We conducted a phase II study evaluating the efficacy and toxicity of pazopanib, a broad spectrum TKI inhibiting KIT, VEGFRs (-1, -2, and -3), and PDGFR (-α and-β) in patients with advanced GIST following failure of at least imatinib and sunitinib. Pazopanib as a single agent has marginal activity in unselected heavily pretreated patients with advanced GIST.
Keywords: GIST, KIT, pazopanib, tyrosine kinase inhibitors
Abstract
Background
Advanced GISTs are incurable, but often treatable for years with tyrosine kinase inhibitors (TKIs). The majority of GISTs harbor an oncogenic activating mutation in KIT or PDGFRA. Inhibition of this activating mutation with TKIs most often leads to durable disease control for many patients. However, almost all patients develop resistance to these TKIs, typically due to the development of secondary mutations, heralding the need for new therapeutic options. We conducted a phase II study evaluating the efficacy and toxicity of pazopanib, a broad spectrum TKI inhibiting KIT, VEGFRs (−1, −2, and −3), and PDGFR (-α and-β) in patients with advanced GIST following failure of at least imatinib and sunitinib.
Methods
Patients received pazopanib 800 mg orally once daily. All patients were assessed for efficacy with CT scans every 8 weeks (two cycles). Patients continued pazopanib until progression or unacceptable toxicity. The primary end point was the 24-week nonprogression [complete response+partial response+stable disease (SD)] rate (NPR) per RECIST 1.1. Secondary end points included PFS, OS, and toxicity.
Results
Between August 2011 and September 2012, a total of 25 patients were treated at two institutions. Median number of prior therapy was 3 (range 2–7). A total of 90 cycles of pazopanib were administered, with a median of two cycles (range 1 to 17+) per patient. Best response of SD at any time was observed in 12 (48%) patients. The NPR was 17% [95% confidence interval (CI) 4.5–37]. All but one patient discontinued protocol either due to PD (n = 19) or intolerance (n = 4). One patient with succinate dehydrogenase (SDH)-deficient GIST exhibited continuing disease control after 17 cycles. The median PFS for the entire cohort was 1.9 months (95% CI 1.6–5.2), and the median OS was 10.7 months (95% CI 3.9–NR).
Conclusions
Pazopanib was reasonably well tolerated with no unexpected toxicities. Pazopanib as a single agent has marginal activity in unselected heavily pretreated patients with advanced GIST.
introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. Patients with metastases are incurable; however, the outcome for these patients has improved dramatically over the past 13 years due to the use of tyrosine kinase inhibitors (TKI) including imatinib mesylate (Gleevec, Novartis, Basel, Switzerland) (IM), sunitinib (Sutent, Pfizer, New York, NY) (SU), and most recently regorafenib (Stivarga, Bayer, Berlin Germany). The majority of GISTs express aberrantly activated transmembrane TK receptors, either KIT or PDGFRα. At the time of the development of this trial, IM and SU were the only two agents approved by the US FDA for the treatment of metastatic or unresectable GIST as first- and second-line therapies, respectively. These drugs have revolutionized the clinical outcomes of these patients, but unfortunately, primary and secondary resistance to both IM and SU eventually develop in virtually all patients, after a median of 24 months in first-line and 6–9 months in second-line setting.
Pazopanib (Votrient, GlaxoSmithKline, Philadelphia, PA) is an oral small molecule TKI, which inhibits KIT as well as VEGFRs (−1, −2, and −3) and PDGFRs (-α and-β). This drug has been recently approved for the treatment of patients with previously treated metastatic soft-tissue sarcomas, excluding GIST, which was not included in the soft-tissue sarcoma clinical trials. In the current study, we evaluated the safety and efficacy of pazopanib in patients with metastatic and/or unresectable GIST following objective failure or intolerance to at least prior IM and SU. The primary objective of this study was to assess progression-free rate (PFR) [defined as the sum of complete response (CR), partial response (PR), and stable disease (SD) per RECIST 1.1] at 24 weeks. Secondary objectives included the 24-week progression-free survival (PFS) rate, overall survival (OS), as well as safety and tolerability.
patients and methods
patient eligibility
Patients with metastatic or unresectable GIST who progressed through or were intolerant of IM and SU were eligible. Additional eligibility criteria included age ≥18 years, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, measurable disease based on RECIST 1.1 criteria, adequate organ function as follows: absolute neutrophil count ≥1500/µl, hemoglobin ≥9 g/dl, platelets ≥100 000/µl, prothrombin time ≤1.2 × upper limit of normal (ULN), total bilirubin ≤1.5 × ULN, AST and ALT ≤2.5 ULN, serum creatinine ≤1.8 mg/dl, urine to protein (UPC) ratio <1. Patient with QT interval (QTc) >480 ms, or those on antiarrythmics or medications known to prolong QTc interval were excluded. Additional exclusion criteria included any of the following vascular events within 6 months before study: coronary angioplasty, stenting or bypass graft surgery, myocardial infarction, unstable angina, symptomatic peripheral vascular disease, class III or IV congestive heart failure as defined by the New York Heart Association (NYHA), and uncontrolled hypertension defined by systolic blood pressure ≥140 mmHg and diastolic ≥90 mmHg. Also, patients with a cerebral vascular event, thrombosis, or bleeding within 6 months before first dose of drug, and those taking strong CYP3A4 inhibitors were excluded. There was no limit to the number of prior treatments. All patients signed an informed consent approved by the institutional review boards at the participating centers.
treatment and measurement of effect
The study planned to administer pazopanib at 800 mg daily for 28 days without break (1 cycle = 28 days) to all patients, continuing unless unacceptable toxicity or disease progression occurred. Dose reductions were allowed in 200 mg increments based on toxicity.
Patients were monitored with complete blood counts (weekly for cycle 1), serum chemistries, and liver enzymes on day 1 of every cycle. Thyroid function tests and UPC were recorded at baseline and every two cycles. All patients had an electrocardiogram at baseline and on day 1, cycle 2 for measurement of QTc interval. The left ventricular ejection fraction was measured at baseline and end of study by an echocardiogram or a multigated acquisition scan. All patients were assessed for toxicity before initiation of the subsequent cycle and graded based on the NCI Common Toxicity Criteria, version 4. The response to treatment was evaluated every two cycles by computed tomography (CT) or magnetic resonance imaging (MRI), and was based on RECIST 1.1.
statistical considerations
The primary end point was the PFR (CR+PR+SD) at 24 weeks per RECIST 1.1. The secondary end points included PFS, OS, and safety and tolerability.
PFR and response rates were calculated with a 90% lower-confidence bound. The null hypothesis was that the PFR at 24 weeks is no larger than 24%. An interim analysis was planned when 15 patients were assessable for PFS; at least four patients were required to be alive and progression free to continue to the second stage; if the study was not stopped, a total of 15 patients would be required to be alive and progression-free at 24 weeks in order to declare statistical significance at the final analysis. With these definitions, this study of 40 patients had 90% power if the true PFS rate at 24 weeks was 45% or larger, using a one-sided >significance level of 10%.
results
Between August 2011 and September 2012, a total of 27 patients were enrolled and 25 treated at Stanford Cancer Institute (n = 11) and Dana-Farber Cancer Institute (n = 14). Two patients did not receive study drug after enrollment due to proteinuria (n = 1) and rapidly progressive disease and decline in the ECOG performance status (n = 1). Patient characteristics are listed in Table 1. The median ECOG performance status was 0 (range 0–2). A prior history of hypertension (HTN), either prediagnosis of GIST or while on prior TKI, was noted in 14 patients. Overall, 9 of 25 (36%) patients had two prior TKIs, 10 (40%) had three TKIs and 6 (24%) had more than three prior therapies (including non-TKI agents).
Table 1.
Patient characteristics (n = 25)
| Age | Median 59 (27–72 years) |
| Sex | |
| Male | 15 |
| Female | 10 |
| Race | |
| White | 18 |
| African America | 3 |
| Asian | 1 |
| Unknown | 3 |
| # Prior treatments | Median 3 (2–7) |
| #Prior Sorafenib | 11 |
| # Prior Regorafenib | 6 |
| Months on IM | Median 14 (0.5–50 months) |
| Reason off IM | 21 PD and 4 Tox |
| Months on SU | Median 5 (1–50 months) |
| Reason off SU | 21 PD and 4 Tox |
| Mutational status (n = 16) | |
| Exon 11 | 8 |
| Exon 9 | 2 |
| Exon 18 | 1 |
| WT | 5 |
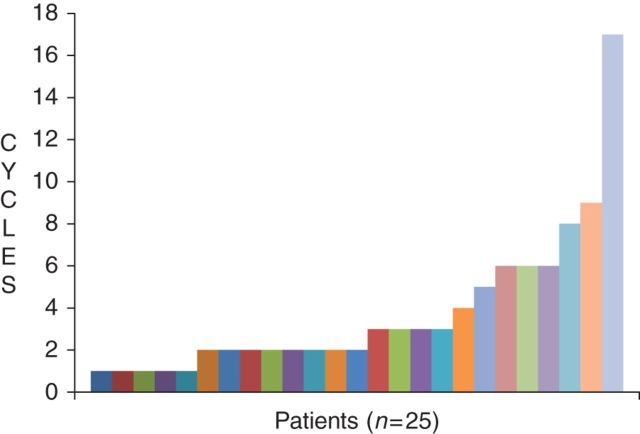
A total of 90 cycles of pazopanib were administered with a median of two cycles per patient (range 1 to 17+), with one patient (SDH-deficient phenotype with ‘wild-type KIT and PDGFRA’ genotype) continuing on study after 17 cycles (Figure 1). Seven patients received more than four cycles of treatment. Best response of SD at any time was observed in 12 (48%) with one patient discontinuing treatment to have surgical resection. Four patients [17% 95% confidence interval (CI) 4.5–37] met the primary end point of PFR at 24 weeks. Five patients were taken off study due to clinical, not radiographic, progression. All but one patient are currently off treatment either due to PD (n = 19) or intolerance (n = 4).
Figure 1.
Number of cycles per patient.
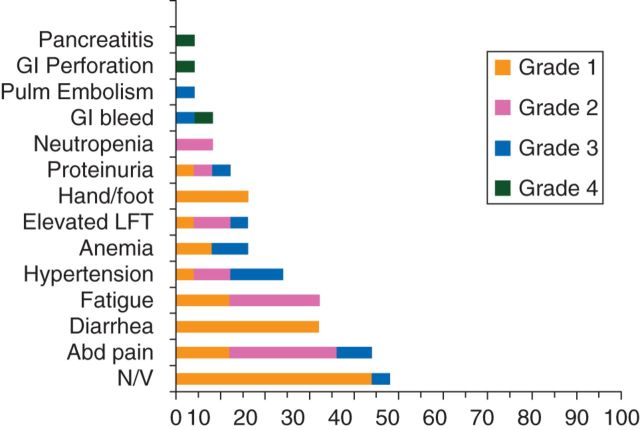
The reasons for intolerance in the four patients were G2 transaminitis, G3 headache, G3 gastrointestinal (GI) symptoms, and G4 GI bleed. Other toxicities occurring in >4% of patients are shown in Figure 2. HTN was well controlled with antihypertensive medication in 17 patients (14 had prior history of HTN). The remaining eight patients did not develop HTN while on pazopanib. Grade 4 toxicities included GI bleed (n = 1), intestinal perforation (n = 1), and pancreatitis (n = 1). Only four patients (16%) developed hand/foot syndrome, and all were grade 1. The patient developing a GI bleed was on day 19 of cycle 1 when she presented with melena. An upper endoscopy showed tumor erosion in to the gastric wall, presumably due to disease progression. One patient developed symptomatic pancreatitis after cycle 2 and was treated conservatively with resolution of symptoms. She was restarted on a lower dose of pazopanib, but opted to be removed from study after cycle 4 with SD when she developed G2 dyspepsia.
Figure 2.
Adverse events.
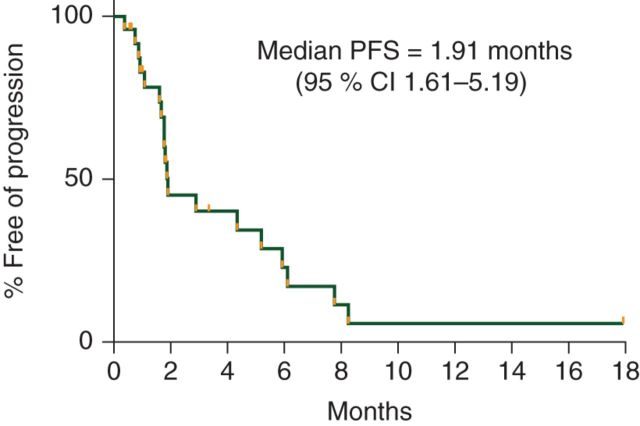
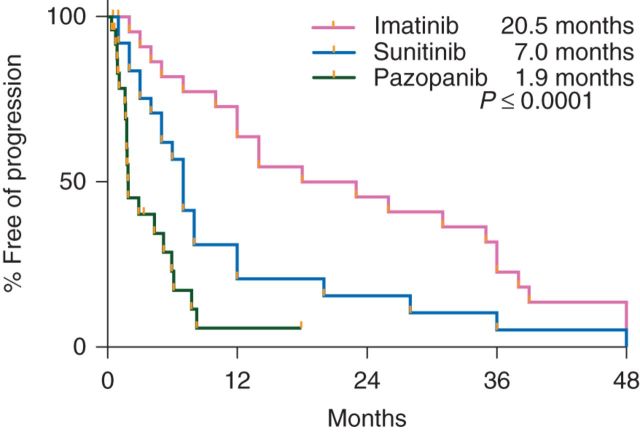
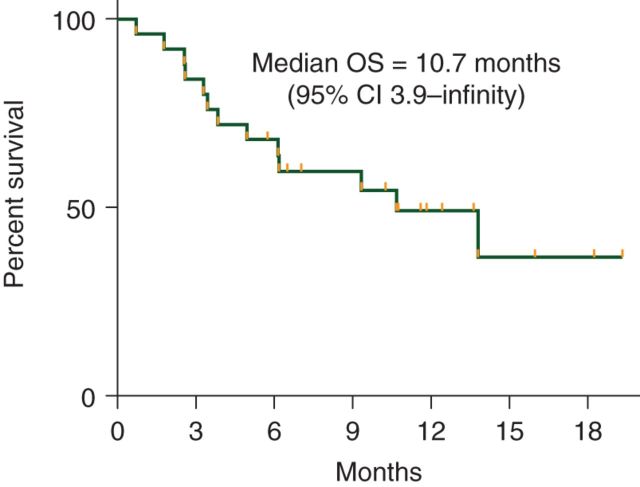
At the interim analysis, only 3 of the first 15 assessable patients were progression-free at 6 months, and therefore, according to the study design, the study was closed to additional enrollment. The median PFS of the total cohort enrolled was 1.9 months (95% CI 1.6–5.2) with a median duration of follow-up of 7 months (range 0.7–19.3 months) in patients alive and progression-free (Figure 3). The PFS of our patients did not differ significantly based on number of prior therapies. The median PFS for our patients during prior treatment with IM, SU, and current pazopanib was 20.5, 7, and 1.9 months, respectively (P < 0.0001) (Figure 4). The median OS was 10.7 months (95% CI 3.9–NR) with a median duration of follow-up of 11.2 months (range 5.8–19.3 months) in patients alive at the time of analysis (Figure 5).
Figure 3.
Progression-free survival for pazopanib.
Figure 4.
Progression-free survival for prior IM, SU, and current pazopanib.
Figure 5.
Overall survival for pazopanib.
Of the two patients with SDH-deficient wild-type GIST, one has experienced a 16% reduction in tumor size and continues on therapy after 17 cycles, and another patient was taken off study after one cycle due to toxicity. An additional patient with unknown KIT or PDGFRA mutational status exhibited a 17% reduction in tumor size.
discussion
Based on high-quality prospective trials, the US FDA has approved IM, SU, and more recently, regorafenib as therapy for advanced GIST, in the first-, second-, and third-line settings, respectively. In this study, we evaluated the efficacy and toxicity of pazopanib in patients with advanced GIST following failure of at least IM and SU. The trial was initially designed for third-line therapy. However, 16 of 25 of patients had been treated with more than two prior TKIs before study entry. The nine patients in our study who were treated with pazopanib as third-line therapy received a median of two cycles [1–9] which is comparable with nilotinib, but inferior to regorafenib [1–3]. The fact that our patients were heavily pretreated with multiple TKIs may have affected the outcome of this trial possibly due to multiple secondary mutations. However, the PFS of our patients did not differ significantly based on number of prior therapies which compares to historical controls for IM and SU. The recently reported randomized phase III GRID study comparing regorafenib to placebo in this patient population resulted in a median PFS 4.8 months compared with 0.9 months in placebo [4]. In our study, the median PFS was 1.9 months with 48% of our patients achieving stabilization of disease at any time point. There were no partial responses (PRs) seen in our study; however, the rate of objective response by RECIST for third or fourth line therapy is <10% [1, 2, 4]. In addition, the recent randomized study evaluating the retreatment with IM versus placebo after initial IM failure resulted in a median PFS of 1.8 months in the IM arm and 0.9 months in the placebo arm [5]. Although we are unable to directly compare results across studies, patients treated with pazopanib had a similar median PFS that IM retreatment but a longer median PFS than placebo’; however, whether this is clinically meaningful remains unknown.
The vast majority of GISTs have activating mutations in KIT and a smaller proportion in PDGFRA. Approximately 10%–15% of GISTs lack mutations in KIT or PDGFRA, and has been designated as ‘wild-type’ GIST. A subgroup of wild-type GIST is deficient in one or more subunits of succinate dehydrogenase (SDH) of the mitochondrial inner membrane protein [6]. Interestingly, in the current study, one patient with SDH-deficient GIST continues on pazopanib past 17 cycles. This patient had progression of disease on sixth line (sorafenib) therapy upon study entry and experienced a 20% reduction in tumor per RECIST 1.1, suggesting some efficacy of pazopanib on the disease process. In this case, it is unclear what the mechanism of pazopanib antitumor activity may be since SDH-deficient GIST has no activating KIT/PDGFRA mutations. We can only speculate that the antiangiogenesis property of pazopanib may play some role. It is shown that hypoxia-induced factor (HIF-1α) is overexpressed in SDH-deficient GIST and can subsequently lead to increased angiogenesis through regulation of VEGF [7, 8]. HIF-1α is also associated with development of metastatic disease in GIST [9].
The current second and third-line TKIs approved for GIST, SU and regorafenib, are potent VEGFR inhibitors in addition to inhibitors of KIT. Similarly, pazopanib is a potent VEGFR inhibitor in addition to KIT inhibitor. The role of VEGF in KIT mutant GIST has not been established. In a small study by Wang et al., serum vascular endothelial growth factor (VEGF) levels were higher in the high-risk GIST patients [10]. Another study showed a positive correlation between high VEGF expression and poor survival independent by KIT genotype [11]. All of our patients enrolled, previously received the antiangiogenesis agent, SU. In addition, 15 of 25 (60%) patients were previously exposed to a second VEGFR inhibitor, sorafenib or regorafenib (2 patients had exposure to both drugs). However, four of the six patients who had more than four cycles of pazopanib had prior exposure to sorafenib or regorafenib, making it difficult to assert a relationship between prior VEGFR inhibition and response in this small study. In one study, patients treated with SU who developed hypertension had a better outcome than those who remained normotensive [12]. The authors concluded that one possible explanation could be that hypertension was a marker of overall drug exposure in these patients, rather than an antitumor impact of VEGFR inhibition. It is likely that the benefit observed in patients with KIT mutant GIST treated with SU and regorafenib after failure to IM is due to the differences in the spectrum of KIT inhibition. The minimal activity we have seen in the current trial may be related to a lack of sufficient KIT inhibition in this heavily pretreated population. Future studies may consider combination of agents to optimize inhibition of the activated KIT pathway in this disease.
In conclusion, our study demonstrates that single-agent pazopanib has marginal activity in heavily pretreated patients with advanced GIST refractory or intolerant to IM and SU. Plans for future studies with pazopanib in combination with other agents in this patient population are perhaps warranted nonetheless, given that pazopanib exhibits very potent KIT inhibition in preclinical laboratory tests.
funding
This work was supported in part by GlaxoSmithKline investigator-initiated trial fund and by Ludwig Center at Dana-Farber/Harvard Cancer Center.
disclosure
KNG, VMV, AK, GAF, JAM, AMM, JEB, DDA—no COI. GDD: Consultant—Bayer, Novartis, Pfizer, Sanofi-Aventis, GlaxoSmithKline, Ariad, Koltan Pharmaceuticals, Foundation Medicine, Blueprint Medicines.
Research Support—Bayer, Novartis, Pfizer, Sanofi-Aventis, GlaxoSmithKline.
Equity—Koltan Pharmaceuticals, Blueprint Medicines. SG:
Consultant—GlaxoSmithKline, Ariad, Pfizer, Bayer.
Research Support—GlaxoSmithKline, Bayer, Ariad, Novartis. AJW: Consultant and research support—Novartis, Pfizer.
references
- 1.Cauchi C, Somaiah N, Engstrom PF, et al. Evaluation of nilotinib in advanced GIST previously treated with imatinib and sunitinib. Cancer Chemother Pharmacol. 2012;69(4):977–982. doi: 10.1007/s00280-011-1785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawaki A, Nishida T, Doi T, et al. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117(20):4633–4641. doi: 10.1002/cncr.26120. [DOI] [PubMed] [Google Scholar]
- 3.George S, Wang Q, Heinrich MC, et al. A multicenter phase II trial efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib. J Clin Oncol. 2012;30(19):2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang YK, Ryu MH, Yoo C, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70453-4. October 18 [epub ahead of print], doi: 10.1016/S1470-2045(13)70453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35(11):1712–1721. doi: 10.1097/PAS.0b013e3182260752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miettinen M, Killian JK, Wang ZF, et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37(2):234–240. doi: 10.1097/PAS.0b013e3182671178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akahashi R, Tanaka S, Hiyama T, et al. Hypoxia-inducible factor-la expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797–802. [PubMed] [Google Scholar]
- 9.Chen WT, Huang CJ, Wu MT, et al. Hypoxia-inducible factor-1a is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35(4):207–213. doi: 10.1093/jjco/hyi067. [DOI] [PubMed] [Google Scholar]
- 10.Wang TB, Qiu WS, Wei B, et al. Serum vascular endothelial growth factor and angiogenesis are related to the prognosis of patients with gastrointestinal stromal tumors. Ir J Med Sci. 2009;178(3):315–320. doi: 10.1007/s11845-009-0315-7. [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe JC, Lazar AJ, Yang D, et al. Association of intratumoral vascular endothelial growth factor expression and clinical outcome for patients with gastrointestinal stromal tumors treated with imatinib mesylate. Clin Cancer Res. 2007;13(22):6727–6734. doi: 10.1158/1078-0432.CCR-07-0895. [DOI] [PubMed] [Google Scholar]
- 12.George S, Reichardt P, Lechner P, et al. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2012;12:3180–3187. doi: 10.1093/annonc/mds179. [DOI] [PubMed] [Google Scholar]