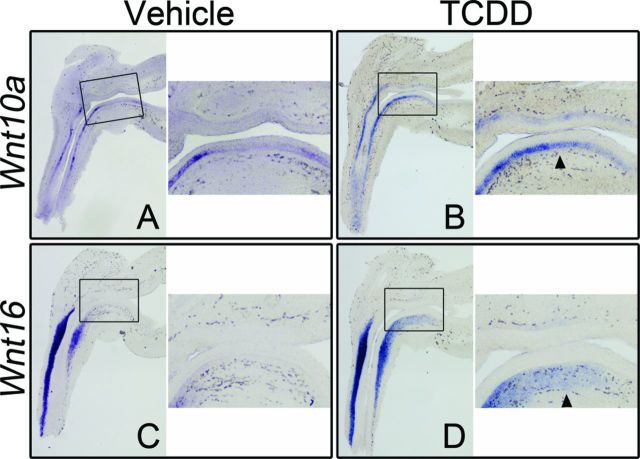
The Wnt family of glycoproteins plays a central role in directing development, and recent studies have demonstrated that exposure to TCDD can alter Wnt signaling in a variety of tissues and cell types (Hrubá et al. 2011; Mathew et al., 2008; Procházková et al., 2011). We dosed pregnant C57BL/6J female mice with vehicle or TCDD (5 μg/kg) at E15.5 and harvested UGSs from male embryos at E16.5, just prior to ventral prostatic budding initiation. ISH was used as an RNA detection method to evaluate both relative abundance and in situ localization. Of the 19 mouse Wnt genes, no staining was observed for five—Wnt1, 3, 8a, 8b, and 10b—indicating that either there was no transcript present, or transcript abundance was below detectable levels (Table 1, Supplementary table 1, and Supplementary figs. 1 and 2). Staining for an additional two genes—Wnt7a and 9b—was found only in the Mullerian ducts and Wolffian structures, respectively (Table 1, Supplementary table 1, and Supplementary fig. 2). Ten genes—Wnt2a, 2b, 3a, 4, 5a, 5b, 6, 7b, 9a, and 11—had detectable levels of staining in control UGSs which was unchanged by exposure to TCDD (Table 1, Supplementary table 1, and Supplementary figs. 1 and 2). Lastly, staining for two genes—Wnt10a and Wnt16—was altered in UGSs exposed to TCDD when compared with controls (Table 1). In control UGSs, strong Wnt10a staining was detected in the basal epithelium of the urethra, but in adjacent basal epithelium of the ventral UGS, staining intensity was markedly weaker to non-existent (Fig. 1A). By comparison, strong Wnt10a staining was detected in the basal epithelium of the urethra and continued all the way through the ventral UGS to the base of the bladder in TCDD-exposed UGSs (Fig. 1B). In control UGSs, Wnt16 staining was only detected in the mesenchyme immediately adjacent to the epithelium (lamina propria) in the urethra (Fig. 1C). In TCDD-exposed UGSs, Wnt16 staining covered a larger region. Like control UGSs, staining was detected in urethral mesenchyme, but also extended cranially into the mesenchyme of the ventral UGS, toward the bladder (Fig. 1D).
TABLE 1. Effect of In Utero TCDD Exposure on Abundance and Localization of RNA from Wnt Genes in Mouse UGS at E16.5a.
aAt least three litter-independent UGSs per treatment group were used to determine RNA abundance and localization for each gene. Corn oil (vehicle) or TCDD (5 μg/kg, po) was administered to pregnant dams on E15.5. A horizontal arrow denotes that RNA was detected in the indicated tissue compartment and that there was no detectable difference in RNA abundance or localization between vehicle- and TCDD-exposed UGSs at E16.5. Upward-pointing arrows indicate that TCDD increased transcript abundance compared with vehicle-exposed control UGSs. No arrow indicates that transcript was not detected.
bBasal epithelium = epithelial cells bordering mesenchyme.
cIntermediate epithelium = epithelial cells that do not border mesenchyme or lumen.
dVentral lamina propria = mesenchyme immediately adjacent to the epithelium in the ventral UGS.
FIG. 1.
TCDD increases Wnt10a and Wnt16 transcript abundance in the ventral UGS. C57BL/6J mouse embryos were exposed in utero to vehicle or TCDD (5 μg/kg dam, po) at E15.5 and UGSs were harvested at E16.5. UGSs were sectioned along the sagittal plane and are shown with the urethra on the left pointing down and the bladder on the right. Images are representative of at least eight litter-independent UGSs per treatment group. Within each panel, the boxed area in the left image is enlarged on the right. The purple stain marks transcript localization as assessed by ISH. Wnt10a transcription was restricted to the epithelium (A, B) whereas Wnt16 transcription was restricted to the mesenchyme (C, D). Staining for Wnt10a and Wnt16 in the ventral region of UGSs from vehicle control embryos is minimal to absent. However, staining for both Wnt10a and Wnt16 is detectable in the ventral UGS after exposure to TCDD (arrowheads).
TCDD Alters RNA Abundance and In Situ Localization of Several Secreted and Transmembrane Modulators of Wnt Signaling
In addition to the Wnts, we used ISH to assess RNA abundance and localization for 27 genes known to play a role in Wnt signaling activation or modulation (Table 2, Supplementary table 2, and Supplementary figs. 3–5). Eleven of these genes encode proteins secreted into the extracellular space, 11 encode transmembrane proteins, and five encode intracellular proteins. At E16.5, there were two genes for which there was no detectable transcript in both vehicle- and TCDD-exposed UGSs, and one gene with staining restricted to the Wolffian structures only. Of the remaining 24 genes, three had detectably altered RNA abundance and in situ localization after exposure to TCDD: Rspo2 (secreted protein), Wif1 (secreted protein), and Ror2 (transmembrane protein) (Table 2). Descriptions of Rspo2 ISH results follow; results for Wif1 and Ror2 are described later.
TABLE 2. Effect of In Utero TCDD Exposure on Abundance and Localization of RNA from Wnt Signaling Modulators in Mouse UGS at E16.5a.
aAt least three litter-independent UGSs per treatment group were used to determine RNA abundance and localization for each gene. Corn oil (vehicle) or TCDD (5 μg/kg, po) was administered to pregnant dams on E15.5. A horizontal arrow denotes that RNA was detected in the indicated tissue compartment and that there was no detectable difference in RNA abundance or localization between vehicle- and TCDD-exposed UGSs at E16.5. Downward-pointing arrows indicate that TCDD decreased transcript abundance compared with vehicle-exposed control UGSs. No arrow indicates that transcript was not detected.
bBasal epithelium = epithelial cells bordering mesenchyme.
cIntermediate epithelium = epithelial cells that do not border mesenchyme or lumen.
dVentral lamina propria = mesenchyme immediately adjacent to the epithelium in the ventral UGS.
eBecause the reported functions of Wnt signaling modulators can vary from system to system, genes were sorted by the subcellular localization of their proteins rather than sorted by function.
At E16.5, in transverse sections of vehicle-exposed control UGSs, staining for Rspo2 transcript was detected bilaterally in the ventral mesenchymal pads, and also as a complete ring running through the mesenchyme of the anterior and ventral budding regions (Supplementary fig. 6A). In sagittal sections, staining for Rspo2 appears in the ventral mesenchymal pad with less intense staining also detectable in the anterior mesenchyme (Fig. 2A). By comparison, staining was less intense in both sagittal and transverse sections from age-matched, TCDD-exposed UGSs, indicating reduced Rspo2 transcript abundance (Fig. 2B and Supplementary fig. 6B).
FIG. 2.
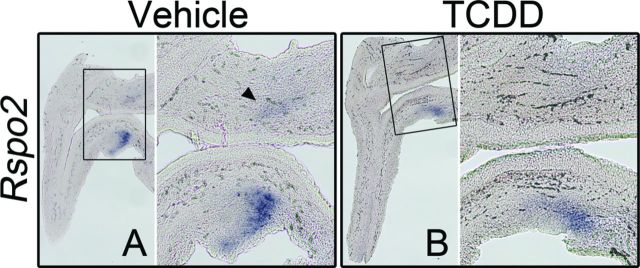
TCDD reduces transcript abundance of Rspo2 in UGS mesenchyme. C57BL/6J mouse embryos were exposed in utero to vehicle or TCDD (5 μg/kg dam, po) at E15.5 and UGSs were harvested at E16.5. UGSs were sectioned along the sagittal plane and are shown with the urethra on the left pointing down and the bladder on the right. Images are representative of at six litter-independent UGSs per treatment group. Within each panel, the boxed area in the left image is enlarged on the right. The purple stain marks transcript localization as assessed by ISH. Rspo2 transcription was restricted to the mesenchyme (A, B). The arrowhead in (A) points to faint staining in the anterior mesenchyme of the UGS. Exposure to TCDD reduces abundance of Rspo2 RNA.
TCDD Reduces Accumulation of Non-Phosphorylated CTNNB1 (NP-CTNNB1) in Basal Epithelium of the Ventral UGS
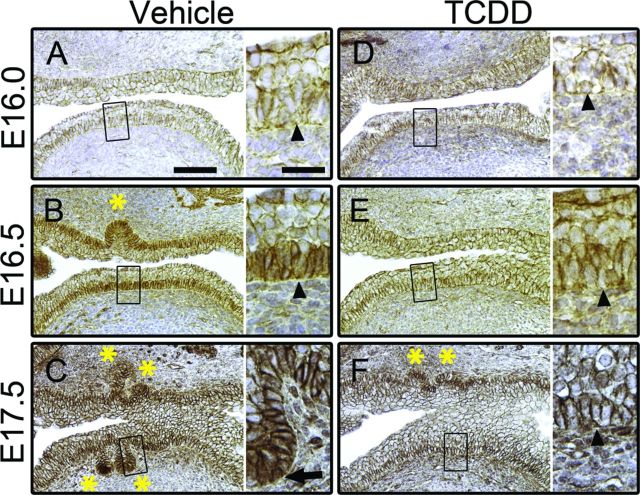
Phosphorylation of CTNNB1 targets the protein for degradation, thus, preventing phosphorylation of CTNNB1 is required to activate CTNNB1 signaling. NP-CTNNB1 is stabilized, accumulates intracellularly, and translocates to the nucleus where it activates transcription of target genes. We dosed pregnant C57BL/6J dams with vehicle or TCDD (5 μg/kg) at E15.5, harvested UGSs from male embryos at E16.0, E16.5, and E17.5, and assessed expression of NP-CTNNB1 by IHC. At E16.0 in control UGSs, NP-CTNNB1 staining was restricted to the cell membrane throughout the epithelium (Fig. 3A). However, around E16.5 we detected an overall increase in NP-CTNNB1 staining intensity in basal epithelial cells of the ventral UGS because, in addition to cell membranes, NP-CTNNB1 was also detected in the cytoplasm and nucleus of basal epithelial cells (Fig. 3B). By comparison, intermediate epithelial cells at E16.5 show no cytoplasmic accumulation or nuclear localization of NP-CTNNB1 (Fig. 3B). Thus, phosphorylation of CTNNB1 subsides around E16.5 in ventral basal epithelial cells in control UGSs and NP-CTNNB1 begins to accumulate and translocate into the nucleus prior to initiation of ventral prostatic budding. By E17.5, prostatic buds are present in all budding regions of control UGSs. In the ventral basal epithelium, overall NP-CTNNB1 staining appears to be reduced compared with E16.5, with significant cytoplasmic accumulation and nuclear localization of NP-CTNNB1 most often observed in cells at the tips of prostatic buds (Fig. 3C).
FIG. 3.
TCDD prevents accumulation and nuclear localization of NP-CTNNB1. C57BL/6J mouse embryos were exposed in utero to vehicle or TCDD (5 μg/kg dam, po) at E15.5 and UGSs were harvested at E16.0, E16.5, or E17.5. UGSs were sectioned along the sagittal plane and the anterior and ventral budding regions are shown (bladder is to the right in each image). Images are representative of at least three litter-independent UGSs per treatment group. In each panel, the image on the left is shown at 100X (scale bar represents 100 μm) and the boxed region is shown on the right at 200X (scale bar represents 25 μm). IHC staining using DAB as the chromogen (brown) shows subcellular localization of NP-CTNNB1; nuclei are counterstained with hematoxylin (blue). Arrowheads point to ventral basal epithelial cells; asterisks mark prostatic buds. In E16.0 vehicle-exposed UGSs, NP-CTNNB1 is restricted to cell membranes (A). By E16.5 in vehicle-exposed UGSs cytoplasmic accumulation and nuclear localization of NP-CTNNB1 is detectable in the ventral basal epithelial cells—note that the nuclei of intermediate epithelial cells are devoid of CTNNB1 staining (B). At E17.5, in vehicle-exposed UGSs, anterior and ventral prostatic buds are visible, with NP-CTNNB1 nuclear localization primarily seen in bud tips (arrow in C). In utero exposure to TCDD has no effect on subcellular localization of NP-CTNNB1 at E16.0 (D). However, at E16.5 (E) and E17.5 (F) TCDD-exposed UGSs show no NP-CTNNB1 accumulation in the cytoplasm, no NP-CTNNB1 nuclear localization, and no ventral buds.
At E16.0 in UGSs from TCDD-exposed embryos, NP-CTNNB1 staining was restricted to the cell membrane throughout the epithelium, and was not detectably different from controls (Fig. 3D). However, at E16.5 overall staining intensity of NP-CTNNB1 in TCDD-exposed UGSs failed to increase as it did in the controls and NP-CTNNB1 nuclear localization was largely absent (Fig. 3E). Thus, in utero TCDD exposure likely causes CTNNB1 phosphorylation to persist inappropriately, preventing CTNNB1 accumulation in basal epithelial cells of the ventral UGS. At E17.5, no ventral buds were present, and there was little to no cytoplasmic accumulation or nuclear localization of NP-CTNNB1 indicating sustained CTNNB1 phosphorylation and degradation after exposure to TCDD.
TCDD Reduces LEF1 Expression in Basal Epithelium of the Ventral UGS
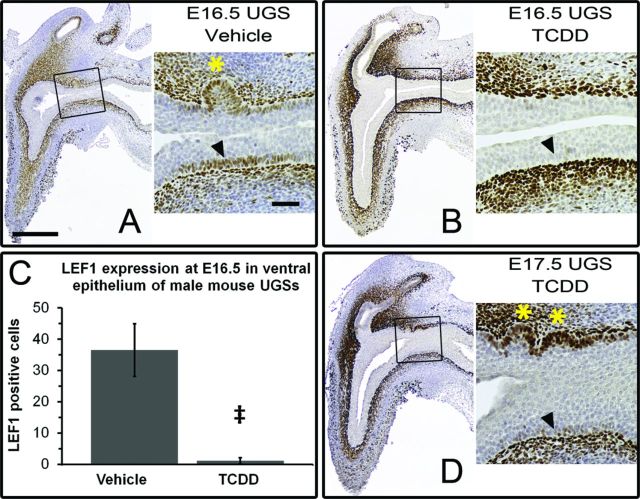
Active CTNNB1 signaling is capable of inducing LEF1 expression in multiple tissues including the UGS (Francis et al., 2013; Mehta et al., 2013). We assessed LEF1 expression in vehicle- and TCDD-exposed UGSs by IHC. In control UGSs, LEF1 expression was detected in the lamina propria of the urethra, UGS, and bladder at E14.5, the earliest time tested, and persisted through E18.5, the latest time tested (Fig. 4 and Schneider, unpublished data). Around E16.5, LEF1 expression was induced in basal epithelial cells of the ventral UGS prior to bud initiation, and continued through the initiation and elongation stages of bud development (Fig. 4A). Thus, induction of epithelial LEF1 expression corresponded with the cytoplasmic accumulation and nuclear localization of NP-CTNNB1 discussed above, and, importantly, we never detected epithelial LEF1 expression without nuclear NP-CTNNB1.
FIG. 4.
TCDD reduces LEF1 expression in basal epithelium of the ventral UGS. C57BL/6J mouse embryos were exposed in utero to vehicle or TCDD (5 μg/kg dam, po) at E15.5 and UGSs were harvested at E16.5 or E17.5. UGSs were sectioned along the sagittal plane and are shown with the urethra on the left pointing down and the bladder on the right. Images are representative of at least six litter-independent UGSs per treatment group. In panels (A), (B), and (D), images on the left are magnified 40X (scale bar represents 300 μm), with the boxed areas shown to the right at 100X (scale bar represents 100 μm). IHC staining using DAB as the chromogen (brown) marks LEF1 positive cells; nuclei are counterstained with hematoxylin (blue). Arrowheads point to ventral basal epithelial cells; asterisks mark prostatic buds. Around E16.5 in vehicle-exposed UGSs, LEF1 expression is initiated in the ventral epithelium (A). TCDD prevents LEF1 expression in the ventral epithelium at E16.5 (B). This difference was quantified by counting and averaging all LEF1-positive cells in the ventral epithelium in a single sagittal section from 10 vehicle-exposed and six TCDD-exposed E16.5 UGSs (C). The double dagger symbol (‡) indicates a statistically significant difference (p < 0.0002). At E17.5 there is still little to no LEF1 expression in the ventral epithelium, however LEF1 staining is seen in the anterior epithelium (D). Correspondingly, prostatic budding has initiated in the anterior UGS but not the ventral UGS.
In TCDD-exposed UGSs, mesenchymal LEF1 expression matched that observed in control UGSs, but epithelial LEF1 expression at E16.5 was greatly reduced compared with vehicle controls (Fig. 4B). The number of basal epithelial cells expressing LEF1 in the ventral UGS was reduced from 37 ± 8 cells (mean ± SE) in control UGSs to 1 ± 1 cell in TCDD-exposed UGSs; p < 0.0002 (Fig. 4C). The ventral region of E17.5 TCDD-exposed UGSs resembled that of E16.5 TCDD-exposed UGSs – no buds and little to no LEF1 expression in basal epithelial cells, which demonstrates persistent downregulation of CTNNB1 signaling by TCDD (Fig.4D). In sharp contrast to the ventral region, LEF1 expression was detected throughout the anterior basal epithelium, including in developing buds (Fig. 4D). LEF1-stained, TCDD-exposed, E17.5 UGSs clearly illustrate that TCDD inhibits CTNNB1 signaling in a region-specific manner, while also providing additional evidence that CTNNB1-signaling is required in the basal epithelium to induce budding.
TCDD Reduces Wif1 Transcript Abundance in Basal Epithelium of the Ventral UGS
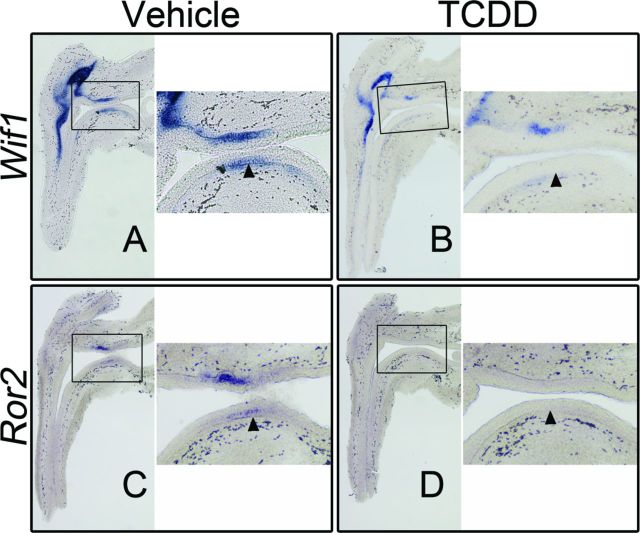
WIF1 is a secreted antagonist of Wnt/CTNNB1 signaling, but has also been identified as a CTNNB1 signaling target gene in several cell lines and tissues, including UGS epithelium (Ha et al., 2012; Mehta et al., 2013). We assessed Wif1 transcript localization and abundance by ISH in vehicle- and TCDD-exposed UGSs. At E16.5, strong mesenchymal staining was detected in both control and TCDD-exposed UGSs; however, Wif1 transcription in the mesenchyme is not regulated by CTNNB1 signaling (Keil et al., 2012). In control UGSs, Wif1 transcript was also detected in basal epithelium of both the anterior and ventral UGS, as well as the epithelium of developing buds (Fig. 5A). In age-matched, TCDD-exposed UGSs, Wif1 transcript abundance was decreased in basal epithelium of the ventral UGS, further demonstrating reduced CTNNB1 signaling after TCDD exposure (Fig. 5B).
FIG. 5.
TCDD reduces transcript abundance of known, Wif1, and putative, Ror2, CTNNB1 signaling target genes in ventral UGS epithelium. C57BL/6J mouse embryos were exposed in utero to vehicle or TCDD (5 μg/kg dam, po) at E15.5 and UGSs were harvested at E16.5. UGSs were sectioned along the sagittal plane and are shown with the urethra on the left pointing down and the bladder on the right. Images are representative of three (Ror2) and six (Wif1) litter-independent UGSs per treatment group. Within each panel, the boxed area in the left image is enlarged on the right. The purple stain marks transcript localization as assessed by ISH and arrowheads point to ventral basal epithelial cells. In vehicle-exposed UGSs, Wif1 transcript is detectable in the ventral epithelium in the same region where nuclear CTNNB1 and LEF1 expression is observed (A). Wif1 RNA abundance is decreased in the ventral epithelium in TCDD-exposed UGSs (B). Similar to Wif1, Ror2 transcript is observed in the ventral epithelium of vehicle-exposed UGSs (C). Exposure to TCDD reduces abundance of Ror2 RNA (D).
TCDD Reduces RNA Abundance for Ror2, a Putative CTNNB1 Signaling Target Gene, in Ventral UGS Epithelium
ROR2 is a single-pass transmembrane receptor that can potentiate multiple Wnt signaling cascades including the Wnt/CTNNB1 signaling cascade (Li et al., 2008; Nishita et al., 2010). At E16.5, in control UGSs, Ror2 is transcribed in the epithelium of all initiated and elongating buds (anterior and dorsolateral regions) as well as in the UGS epithelium of anterior, dorsolateral, and ventral budding regions (Fig. 5C and Schneider et al., unpublished data). Notably, the basal cell layer is generally devoid of staining whereas the intermediate cell layer shows staining (Table 2). In age-matched UGSs exposed to TCDD, little to no Ror2 transcript was observed (Fig. 5D).
Like Lef1 and Wif1, Ror2 might also be a CTNNB1 signaling target gene. UGSs genetically engineered to overexpress CTNNB1 in the epithelium develop clusters of cells that express high levels of CTNNB1 (Supplementary fig. 7A and Mehta et al., 2013). When these UGSs are probed by ISH for known target genes Lef1 and Wif1, strong staining is observed in the CTNNB1-positive cell clusters (Mehta et al., 2013). Similarly, these CTNNB1-positive cell clusters also stain positively for Ror2 suggesting that Ror2 is a CTNNB1 signaling target gene in UGS epithelium (Supplementary fig. 7B).
DISCUSSION
TCDD is a potent teratogen in the mouse, and one of the consequences of in utero exposure is complete inhibition of ventral prostatic budding, culminating in ventral prostate agenesis (Lin et al., 2003; Vezina et al., 2008). We previously established that budding inhibition occurs when TCDD activates AHR in the UGS mesenchyme (Ko et al., 2004a), yet the subsequent alterations to the mesenchymally derived paracrine signaling that induces prostatic budding are not fully understood. Wnt signaling is required for prostate development, and given that most Wnts are expressed in the UGS (Mehta et al., 2011; Zhang et al., 2006), it is likely that multiple Wnt signaling cascades are active during the budding process. Importantly, a well-regulated balance must be attained as both downregulation and upregulation of Wnt signaling can impair normal prostatic bud formation (Allgeier et al., 2008; Branam et al., 2013, Francis et al., 2013; Huang et al., 2009; Mehta et al., 2013; Simons et al., 2012). Recently, we and others have demonstrated that exposure to TCDD can downregulate the Wnt/CTNNB1 signaling cascade in vitro (Branam et al., 2013; Hrubá et al. 2011; Procházková et al., 2011). In this study, we assessed Wnt signaling in vivo, using UGSs from both vehicle- and TCDD-exposed male mouse embryos. We found that (1) Wnt signaling is active in the UGS at E16.5 as we detected RNA from a majority of the 46 genes surveyed; (2) in control UGSs, CTNNB1 signaling is activated in the ventral epithelium around E16.5, just before ventral budding initiation; (3) in utero TCDD exposure altered RNA abundance for five of the 46 genes surveyed by ISH; and (4) TCDD blocked activation of CTNNB1 signaling.
TCDD Prevents Accumulation of NP-CTNNB1 and Activation of CTNNB1 Signaling
Around E16.5, just prior to ventral bud initiation, NP-CTNNB1 begins to accumulate and translocate into the nucleus of basal epithelial cells of the ventral UGS. Coincident with nuclear localization of NP-CTNNB1 is an upregulation of LEF1 expression. Lef1 has previously been identified as a CTNNB1 target gene in the UGS epithelium (Branam et al., 2013; Francis et al., 2013; Mehta et al., 2013), and, correspondingly, we never observed epithelial LEF1 expression without CTNNB1 nuclear localization. Though these data do not elucidate the activating stimulus that prevents CTNNB1 phosphorylation, they do demonstrate that there is active signaling through CTNNB1 in the basal epithelium of the ventral UGS prior to budding.
In utero exposure to TCDD at E15.5 caused a pronounced decrease in NP-CTNNB1 accumulation and a concomitant decrease in expression and transcript abundance, respectively, of CTNNB1 target genes Lef1 and Wif1 in the ventral epithelium of UGSs at E16.5. Furthermore, Ror2, which is a putative CTNNB1 target gene in the UGS epithelium, also demonstrated decreased transcript abundance after exposure to TCDD. We and others have demonstrated that knockout of Ctnnb1 in the UGS prevents prostatic budding; therefore, this downregulation of CTNNB1 signaling by in utero TCDD exposure could explain why ventral buds do not develop (Francis et al., 2013; Lin et al., 2012; Mehta et al., 2013; Simons et al., 2012). We attempted to restore ventral budding to the UGSs of TCDD-exposed embryos, by overexpressing a stabilized form of CTNNB1 in the UGS epithelium, but we found that, similar to other reports, excess CTNNB1 signaling is also capable of inhibiting prostatic budding (Francis et al., 2013; Lin et al., 2012; Mehta et al., 2013).
TCDD Alters RNA Abundance and In Situ Localization of Several Wnt Signaling Modulators
ISH staining allowed us to detect changes in both relative abundance and localization of RNA after exposure to TCDD. TCDD altered RNA abundance and localization for five genes, generally increasing staining for Wnt10a and Wnt16, while simultaneously decreasing staining for Ror2, Rspo2, and Wif1. However, there are several caveats to consider when interpreting these ISH findings: (1) we assessed RNA abundance and localization at only one developmental time point (E16.5); (2) initiation of transcription for Ror2, Rspo2, and Wif1 in the ventral UGS appears to be around E16.5; and (3) we previously reported that in utero exposure to TCDD delays prostatic budding in the anterior and dorsolateral regions of the UGS by about 24 h (Lin et al., 2003). Therefore, we cannot say if the decreases in RNA abundance are sustained indefinitely, or are due to a TCDD-induced developmental delay. Nonetheless, delayed Wnt signaling could be sufficient to cause ventral prostate agenesis because Simons et al. (2012) have proposed that CTNNB1 signaling is required between E14.5 and E16.5 for normal prostatic differentiation to occur. TCDD could delay activation of CTNNB1 signaling until this developmental window has passed.
Significance of Wnt16 Transcription in the Ventral UGS after Exposure to TCDD
TCDD-induced activation of AHR in the UGS mesenchyme is the initial stimulus that causes prostatic budding failure in the ventral UGS epithelium (Ko et al., 2004a). We show here that TCDD subsequently blocks CTNNB1 signaling in the ventral basal epithelium. We have also presented data that make Wnt16 a putative link between the mesenchymal site of action for TCDD and the epithelial outcome of downregulated CTNNB1 signaling. At E16.5 in control UGSs, Wnt16 transcript is detected only in the mesenchyme around the urethra, but in age-matched TCDD-exposed UGSs, transcript is also detected in the ventral UGS mesenchyme. This finding suggests TCDD induces ectopic WNT16 expression in the ventral mesenchyme, thereby generating a known paracrine signal (Sun et al., 2012) that could alter Wnt signaling in the ventral epithelium. Multiple studies have shown that WNT16 either does (Jiang et al., 2014; Mazieres et al., 2005; Sun et al., 2012) or does not (Binet et al., 2009; Clements et al., 2011; Lu et al., 2004; Nygren et al., 2009; Teh et al., 2007) activate CTNNB1 signaling, with the end result likely dependent upon which other regulators of Wnt signaling are also present in the tissue. There are at least two plausible mechanisms by which WNT16 could alter Wnt signaling so as to inhibit CTNNB1 signaling: by out-competing ligands that activate CTNNB1 signaling for cell surface receptors or by activating a signaling cascade that prevents CTNNB1 stabilization and accumulation. Thus, WNT16 is an attractive candidate as a protein that has a direct role in inhibiting ventral budding because TCDD induces its expression in the ventral UGS mesenchyme, and WNT16 could be capable of blocking activation of CTNNB1 signaling.
Significance of Reduced Rspo2 Transcript Abundance after Exposure to TCDD
At E16.5, Rspo2 is transcribed in a ring around the epithelium of the anterior and ventral budding regions. In sagittal sections, this appears as staining in the anterior mesenchyme and ventral mesenchymal pads, which are subcompartments of the UGS mesenchyme known to produce signaling factors essential for ventral prostate development (reviewed in Thomson, 2008). ISH data from E17.5 UGSs (Mehta et al., 2011) show that staining for Rspo2 transcript is more intense and covers a moderately larger area, suggesting that Rspo2 transcription starts not long before E16.5. Therefore, the reduced Rspo2 transcript abundance observed at E16.5 in TCDD-exposed UGSs could be due to a delay in the onset of Rspo2 transcription rather than a sustained transcriptional decrease. In vitro experiments showed reduced Rspo2 transcript abundance in UGSs cultured with TCDD for two days, but no difference was observed in control versus TCDD-exposed UGSs after three or four days in culture, suggesting TCDD delays the onset of Rspo2 transcription (Branam et al., 2013).
RSPO2 is a paracrine signaling protein that can activate CTNNB1 signaling (reviewed in Jin and Yoon, 2012). Given the robust Rspo2 transcription observed in the ventral mesenchymal pads, it is likely that the ventral epithelium is the target tissue for RSPO2 protein. In vitro, RSPO2 promotes prostatic budding. UGSs cultured with exogenous RSPO2 developed significantly more buds compared with controls. Furthermore, although UGSs cultured with TCDD had reduced bud number and epithelial CTNNB1 signaling compared with control UGSs, culture with TCDD plus RSPO2 restored bud number to that of controls and increased epithelial CTNNB1 signaling (Branam et al., 2013). Other TCDD-induced developmental defects could also be linked to the downregulaton of RSPO2 expression because Rspo2 knockout mice develop with a cleft palate and hypomorphic lungs which are also teratogenic outcomes of in utero TCDD exposure (Couture et al., 1990; Kransler et al., 2009; Yamada et al., 2009). Thus, like Wnt16, Rspo2 is another attractive candidate gene with a potential role in the impaired ventral prostatic budding phenotype. However, opposite of Wnt16, it is the TCDD-induced downregulation of RSPO2 in UGS mesenchyme that could directly result in decreased CTNNB1 signaling in the epithelium.
TCDD and Wnt Signaling
Wnt signaling encompasses a number of different signaling cascades and is capable of regulating diverse biological responses. How Wnt signaling affects a biological response seems to depend on the combination of Wnt ligands, receptors, co-receptors, agonists, and antagonists that are present. Therefore, TCDD-induced inhibition of ventral prostatic budding might not be due to the altered expression of any one gene in the Wnt signaling pathway, but rather, due to the multiple changes we have reported here that, cumulatively, alter the well-regulated balance of all Wnt signaling cascades.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES01332). Funding for open access charge: National Institute of Environmental Health Sciences.
Supplementary Material
Acknowledgments
We would like to thank Joan Palmer for her assistance with manuscript formatting.
REFERENCES
- Abbott B. D., Lin T. M., Rasmussen N. T., Albrecht R. M., Schmid J. E., Peterson R. E. Lack of expression of EGF and TGF-α in the fetal mouse alters formation of prostatic epithelial buds and influences the response to TCDD. Toxicol. Sci. 2003;76:427–436. doi: 10.1093/toxsci/kfg238. [DOI] [PubMed] [Google Scholar]
- Abler L. L., Mehta V., Keil K. P., Joshi P. S., Flucus C. L., Hardin H. A., Schmitz C. T., Vezina C. M. A high throughput in situ hybridization method to characterize mRNA expression patterns in the fetal mouse lower urogenital tract. J. Vis. Exp. 2011;54:e2912. doi: 10.3791/2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgeier S. H., Lin T. M., Vezina C. M., Moore R. W., Fritz W. A., Chiu S. Y., Zhang C., Peterson R. E. WNT5A selectively inhibits mouse ventral prostate development. Dev. Biol. 2008;324:10–17. doi: 10.1016/j.ydbio.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet R., Ythier D., Robles A. I., Collado M., Larrieu D., Fonti C., Brambilla E., Brambilla C., Serrano M., Harris C. C., et al. WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2009;69:9183–9191. doi: 10.1158/0008-5472.CAN-09-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Fenton S. E. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branam A. M., Davis N. M., Moore R. W., Schneider A. J., Vezina C. M., Peterson R. E. TCDD inhibition of canonical Wnt signaling disrupts prostatic bud formation in mouse urogenital sinus. Toxicol. Sci. 2013;133:42–53. doi: 10.1093/toxsci/kft027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements W. K., Kim A. D., Ong K. G., Moore J. C., Lawson N. D., Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–225. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture L. A., Abbott B. D., Birnbaum L. S. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: recent advances toward understanding the mechanism. Teratology. 1990;42:619–627. doi: 10.1002/tera.1420420606. [DOI] [PubMed] [Google Scholar]
- Craig Z. R., Wang W., Flaws J. A. Endocrine-disrupting chemicals in ovarian function: effect on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- Cunha G. R. Mesenchymal-epithelial interactions: Past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Francis J. C., Thomsen M. K., Taketo M. M., Swain A. β-catenin Is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9:e1003180. doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha A., Perez-Iratxeta C., Liu H., Mears A. J., Wallace V. A. Identification of Wnt/beta-catenin modulated genes in the developing retina. Mol. Vis. 2012;18:645–656. [PMC free article] [PubMed] [Google Scholar]
- Hrubá E., Vondráček J., Líbalová H., Topinka J., Bryja V., Souček K., Machala M. Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol. Lett. 2011;206:178–188. doi: 10.1016/j.toxlet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Huang L., Pu Y., Hu W. Y., Birch L., Luccio-Camelo D., Yamaguchi T., Prins G. S. The role of Wnt5a in prostate gland development. Dev. Biol. 2009;328:188–199. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Von den Hoff J. W., Torensma R., Meng L., Bian Z. Wnt16 is involved in intramembranous ossification and suppresses osteoblast differentiation through the Wnt/β-catenin pathway. J. Cell. Physiol. 2014;229:384–392. doi: 10.1002/jcp.24460. [DOI] [PubMed] [Google Scholar]
- Jin Y. R., Yoon J. K. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. 2012;44:2278–2287. doi: 10.1016/j.biocel.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K., Kobayashi Y., Ohtake F., Ikuta T., Matsushima Y., Mimura J., Pettersson S., Pollenz R. S., Sakaki T., Hirokawa T., et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil K. P., Mehta V., Branam A. M., Abler L. L., Buresh-Stiemke R. A., Joshi P. S., Schmitz C. T., Marker P. C., Vezina C. M. Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology. 2012;153:6091–6103. doi: 10.1210/en.2012-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N. I. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaishvili G., Simkova D., Makharoblidze E., Trtkova K., Kolar Z., Bouchal J. Wnt signaling in prostate development and carcinogenesis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011;155:11–18. doi: 10.5507/bp.2011.016. [DOI] [PubMed] [Google Scholar]
- King-Heiden T. C., Mehta V., Xiong K. M., Lanham K. A., Antkiewicz D. S., Ganser A., Heideman W., Peterson R. E. Reproductive and developmental toxicity of dioxin in fish. Mol. Cell. Endocrinol. 2012;354:121–138. doi: 10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr S., Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- Ko K., Moore R. W., Peterson R. E. Aryl hydrocarbon receptors in urogenital sinus mesenchyme mediate the inhibition of prostatic epithelial bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 2004a;196:149–155. doi: 10.1016/j.taap.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Ko K., Theobald H. M., Moore R. W., Peterson R. E. Evidence that inhibited prostatic epithelial bud formation in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed C57BL/6J fetal mice is not due to interruption of androgen signaling in the urogenital sinus. Toxicol. Sci. 2004b;79:360–369. doi: 10.1093/toxsci/kfh111. [DOI] [PubMed] [Google Scholar]
- Kransler K. M., McGarrigle B. P., Swartz D. D., Olson J. R. Lung development in the Holtzman rat is adversely affected by gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2009;107:498–511. doi: 10.1093/toxsci/kfn235. [DOI] [PubMed] [Google Scholar]
- Leng L., Chen X., Li C.-P., Luo X.-Y., Tang N.-J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure and prostate cancer: A meta-analysis of cohort studies. Public Health. 2014;128:207–213. doi: 10.1016/j.puhe.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Li C., Chen H., Hu L., Xing Y., Sasaki T., Villosis M. F., Li J., Nishita M., Minami Y., Minoo P. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol. Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-M., Bai J., Peterson R.E. Early stabilization of β-catenin in urogenital sinus (UGS) epithelia disrupts mouse ventral prostate (VP) development. Toxicol. Sci. 2012;126(Suppl. 1) 2357 (Abstract) [Google Scholar]
- Lin T.-M., Ko K., Moore R. W., Simanainen U., Oberley T. D., Peterson R.E. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol. Sci. 2002;68:479–487. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- Lin T.-M., Rasmussen N. T., Moore R. W., Albrecht R. M., Peterson R. E. Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2003;76:171–181. doi: 10.1093/toxsci/kfg218. [DOI] [PubMed] [Google Scholar]
- Lu D., Zhao Y., Tawatao R., Cottam H. B., Sen M., Leoni L. M., Kipps T. J., Corr M., Carson D. A. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M. K. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012;7:1–15. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. M., DeVito M. J., Birnbaum L. S., Walker N. J. Toxicology of dioxin and dioxin-like compounds. In: Schecter A., Gasiewicz T., editors. Dioxins and Health. Hoboken, NJ: John Wiley & Sons, Inc; 2003. pp. 137–157. [Google Scholar]
- Mathew L. K., Sengupta S. S., Ladu J., Andreasen E. A., Tanguay R. L. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 2008;22:3087–3096. doi: 10.1096/fj.08-109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J., You L., He B., Xu Z., Lee A. Y., Mikami I., McCormick F., Jablons D. M. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene. 2005;24:5396–5400. doi: 10.1038/sj.onc.1208568. [DOI] [PubMed] [Google Scholar]
- Mehta V., Abler L. L., Keil K. P., Schmitz C. T., Joshi P. S., Vezina C. M. Atlas of Wnt and R-spondin gene expression in the developing male mouse lower urogenital tract. Dev. Dyn. 2011;240:2548–2560. doi: 10.1002/dvdy.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Schmitz C. T., Keil K. P., Joshi P. S., Abler L. L., Lin T. M., Taketo M. M., Sun X., Vezina C. M. Beta-catenin (CTNNB1) induces Bmp expression in urogenital sinus epithelium and participates in prostatic bud initiation and patterning. Dev. Biol. 2013;376:125–135. doi: 10.1016/j.ydbio.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M., Enomoto M., Yamagata K., Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–354. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Nygren M. K., Døsen-Dahl G., Stubberud H., Wälchli S., Munthe E., Rian E. β-catenin is involved in N-cadherin-dependent adhesion, but not in canonical Wnt signaling in E2A-PBX1-positive B acute lymphoblastic leukemia cells. Exp. Hematol. 2009;37:225–233. doi: 10.1016/j.exphem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Procházková J., Kabátková M., Bryja V., Umannová L., Bernatík O., Kozubík A., Machala M., Vondráček J. The interplay of the aryl hydrocarbon receptor and beta-catenin alters both AhR-dependent transcription and Wnt/beta-catenin signaling in liver progenitors. Toxicol. Sci. 2011;122:349–360. doi: 10.1093/toxsci/kfr129. [DOI] [PubMed] [Google Scholar]
- Simons B. W., Hurley P. J., Huang Z., Ross A. E., Miller R., Marchionni L., Berman D. M., Schaeffer E. M. Wnt signaling though beta-catenin is required for prostate lineage specification. Dev. Biol. 2012;371:246–255. doi: 10.1016/j.ydbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulentic C. E. W., Kaminski N. E. The long winding road toward understanding the molecular mechanisms for B-cell suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2011;120(Suppl. 1):S171–S191. doi: 10.1093/toxsci/kfq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Campisi J., Higano C., Beer T. M., Porter P., Coleman I., True L., Nelson P. S. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh M-T., Blaydon D., Ghall L. R., Briggs V., Edmunds S., Pantazi E., Barnes M. R., Leigh I. M., Kelsell D. P., Philpott M. P. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J. Cell Sci. 2007;120:330–339. doi: 10.1242/jcs.03329. [DOI] [PubMed] [Google Scholar]
- Thomson A. A. Mesenchymal mechanisms in prostate organogenesis. Differentiation. 2008;76:587–598. doi: 10.1111/j.1432-0436.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- Tian Y. Ah receptor and NF-κB interplay on the stage of epigenome. Biochem. Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Vezina C. M., Allgeier S. H., Moore R. W., Lin T. M., Bemis J. C., Hardin H. A., Gasiewicz T. A., Peterson R. E. Dioxin causes ventral prostate agenesis by disrupting dorsoventral patterning in developing mouse prostate. Toxicol. Sci. 2008;106:488–496. doi: 10.1093/toxsci/kfn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C. M., Hardin H. A., Moore R. W., Allgeier S. H., Peterson R. E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits fibroblast growth factor 10-induced prostatic bud formation in mouse urogenital sinus. Toxicol. Sci. 2010;113:198–206. doi: 10.1093/toxsci/kfp226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C. M., Lin T-M., Peterson R. E. AHR signaling in prostate growth, morphogenesis, and disease. Biochem. Pharmacol. 2009;77:566–576. doi: 10.1016/j.bcp.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada W., Nagao K., Horikoshi K., Fujikura A., Ikeda E., Inagaki Y., Kakitani M., Tomizuka K., Miyazaki H., Suda T., et al. Craniofacial malformation in R-spondin2 knockout mice. Biochem. Biophys. Res. Commun. 2009;381:453–458. doi: 10.1016/j.bbrc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Zhang T. J., Hoffman B. G., Ruiz de Algara T., Helgason C. D. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expr. Patterns. 2006;6:310–324. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.