Abstract
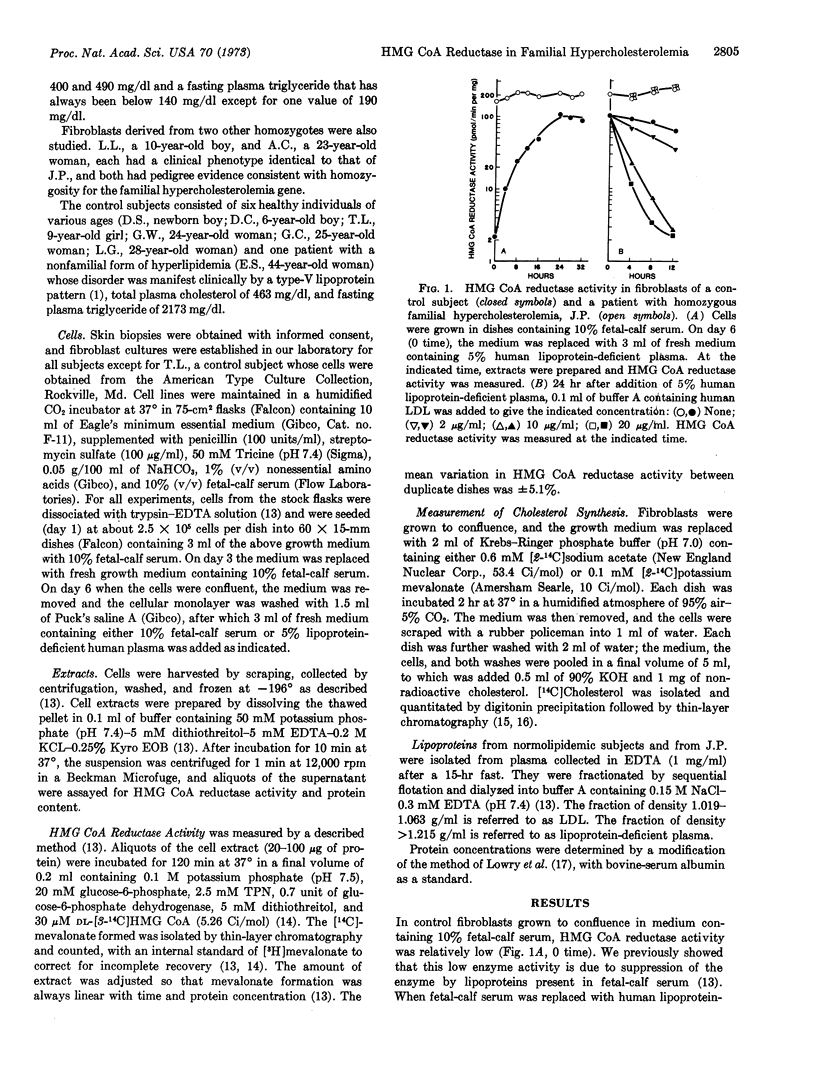
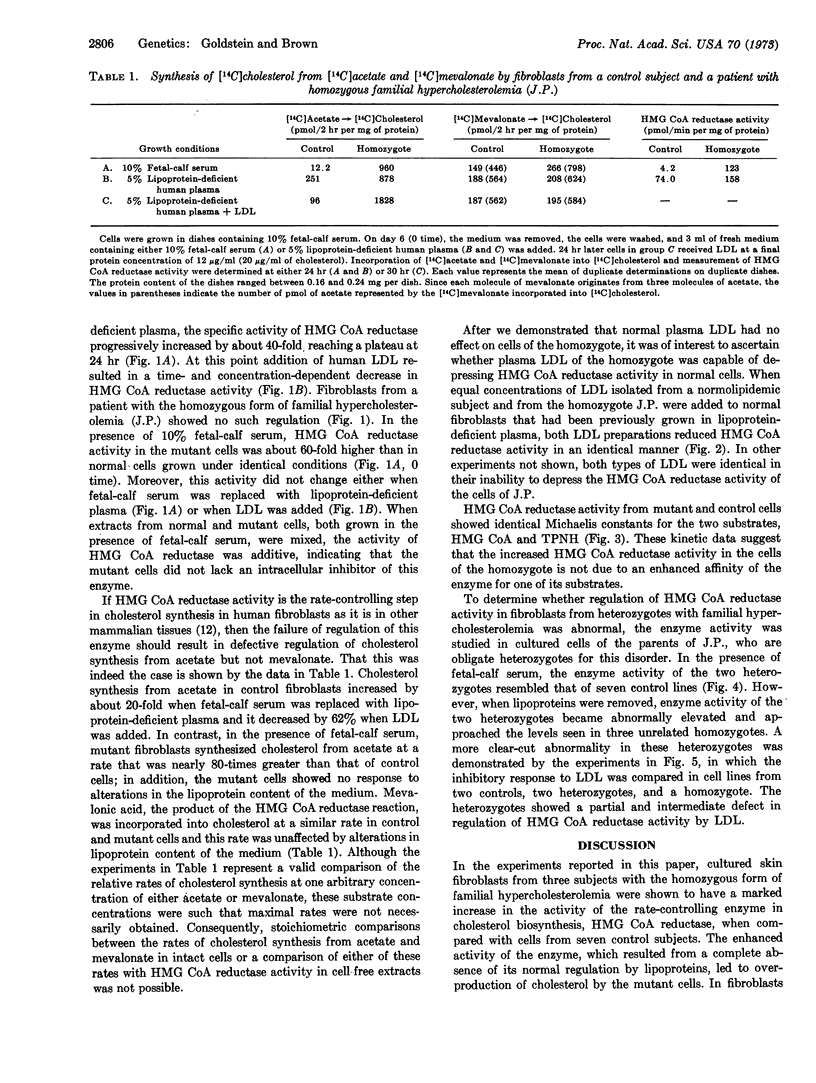
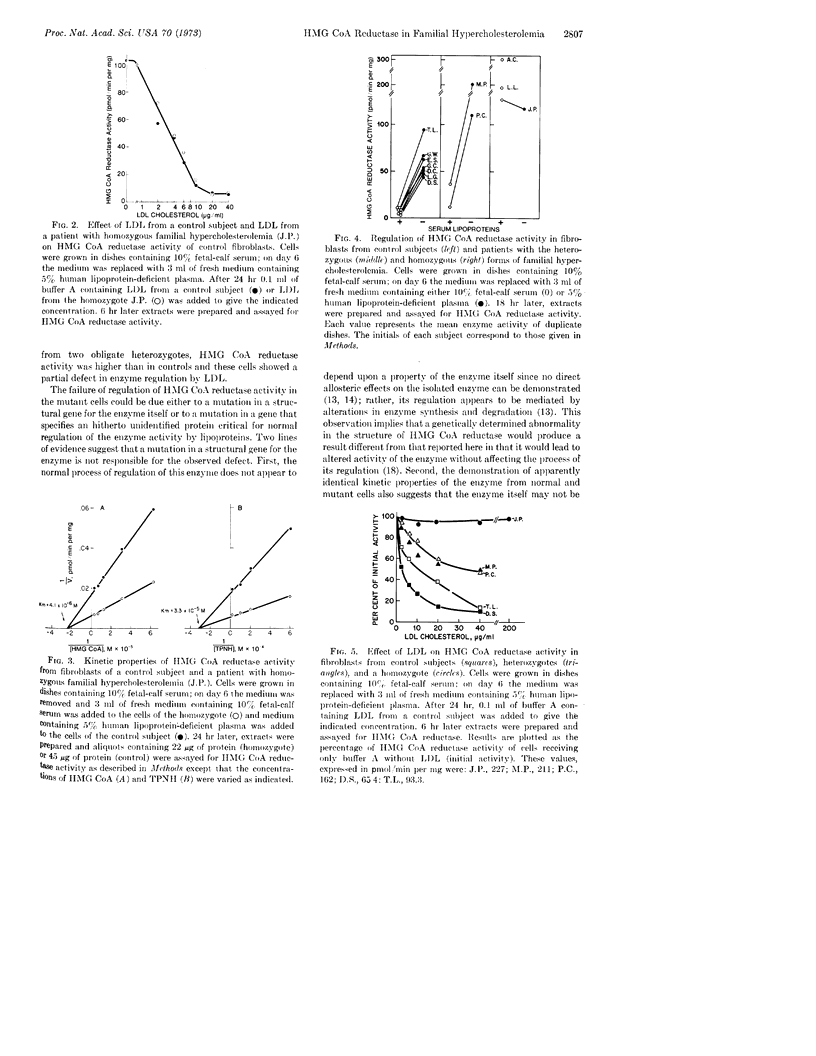
The homozygous form of the autosomal dominant disorder, familial hypercholesterolemia, is characterized by the presence in children of profound hypercholesterolemia, cutaneous planar xanthomas, and rapidly progressive coronary vascular disease that usually results in death before age 30 years. Cultured skin fibroblasts from three unrelated subjects with this disorder showed 40- to 60-fold higher activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (EC 1.1.1.34), the rate-controlling enzyme in cholesterol biosynthesis, when compared with fibroblasts of seven control subjects. Enhanced enzyme activity resulted from a complete absence of normal feedback suppression by low-density lipoproteins, which led to a marked overproduction of cholesterol by the mutant cells. The demonstration of apparently identical kinetic properties of the reductase activity of control and mutant cells, coupled with the evidence that this enzyme is normally regulated not by allosteric effectors but by alterations in enzyme synthesis and degradation, suggests that the primary genetic abnormality does not involve the structural gene for the enzyme itself, but a hitherto unidentified gene whose product is necessary for mediation of feedback control by lipoproteins. The fibroblasts of two obligate heterozygotes, the parents of one of the homozygotes, showed a pattern of enzyme regulation intermediate between that of controls and homozygotes.
Keywords: cholesterol synthesis, hyperlipidemia, low-density lipoproteins, enzyme regulation, coronary heart disease
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida N., Okamura T. Increased cholesterol-biosynthesis in familial hypercholesterolemia. Tohoku J Exp Med. 1971 Oct;105(2):147–155. doi: 10.1620/tjem.105.147. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Goldstein J. L., Schrott H. G., Hazzard W. R., Bierman E. L., Motulsky A. G. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973 Jul;52(7):1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHACHADURIAN A. K. THE INHERITANCE OF ESSENTIAL FAMILIAL HYPERCHOLESTEROLEMIA. Am J Med. 1964 Sep;37:402–407. doi: 10.1016/0002-9343(64)90196-2. [DOI] [PubMed] [Google Scholar]
- Khachadurian A. K. Lack of inhibition of hepatic cholesterol synthesis by dietary cholesterol in cases of familial hypercholesterolaemia. Lancet. 1969 Oct 11;2(7624):778–780. doi: 10.1016/s0140-6736(69)90484-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen T. A., Pelkonen R., Nikkilä E. A., Heinonen O. Low excretion of fecal bile acids in a family with hypercholesterolemia. Acta Med Scand. 1967 Nov;182(5):645–650. doi: 10.1111/j.0954-6820.1967.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Myant N. B. The regulation of cholesterol metabolism as related to familial hypercholesterolaemia. Sci Basis Med Annu Rev. 1970:230–259. [PubMed] [Google Scholar]
- Nevin N. C., Slack J. Hyperlipidaemic xanthomatosis. II. Mode of inheritance in 55 families with essential hyperlipidaemia and xanthomatosis. J Med Genet. 1968 Mar;5(1):9–28. doi: 10.1136/jmg.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott H. G., Goldstein J. L., Hazzard W. R., McGoodwin M. M., Motulsky A. G. Familial hypercholesterolemia in a large indred. Evidence for a monogenic mechanism. Ann Intern Med. 1972 May;76(5):711–720. doi: 10.7326/0003-4819-76-5-711. [DOI] [PubMed] [Google Scholar]
- Uhlendorf B. W., Mudd S. H. Cystathionine synthase in tissue culture derived from human skin: enzyme defect in homocystinuria. Science. 1968 May 31;160(3831):1007–1009. doi: 10.1126/science.160.3831.1007. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The relation between cholesterol absorption and cholesterol synthesis in the baboon. J Clin Invest. 1972 Jun;51(6):1450–1458. doi: 10.1172/JCI106941. [DOI] [PMC free article] [PubMed] [Google Scholar]



