Abstract
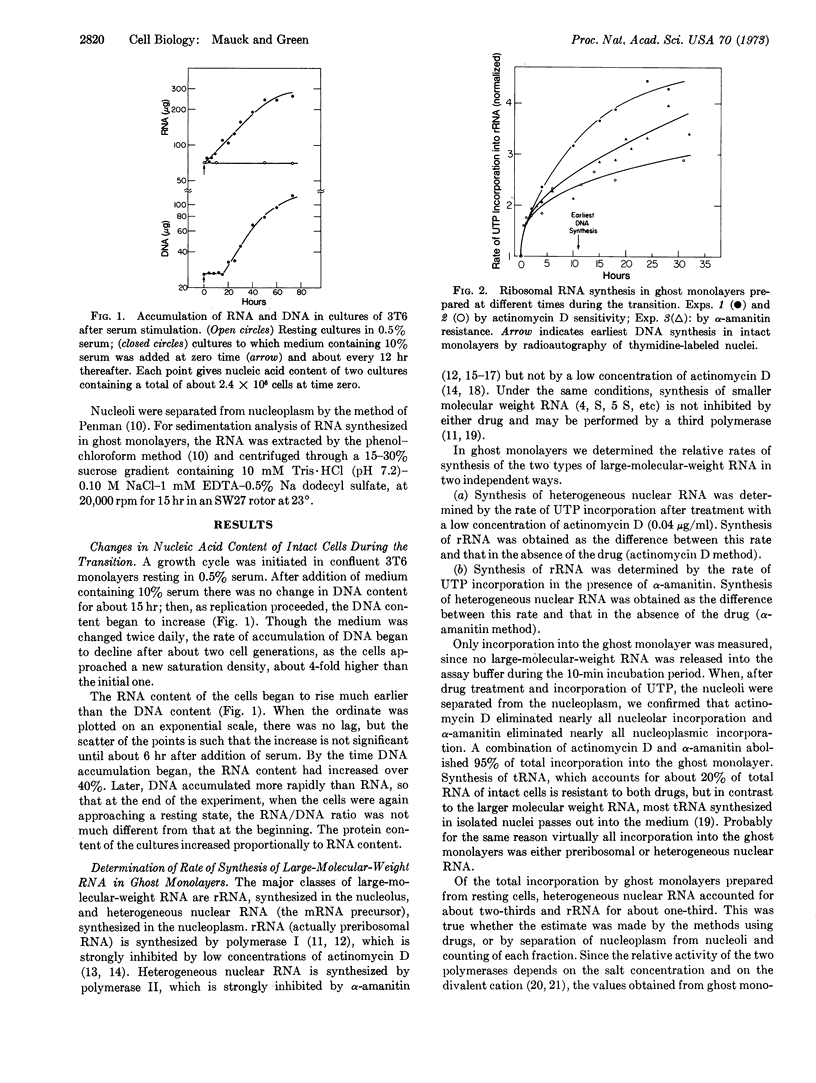
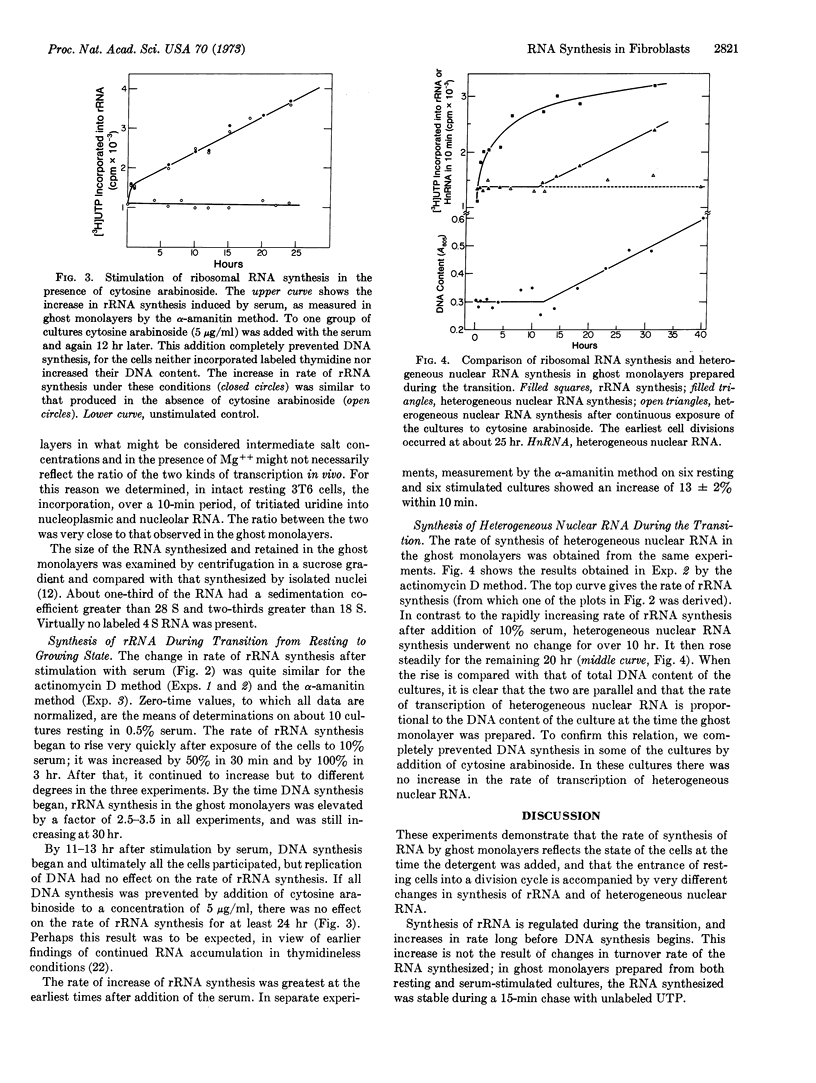
Addition of serum, containing fibroblast growth factors, to a culture of resting 3T6 cells stimulates a transition to the growing state. Studies of ghost monolayers prepared with the aid of detergent at intervals after stimulation showed an increase in the rate of ribosomal RNA synthesis within 10 min. The rate continued to increase for many hours and reached a level 2.5- to 3.5-fold higher by the time DNA synthesis began. The increasing rate of ribosomal RNA synthesis appeared independent of an increase in the number of ribosomal genes, since it was not affected by prevention of DNA synthesis with cytosine arabinoside.
In contrast to ribosomal RNA, the overall rate of transscription of heterogeneous nuclear RNA was not directly affected by serum growth factors and does not appear to be regulated during the transition from resting to growing state. It seems, instead, to be fixed in relation to the amount of template, for it increases proportionally to DNA content.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L. Studies on RNA metabolism during lymphocyte activation. Transplant Rev. 1972;11:3–38. doi: 10.1111/j.1600-065x.1972.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyházi E., D'Monte B., Edström J. E. Effects of -amanitin on in vitro labeling of RNA from defined nuclear components in salivary gland cells from Chironomus tentans. J Cell Biol. 1972 May;53(2):523–531. doi: 10.1083/jcb.53.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C. P. Regulation of the synthesis and the stability of ribosomal RNA during contact inhibition of growth. Nat New Biol. 1971 Jul 28;232(30):101–106. doi: 10.1038/newbio232101a0. [DOI] [PubMed] [Google Scholar]
- Giacomoni D., Finkel D. Time of duplication of ribosomal RNA cistrons in a cell line of Potorous tridactylis (rat kangaroo). J Mol Biol. 1972 Oct 14;70(3):725–728. doi: 10.1016/0022-2836(72)90570-0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Specific action of alpha-amanitin on mammalian RNA polymerase protein. Nature. 1970 Jan 3;225(5227):60–62. doi: 10.1038/225060b0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- RUECKERT R. R., MUELLER G. C. Studies on unbalanced growth in tissue culture. I. Induction and consequences of thymidine deficiency. Cancer Res. 1960 Dec;20:1584–1591. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Becker H. Control of macromolecular synthesis in proliferating and resting Syrian hamster cells in monolayer culture. I. Ribosome function. J Cell Physiol. 1971 Feb;77(1):31–42. doi: 10.1002/jcp.1040770105. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. The formation and distribution of ribosomes during hormone-induced growth and development. Biochem J. 1967 Jul;104(1):1–16. doi: 10.1042/bj1040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Rate of RNA synthesis in ghost monolayers obtained from fibroblasts preparing for division. Nat New Biol. 1973 Jun 6;243(127):168–170. doi: 10.1038/newbio243168a0. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Ribosomal RNA turnover in contact inhibited cells. Nat New Biol. 1972 Jan 12;235(54):58–61. doi: 10.1038/newbio235058a0. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Rubin H. Uridine transport and RNA synthesis in growing and in density-inhibited animal cells. J Cell Physiol. 1971 Apr;77(2):157–168. doi: 10.1002/jcp.1040770205. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



