Abstract
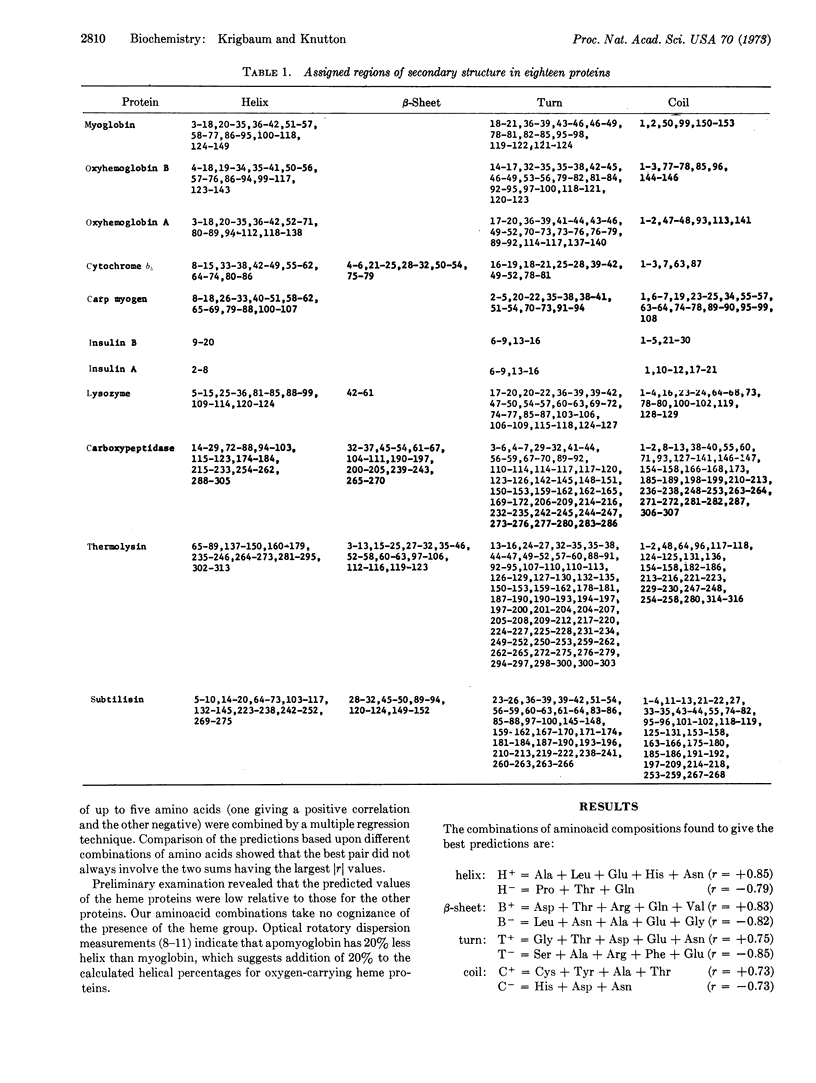
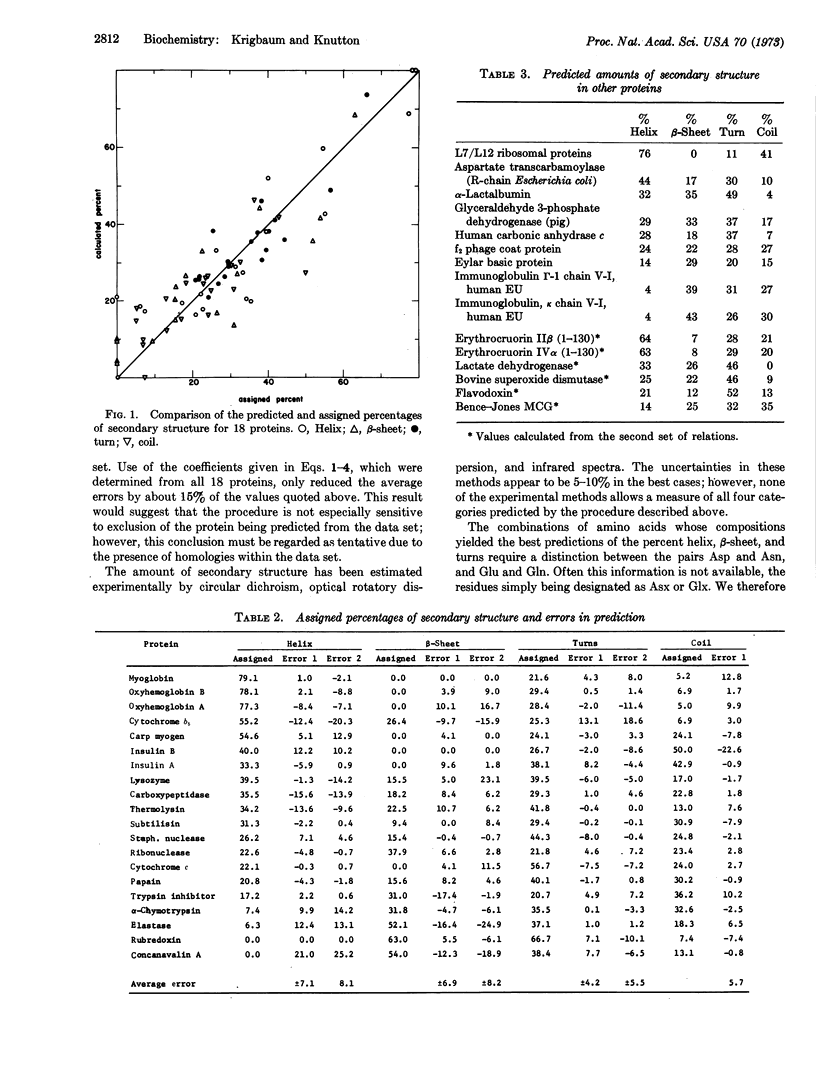
Multiple regression is used to obtain relationships for predicting the amount of secondary structure in a protein molecule from a knowledge of its aminoacid composition. We tested these relations using 18 proteins of known structure, but omitting the protein to be predicted. Independent predictions were made for the two subchains of hemoglobin and insulin. The average errors for these 20 chains or subchains are: helix ± 7.1%, β-sheet ± 6.9%, turn ± 4.2%, and coil ± 5.7%. A second set of relations yielding somewhat inferior predictions is given for the case in which Asp and Asn, and Glu and Gln, are not differentiated. Predictions are also listed for 15 proteins for which the aminoacid sequence or tertiary structure is unknown.
Keywords: helix, β-sheet, turns
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRESLOW E., BEYCHOK S., HARDMAN K. D., GURD F. R. RELATIVE CONFORMATIONS OF SPERM WHALE METMYOGLOBIN AND APOMYOGLOBIN IN SOLUTION. J Biol Chem. 1965 Jan;240:304–309. [PubMed] [Google Scholar]
- Epand R. M., Scheraga H. A. The influence of long-range interactions on the structure of myoglobin. Biochemistry. 1968 Aug;7(8):2864–2872. doi: 10.1021/bi00848a024. [DOI] [PubMed] [Google Scholar]
- HARRISON S. C., BLOUT E. R. REVERSIBLE CONFORMATIONAL CHANGES OF MYOGLOBIN AND APOMYOGLOBIN. J Biol Chem. 1965 Jan;240:299–303. [PubMed] [Google Scholar]
- Hermans J., Jr, Puett D. Relative effects of primary and tertiary structure on helix formation in myoglobin and alpha-lactalbumin. Biopolymers. 1971;10(5):895–914. doi: 10.1002/bip.360100512. [DOI] [PubMed] [Google Scholar]
- Leberman R. Secondary structure of tobacco mosaic virus protein. J Mol Biol. 1971 Jan 14;55(1):23–30. doi: 10.1016/0022-2836(71)90277-4. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Scheraga H. A. Predictions of structural homologies in cytochrome c proteins. Arch Biochem Biophys. 1971 Jun;144(2):576–583. doi: 10.1016/0003-9861(71)90363-8. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Rubin B., Richardson J. S. The simple construction of protein alpha-carbon models. Biopolymers. 1972;11(11):2381–2385. doi: 10.1002/bip.1972.360111116. [DOI] [PubMed] [Google Scholar]
- Troitskii G. V., Zav'yalov V. P. Calculation of the conformation of proteins with the aid of a modified nomogram. Establishment of the interrelationship between the primary and secondary structures of the polypeptide chain. Mol Biol. 1972 Sep-Oct;6(5):509–519. [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]


