Abstract
Background
In resource-poor regions of the world, HIV virologic testing is not available.
Objective
We sought to evaluate the diagnostic usefulness of the CD4/CD8 T-cell ratio in predicting HIV infection in infants.
Methods
Data from the 3- and 9-month visits for non–breastfed infants born to HIV-infected mothers enrolled (1990–1994) in the Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study (mother-to-child transmission of HIV, 17%) were analyzed. Data from the 3-month visit for infants enrolled (1985–1996) in the Perinatal AIDS Collaborative Transmission Study (mother-to-child transmission of HIV, 18%) were used for validation.
Results
At 3 months of age, data were available on 79 HIV-infected and 409 uninfected non–breast-fed infants in the Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study. The area under the curve (AUC) of the receiver operating characteristic curve at 3 months was higher for the CD4/CD8 ratio compared with the CD4+ T-cell count (AUC, 0.83 and 0.75; P = .03). The mean CD4/CD8 ratio at the 3-month visit was 1.7 for HIV-infected infants and 3.0 for uninfected infants. A CD4/CD8 ratio of 2.4 at 3 months of age was almost 2.5 times more likely to occur in an HIV-infected infant compared with an uninfected infant (test sensitivity, 81%; posttest probability of HIV, 33%). Model performance in the Centers for Disease Control and Prevention Perinatal AIDS Collaborative Transmission Study validation test (224 HIV-infected and 1015 uninfected 3-month-old infants) was equally good (AUC, 0.78 for CD4/CD8 ratio).
Conclusion
The CD4/CD8 T-cell ratio is a more sensitive predictor of HIV infection in infants than the CD4+ T-cell count.
Clinical implications
The CD4/CD8 T-cell ratio can be used with caution to predict HIV infection in children.
Keywords: CD4/CD8 T-cell ratio, mother-to-child transmission of HIV, HIV infection
Because early treatment intervention can dramatically alter the course of disease in HIV-infected infants, early diagnosis of HIV infection is critically important for infants born to HIV-infected women.1,2 Although specific diagnosis of HIV infection requires virologic assays, such as DNA PCR,3 these tests are not available in many parts of the world, and simpler alternative surrogate tests are being investigated. Immunologic assessment of CD4+ T cells often is more readily available than virologic assays. CD4+ T-cell depletion is a hallmark of HIV infection, and a significant difference in CD4+ T-cell counts between infected and uninfected infants has been reported in early infancy.4,5 However, infants normally have high CD4+ T-cell counts at birth, and because most mother-to-child transmission (MTCT) of HIV occurs around the time of birth, the CD4+T-cell counts of HIV-infected infants are equivalent to those of HIV-uninfected infants.6 HIV also influences CD8+ T cells, causing immune activation and expansion of this cell population.7 Establishing the diagnosis of HIV infection by using a surrogate assay, such as CD4/CD8 T-cell ratio, could allow early identification of HIV-infected infants, prompting timely treatment and thus a favorable modification of the disease course. A prior study conducted in sub-Saharan Africa evaluated CD4/CD8 T-cell ratios in infants in whom virologic diagnosis of infection was established by means of PCR and found a concordance between the two.8 Also, an analysis of the CD4/CD8 ratio as a predictor of HIV infection in North American infants has been suggested.9 Our objectives were to confirm these observations by using the National Institutes of Health’s National Heart, Lung, and Blood Institute Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV (P2C2) Study database to evaluate the utility of CD4/CD8 T-cell ratio for the diagnosis of HIV infection among HIV-exposed infants. In addition, we validated our methods of analysis by testing them in the Centers for Disease Control and Prevention Perinatal AIDS Collaborative Transmission Study (PACTS). A preliminary report of our findings has been presented at the Federation of Clinical Immunology Societies meeting on June 8, 2007.10
METHODS
Study populations
The P2C2 Study collected clinical and laboratory data from a birth cohort of 600 children born to HIV-infected women who were enrolled at birth or by 28 days of life beginning in 1990 and followed prospectively for up to 6 years in 5 centers located in Boston, Houston, New York (2 centers), and Los Angeles.11 Breast-feeding was not recommended, and study visits occurred at birth and at 3, 9, 15, 21, and 30 months of life. HIV infection was determined by means of HIV culture. Overall, there were 93 HIV-infected children, 463 HIV-uninfected children, and 44 children of indeterminate HIV infection status, and the rate of MTCT of HIV was 17%. The final assessment of the 600 patients was made at 30 months after the last infant was born. None of the 44 indeterminate children had remained in the study, 9 had died, and the remaining 35 were lost to follow-up.12 The PACTS database used for validation purposes was derived from 1454 children born to HIV-infected women from 1985 through 1996 in 4 centers located in New York City; Newark, New Jersey; Baltimore, Maryland; and Atlanta, Georgia. The rate of MTCT of HIV was 18%.13 Both P2C2 and PACTS protocols for human study were approved by local and central institutional review boards, and informed consent was obtained from parents.
Laboratory methods
The P2C2 Study and the PACTS clinical sites used virologic tests and lymphocyte subset analysis recommended by the Pediatric AIDS Clinical Trial Group Quality Control Program.14,15 During study visits for the P2C2 Study, anticoagulated blood specimens were obtained, and lymphocyte subset analysis was performed within 24 hours by using whole-blood lysis and commercially available antihuman CD-specific antibody conjugated to fluorescein isothiocyanate or phycoerythrin. For PACTS, CD4+ T-cell counts were obtained at birth and every 2 to 3 months of life throughout the study.
Statistical methods
Rates of decrease of CD4+ T-lymphocyte cell counts in the first year of life were obtained by using a mixed-effects model specifying that CD4+ T-lymphocyte counts follow a linear regression over time, with random intercept and slope for each infant. The same model was fit for the CD4/CD8 ratio.
Longitudinal profiles of CD4/CD8 ratio (Box-Cox transformation of ratios) up to 14 months of age for HIV-infected and HIV-uninfected infants were generated. The second-degree fractional polynomials16 were applied to obtain the best-fitting models with respect to age and CD4/CD8 ratio. Based on the methods of Royston,17 smoothed percentile curves of the CD4/CD8 ratio for age were estimated by using random-effects modeling.
Two approaches were used to evaluate the CD4/CD8 ratio and the CD4+ T-cell count as markers of HIV infection. First, the risk of HIV infection was modeled as a function of the CD4/CD8 ratio (and separately using the CD4+ cell count) by using logistic regression performed separately for the 3- and 9-month visit data. Second, marker performance was summarized with classification performance measures, such as sensitivity, specificity, predictive values, receiver operating characteristic (ROC) curves, and likelihood ratios and the corresponding posttest probabilities. Interpretation of likelihood ratios followed the guidelines provided by Jaeschke et al.18 ROC curves were constructed for each marker at 3 study visits: birth (0–44 days), 3 months (45–150 days), and 9 months (151–365 days). The areas under the curve (AUCs) for the 2 correlated ROC curves were compared at each age interval by using a nonparametric approach described by DeLong et al.19 The validation data set included data from the 3-month visit for infants enrolled in the PACTS cohort (45–150 days).
The same statistical methods described in the previous paragraph were used for the validation data set to compare the AUCs for the 2 markers and to summarize the diagnostic accuracy of the CD4/CD8 ratio as a predictor of HIV infection. Calibration evaluates the degree of correspondence between the model’s estimated probabilities of HIV infection risk for the P2C2 data compared with the PACTS validation data set. To assess model calibration after initial review, the CD4/CD8 ratio data from the P2C2 Study were divided into 7 equivalent categories (<0.5, 0.5–1.0, 1.0–1.5, 1.5–2.0, 2.0–2.5, 2.5–3.0, and >3.0), and the logistic regression refit provided HIV risk estimates and 95% CIs for each of the 7 categories. The observed risk estimate from the validation data for each CD4/CD8 category was compared with the P2C2 estimates to determine whether the observed results fell within the CIs of the values predicted from the P2C2 HIV group.
RESULTS
Derivation of the study populations
Of 600 P2C2 subjects, data from 79 HIV-infected infants and 409 HIV-uninfected non–breast-fed infants at the 3-month visit were available for analysis. Similarly, data from 78 HIV-infected infants and 372 HIV-uninfected infants at the 9-month visit were available. Of the 1454 PACTS subjects, data from 224 HIV-infected infants and 1015 HIV-uninfected infants at the 3-month visit were available.
Performance of CD4/CD8 ratio in predicting HIV infection
The mean CD4/CD8 ratios at the 3- and 9-month visits were 1.7 and 1.1 for HIV-infected infants and 3.0 and 2.6 for HIV-uninfected infants, respectively. Diagnostic accuracy statistics for the CD4/CD8 ratio at the 3- and 9-month visits can be found in Tables I and II. At the 3-month visit, a CD4/CD8 ratio of 2.4 was almost 2.5 times (95% CI for the likelihood ratio of a positive test, 2.1–2.9) more likely to occur in an HIV-infected infant compared with an HIV-uninfected infant (test sensitivity, 81%; post-test probability of HIV, 33%; Table I). At the 9-month visit, a CD4/CD8 ratio of 1.8 was almost 3.5 times (95% CI for the likelihood ratio, 2.9–4.3) more likely to occur in an HIV-infected infant compared with an HIV-uninfected infant (test sensitivity, 83%; posttest probability of HIV, 42%; Table II). According to the guidelines reported by Jaeschke et al,18 a likelihood ratio of 2 to 5 is suggestive of a small but sometimes important change from pretest to posttest probability. For an infant 3 months of age with a CD4/CD8 ratio of 2.4 (1 SD greater than the mean of 1.7 for P2C2 HIV-infected infants) whose risk of HIV infection is in the equivocal range, a likelihood ratio of 2.5 is suggestive of a potentially important increase in the probability of HIV infection.
TABLE I.
Diagnostic accuracy of the CD4/CD8 ratio in the P2C2 HIV cohort at the 3-month study visit (45–150 days; n = 79 HIV-infected and n = 409 HIV-uninfected subjects)
| Diagnostic accuracy measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4/CD8 ratio |
HIV-positive group No.* |
HIV-negative group No.* |
SE | SP | PPV | NPV | DLR+ (95% CI) | DLR− (95% CI) | Posttest probability (% [95% CI]) |
| 1.00 | 25 | 4 | 0.32 | 0.99 | 0.86 | 0.88 | 32.3 (11.6–90.2) | 0.7 (0.6–0.8) | 87 (84–89) |
| 1.20 | 30 | 8 | 0.38 | 0.98 | 0.79 | 0.89 | 19.4 (9.2–40.7) | 0.6 (0.5–0.8) | 80 (76–83) |
| 1.40 | 36 | 21 | 0.46 | 0.95 | 0.63 | 0.90 | 8.9 (5.5–14.4) | 0.6 (0.5–0.7) | 65 (59–69) |
| 1.60 | 41 | 37 | 0.52 | 0.91 | 0.53 | 0.91 | 5.7 (4.0–8.3) | 0.5 (0.4–0.7) | 54 (48–59) |
| 1.80 | 48 | 57 | 0.61 | 0.86 | 0.46 | 0.92 | 4.4 (3.2–5.9) | 0.5 (0.4–0.6) | 47 (42–52) |
| 2.00 | 51 | 82 | 0.65 | 0.80 | 0.38 | 0.92 | 3.2 (2.5–4.2) | 0.4 (0.3–0.6) | 40 (34–45) |
| 2.20 | 58 | 105 | 0.73 | 0.74 | 0.36 | 0.94 | 2.9 (2.3–3.5) | 0.4 (0.3–0.5) | 37 (32–42) |
| 2.40 | 64 | 135 | 0.81 | 0.67 | 0.32 | 0.95 | 2.5 (2.1–2.9) | 0.3 (0.2–0.5) | 33 (29–38) |
| 2.60 | 65 | 163 | 0.82 | 0.60 | 0.29 | 0.95 | 2.1 (1.8–2.4) | 0.3 (0.2–0.5) | 30 (25–34) |
| 2.80 | 71 | 202 | 0.90 | 0.51 | 0.26 | 0.96 | 1.8 (1.6–2.1) | 0.2 (0.1–0.4) | 27 (23–31) |
| 3.00 | 73 | 229 | 0.92 | 0.44 | 0.24 | 0.97 | 1.7 (1.5–1.8) | 0.2 (0.1–0.4) | 25 (21–29) |
| Total† | 79 | 409 | |||||||
The pretest probability is 17% (95% CI, 14% to 20%).
SE, Sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; DLR+, diagnostic positive likelihood ratio; DLR−, diagnostic negative likelihood ratio.
Number of infants having a CD4/CD8 ratio of less than the cutoff point within each HIV group.
Total, Number of infants in this age interval within each HIV group.
TABLE II.
Diagnostic accuracy of the CD4/CD8 ratio in the P2C2 HIV cohort at the 9-month age interval (151–365 days; n = 78 HIV-positive and n = 372 HIV-negative patients)
| Diagnostic accuracy measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4/CD8 ratio |
HIV-positive group No.* |
HIV-negative group No.* |
SE | SP | PPV | NPV | DLR+ (95% CI) | DLR− (95% CI) | Posttest probability (% [95% CI]) |
| 1.00 | 50 | 10 | 0.64 | 0.97 | 0.83 | 0.93 | 23.8 (12.7–44.9) | 0.4 (0.3–0.5) | 83 (80–86) |
| 1.20 | 55 | 22 | 0.71 | 0.94 | 0.71 | 0.94 | 11.9 (7.8–18.3) | 0.3 (0.2–0.4) | 71 (66–75) |
| 1.40 | 56 | 37 | 0.72 | 0.90 | 0.60 | 0.94 | 7.2 (5.2–10.1) | 0.3 (0.2–0.5) | 60 (54–64) |
| 1.60 | 58 | 56 | 0.74 | 0.85 | 0.51 | 0.94 | 4.9 (3.8–6.5) | 0.3 (0.2–0.4) | 50 (45–55) |
| 1.80 | 65 | 88 | 0.83 | 0.76 | 0.42 | 0.96 | 3.5 (2.9–4.3) | 0.2 (0.1–0.4) | 42 (36–47) |
| 2.00 | 68 | 115 | 0.87 | 0.69 | 0.37 | 0.96 | 2.8 (2.4–3.4) | 0.2 (0.1–0.3) | 37 (31–41) |
| 2.20 | 71 | 145 | 0.91 | 0.61 | 0.33 | 0.97 | 2.3 (2.0–2.7) | 0.2 (0.1–0.3) | 32 (28–37) |
| 2.40 | 74 | 175 | 0.95 | 0.53 | 0.30 | 0.98 | 2.0 (1.8–2.3) | 0.1 (0.0–0.3) | 29 (25–34) |
| 2.60 | 77 | 207 | 0.99 | 0.44 | 0.27 | 0.99 | 1.8 (1.6–2.0) | 0.03 (0.0–0.2) | 27 (22–31) |
| Total† | 78 | 372 | |||||||
The pretest probability is 17% (95% CI, 14% to 20%).
SE, Sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; DLR+, diagnostic positive likelihood ratio; DLR−, diagnostic negative likelihood ratio.
Number of infants having a CD4/CD8 ratio of less than the cutoff point within each HIV group.
Total, Number of infants in this age interval within each HIV group.
Clinical decision criteria are also formulated in terms of risk. We defined the risk of HIV infection between 10% and 30% (CD4/CD8, >1.70 and <2.62) as being in the indeterminate range. Based on this rule, 18% of infants at the 3-month visit were HIV infected (risk, ≥30%; CD4/CD8, ≤1.70), 50% of infants were HIV uninfected (risk, ≤10%; CD4/CD8, ≥2.62), and 32% of infants were indeterminate. By the 9-month visit, 21% of infants were HIV infected (risk, ≥30%; CD4/CD8, ≤1.40), 62% of infants were HIV uninfected (risk, ≤10%; CD4/CD8, ≥1.94), and 17% of infants were indeterminate.
Comparison of CD4+ T-cell count versus CD4/CD8 ratio as a predictor of HIV infection
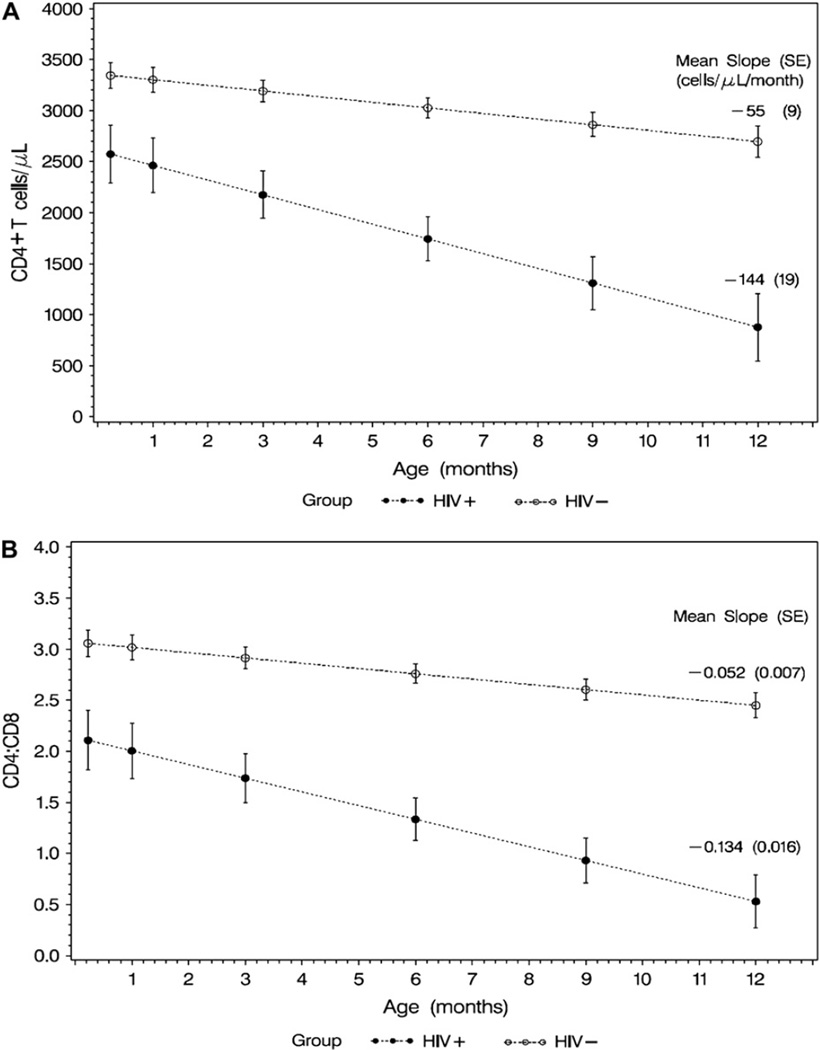
The average rate of decrease of CD4+ T-cell counts during the first year of life (mean ± SE) was 144 ± 19 cells/µL per month in the HIV-infected group versus 55 ± 9 cells/µL per month in the HIV-uninfected group (P < .001; Fig 1, A). Similarly, the average rate of decrease of the CD4/CD8 ratio in the first year of life was greater in HIV-infected infants (0.134 ± 0.016) compared with that seen in the HIV-uninfected infants (0.052 ± 0.007, P < .001; Fig 1, B). The P value between HIV-positive and HIV-negative infants’ values at all time points (1 week and 1, 3, 6, 9, and 12 months) is less than .001 for CD4 T-cell count and CD4/CD8 ratio (Fig 1).
Figure 1.
Mean CD4 cell count versus age (A) and mean CD4/CD8 ratio versus age (B) in infants born to HIV-infected mothers. In Fig 1, A, the mean CD4 cell count (in cells per microliter) at 1 week and 1, 3, 6, 9, and 12 months of age and the average rate of decrease of CD4 T-cell counts in the first year of life for HIV-infected infants and HIV-uninfected infants is shown. In Fig 1, B, similar data for CD4/CD8 ratio by HIV infection status are shown. Rates of CD4 T-cell count decrease and CD4/CD8 ratio decrease were significantly greater in HIV-infected infants compared with those seen in HIV-uninfected infants. Vertical bars, 95% CI.
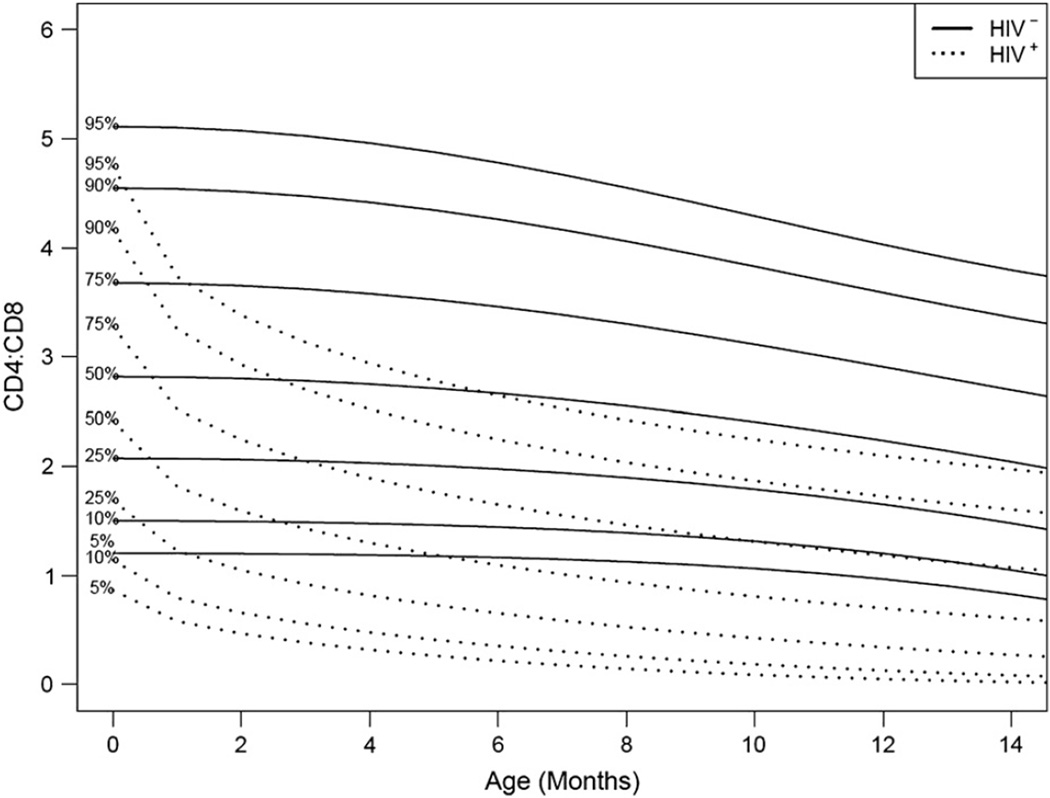
Fig 2 provides longitudinal profiles of the CD4/CD8 ratio up to 14 months of age for HIV-infected and HIV-uninfected infants. From birth to the 3-month visit, the CD4/CD8 ratio decreased to about 1.5 (50th percentile) for the HIV-infected infants. However, the decrease was much less for HIV-uninfected infants (50th percentile = 2.8). By the 9-month visit, the CD4/CD8 ratio decreased to about 1.1 for the HIV-infected infants (50th percentile; ie, less than the 50th percentile for the HIV-uninfected infants).
Figure 2.
Reference percentile curves for CD4/CD8 ratio by HIV status in the first 14 months of life.
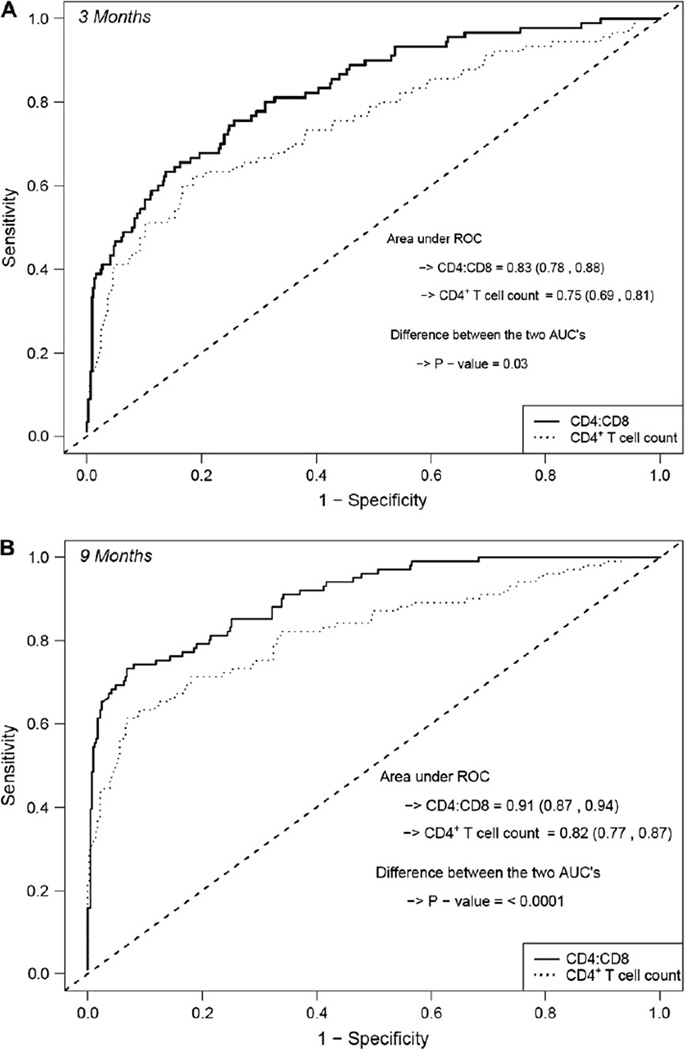
The CD4/CD8 ratio performed better than the CD4+ T-cell count as an early diagnostic marker of HIV infection (data not shown). The AUC estimate at the 3-month visit was higher for the CD4/CD8 ratio compared with the CD4+ T-cell count (AUC, 0.83 and 0.75, P = .03; Fig 3, A). By the 9-month visit, the AUC estimate of 0.91 for the CD4/CD8 ratio exceeded the AUC estimate of 0.83 for the CD4+ T-cell count (P < .0001; Fig 3, B). The AUC estimate at the 3-month visit for the CD4/CD8 ratio reflects the proportion (0.83) of infant pairs for which the logistic regression model assigned a higher probability to an infant who will be HIV infected than to an infant who will not be HIV infected.20 The estimate of the AUC increased to 0.87 after adjusting for weight (weight z score at the 3-month visit) and hemoglobin value (at the 6-month visit) in addition to the CD4/CD8 ratio.
Figure 3.
ROC curves based on the P2C2 data for CD4 T-cell count and CD4/CD8 ratio. A, Three-month study visit. B, Nine-month study visit.
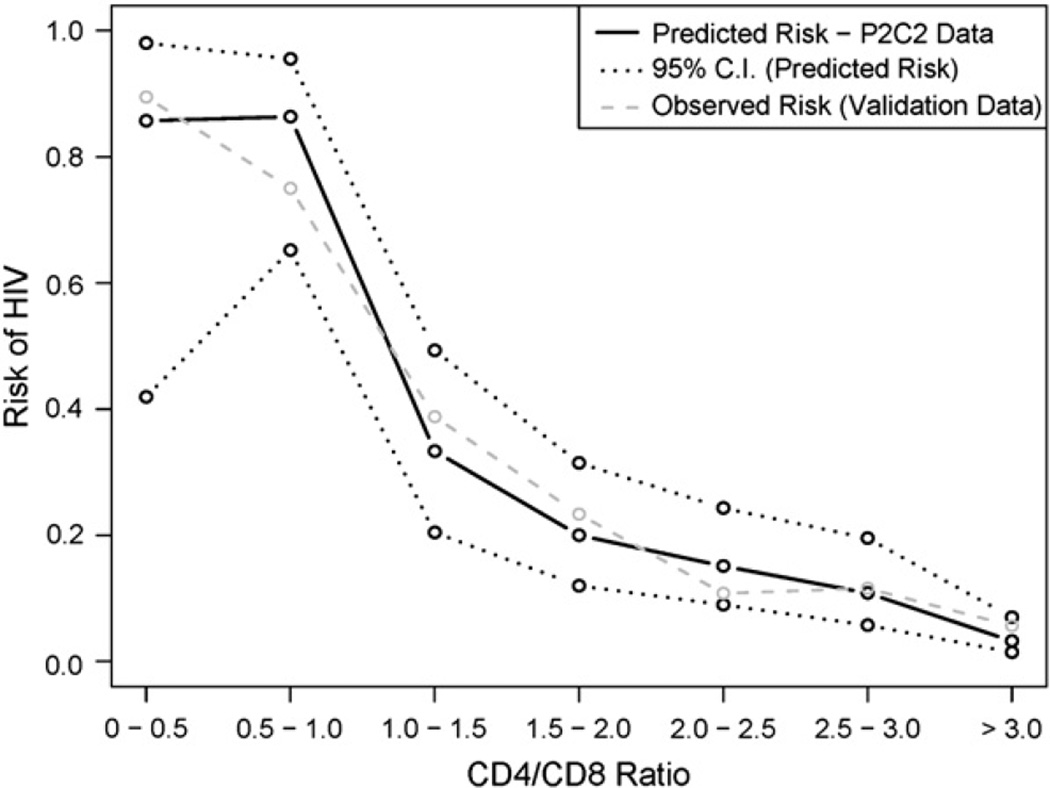
Validation of method analysis with PACTS data
Table III and Fig 4 contain the validation-of-method data by the PACTS database. Diagnostic accuracy statistics were similar for the P2C2 Study and PACTS (Table III). For PACTS, the AUC estimate for the CD4/CD8 ratio was 0.78 (95% CI, 0.74–0.81; data not shown). Fig 4 indicates that the observed risk of HIV infection based on the PACTS validation data tracks closely to the predicted HIV risk based on the P2C2 Study data.
TABLE III.
Diagnostic accuracy of the CD4/CD8 ratio in the P2C2 HIV and PACTS (validation data) cohorts at 3 months of age
| CD4/CD8 ratio |
Percentage of HIV-positive subjects below cutoff point |
Percentage of HIV-negative subjects below cutoff point |
Sensitivity (%) |
Specificity (%) |
DLR+ | Posttest probability* (%) |
DLR− | Posttest probability† (%) |
|---|---|---|---|---|---|---|---|---|
| 1.00 | 86 (79) | 14 (21) | 32 (26) | 99 (98) | 32.3 (16.7) | 87 (79) | 0.7 (0.8) | 12 (14) |
| 1.20 | 79 (68) | 21 (32) | 38 (33) | 98 (97) | 19.4 (9.4) | 80 (68) | 0.6 (0.7) | 11 (13) |
| 1.40 | 63 (57) | 37 (43) | 46 (41) | 95 (93) | 8.9 (6.1) | 65 (57) | 0.6 (0.6) | 11 (12) |
| 1.60 | 53 (51) | 47 (49) | 52 (51) | 91 (89) | 5.7 (4.7) | 54 (51) | 0.5 (0.6) | 10 (11) |
| 1.80 | 46 (42) | 54 (58) | 61 (57) | 86 (83) | 4.4 (3.4) | 47 (42) | 0.5 (0.5) | 9 (10) |
| 2.00 | 38 (38) | 62 (62) | 65 (68) | 80 (76) | 3.2 (2.8) | 40 (38) | 0.4 (0.4) | 8 (8) |
| 2.20 | 36 (33) | 64 (67) | 73 (74) | 74 (67) | 2.9 (2.2) | 37 (33) | 0.4 (0.4) | 7 (8) |
| 2.40 | 32 (29) | 68 (71) | 81 (79) | 67 (58) | 2.5 (1.9) | 33 (30) | 0.3 (0.4) | 5 (7) |
| 2.60 | 29 (27) | 71 (73) | 82 (82) | 60 (51) | 2.1 (1.6) | 30 (27) | 0.3 (0.4) | 6 (7) |
| 2.80 | 26 (25) | 74 (75) | 90 (86) | 51 (44) | 1.8 (1.5) | 27 (25) | 0.2 (0.3) | 4 (7) |
| 3.00 | 24 (24) | 76 (76) | 92 (90) | 44 (38) | 1.7 (1.4) | 25 (24) | 0.2 (0.3) | 3 (6) |
The pretest probabilities for P2C2 data and validation data sets are 17% and 18%, respectively. For each column, the first number is based on the P2C2 data set, and the number within parentheses is calculated from the validation data set (PACTS).
Posttest probability for a positive test result.
Posttest probability for a negative test result.
Figure 4.
Predicted risk of HIV infection by CD4/CD8 ratio: P2C2 Study data versus validation (PACTS) data.
DISCUSSION
In resource-replete countries the diagnosis of HIV infection in infants is rapid, accurate, and definitive, thus enabling the clinician to have confidence in relating infants’ HIV infection status to their parents. The authoritative pronouncements of HIV infection in very young children have been brought about by the application of molecular virology to clinical medicine, which has produced the HIV DNA PCR or similar assays. Such assays, however, are not immediately available in many parts of the resource-limited countries, thus leading to a search for alternative technologies. In some settings access to determination of peripheral blood CD4+ T-cell and CD8+ T-cell counts/percentages is available, thus prompting a reinvestigation of the use of these lymphocyte subset values to assess HIV infection in children. Indeed, low-cost methodology can be used in small laboratories and rural villages to assess CD4+ and CD8+ T-cell counts.21 Previously, analysis of the CD4+ or CD3+ T-cell counts has been proposed either as an alternate measure of diagnosis or prediction of HIV disease progression. Although it is possible to wait until maternal anti-HIV antibodies (basis for immunoassay and Western blotting) disappear in infants (ie, 12–18 months of age), an earlier diagnosis of HIV infection is much preferred. Mofenson et al22 have proposed using total lymphocyte cell count and serum albumin concentration to predict mortality in children in resource-poor settings.
Our analysis of the CD4/CD8 T-cell ratio as a surrogate method of determining HIV infection in a large US cohort of prospectively followed HIV-infected children (P2C2 Study) has led to some interesting results. The confirmation of these findings with a second large US cohort of HIV-infected children (PACTS) has suggested this alternative method of assessing HIV infection in children as a reliable and simple diagnostic tool. As early as 3 months of age, it is possible to assess with some certainty the state of HIV infection in the P2C2 Study cohort by using the CD4/CD8 ratio. This assessment can be expressed based on the strong association of the CD4/CD8 T-cell ratio with the risk of HIV infection and by summarizing the CD4/CD8 T-cell performance with classification performance measures, such as sensitivity, specificity, likelihood ratios, and ROC curves. Setting the indeterminate HIV risk range at a modest level actually observed with P2C2 patients (ie, 10% to 30%) results in the ability to diagnose HIV infection in 18% of infants and rule out infection at 3 months of age in 50% of infants, leaving 32% of infants in the indeterminate category. By 9 months of age, this cohort would have 21% HIV-infected, 62% uninfected, and 17% indeterminate infants. Thus a treatment decision (ie, treat vs no treatment) for a population with approximately 20% HIV prevalence (treat or not treat) for 3-month old infants born to HIV-infected women could be made reasonably in two thirds of cases.
Because the prevalence of HIV is approximately 17% in the population studied, a treatment decision is straightforward if the calculated risk of HIV infection is close to 0 or 1.We are assuming that greater than 30% risk of HIV infection is sufficiently high to recommend treatment and that a risk of less than 10% is sufficiently low to decide against treatment. If the calculated risk is in the equivocal or indeterminate range, it is not helpful. Treatment recommendations are most difficult for infants whose risks are calculated in the range of 10% to 30%. A risk model will be most useful for individual decision making if calculated risks of HIV tend to exceed 30% or are less than 10%. Other thresholds might be chosen for defining low and high risk of HIV infection.
Table IV indicates that HIV risk decreases as the positive diagnostic likelihood ratio (DLR+) decreases and as sensitivity increases. Treatment of a 3-month-old infant with a CD4/CD8 T-cell ratio of 1.0 has a very high HIV risk (0.54) and DLR+ (32.3). However, because the sensitivity (0.32) is low, many HIV-positive infants would not be treated. Not treating infants with a CD4/CD8 ratio of 3.00 is appealing for both risk (0.06) and sensitivity (0.92). However, the DLR+ is somewhat low (1.7 < 2).
TABLE IV.
Linking risk estimates and performance measures
| CD4/CD8 ratio |
Risk (%) | DLR+ | Sensitivity | Specificity |
|---|---|---|---|---|
| Summary of P2C2 data (3 mo): | ||||
| 1.00 | 54 | 32.3 | 0.32 | 0.99 |
| 1.20 | 46 | 19.4 | 0.38 | 0.98 |
| 1.40 | 39 | 8.9 | 0.46 | 0.95 |
| 1.60 | 33 | 5.7 | 0.52 | 0.91 |
| 1.80 | 27 | 4.4 | 0.61 | 0.86 |
| 2.00 | 21 | 3.2 | 0.65 | 0.80 |
| 2.20 | 17 | 2.9 | 0.73 | 0.74 |
| 2.40 | 13 | 2.5 | 0.81 | 0.67 |
| 2.60 | 10 | 2.1 | 0.82 | 0.60 |
| 2.80 | 8 | 1.8 | 0.90 | 0.51 |
| 3.00 | 6 | 1.7 | 0.92 | 0.44 |
| For 3-mo age window: | ||||
| <1.80 | >30% (approx.) | >4.4 | <0.61 | >0.86 |
| 1.80–2.60 | 10% to 30% | 2.1–4.4 | 0.61–0.82 | 0.60–0.86 |
| ≥2.60 | <10% | <2.1 | >0.82 | <0.60 |
Treatment decisions based solely on risk (10% and 30% are the threshold values) and DLR+ suggest treatment when the CD4/CD8 T-cell ratio is 1.8 or less. However, because the sensitivity (<0.61) is low, many HIV-infected infants would not be treated. If the sensitivity is increased to at least 80%, then treating a 3-month infant with a CD4/CD8 ratio of 2.4 to 2.6 or less might be reasonable. Even though the risk (10% to 13%) is in the indecisive region, it, at least, is not suggestive against treatment. If the CD4/CD8 T-cell ratio is as high as 2.80, the risk is low (<8%) and therefore suggestive against treatment. Appendix E1 in the Online Repository (available at www.jacionline.org) contains additional illustrations of the calculations of risk of infection by using a CD4/CD8 ratio of 1.00, 2.00, and 3.00.
These methods of calculating the risk of infection are obviously much less accurate than using the HIV DNA PCR assay, in which sensitivity and specificity are near 100%. Nevertheless, in circumstances in which testing such as DNA PCR assays cannot be performed and CD4+ and CD8+ T-cell measurements are available, decisions to treat or not treat high-risk infants on the basis of the CD4/CD8 ratio reasonably can be made in 68% of infants at 3 months and 83% of infants at 9months of age. Of course this calculation assumes the infant will not be breast-fed, which adds a risk factor of infection of up to 15%.23 Although we did not study HIV infection through breast milk in the P2C2 Study, it is possible that our findings would be applicable to children infected through that route of transmission. Another caveat to our findings is that CD4/CD8 ratios might be altered in other congenital infections, such as cytomegalovirus (CMV) infection, although the incidence of congenital CMV infection in the newborn population in resource-poor countries (estimated at ≤1%) would be low compared with that of perinatal HIV infection.24
We conclude that in circumstances in which HIV DNA PCR testing is not available, assessment of CD4+ and CD8+ T-cell values will permit the calculation of CD4/CD8 ratios that are helpful in making treatment decisions in a majority of infants at 3 to 9 months of age.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grants and contracts HL96040, HL079533, HL72705, AI27551, AI36211, HD41983, RR0188, and AI41089; the Emory Center for AIDS Research (P30 A1050409); the Pediatric Research and Education Fund, Baylor College of Medicine; and the David Fund, Pediatrics AIDS Fund, and Immunology Research Fund, Texas Children’s Hospital.
We thank the investigators, study staff, and families who participated in the P2C2 Study and the National Heart, Lung, and Blood Institute; the Perinatal AIDS Collaborative Transmission Study; and the Centers for Disease Control and Prevention for the use of their databases. A complete list of study participants can be found in reference 11. Ms Carolyn Jackson rendered assistance with the preparation of the manuscript.
Abbreviations used
- AUC
Area under the curve
- DLR
Diagnostic likelihood ratio
- MTCT
Mother-to-child transmission
- PACTS
Perinatal AIDS Collaborative Transmission Study
- P2C2 Study
Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study
- ROC
Receiver operating characteristic
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Gortmaker SL, Hughes M, Cervia J, Brady M, Johnson GM, Seage GR., 3rd Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 2.Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo P. Diagnostic methods for infants born to HIV-infected women. In: Shearer WT, Hanson IC, editors. Medical management of AIDS in children. Philadelphia: Elsevier/Saunders; 2003. pp. 107–116. [Google Scholar]
- 4.Shearer WT, Easley KA, Goldfarb J, Rosenblatt HM, Jenson HB, Kovacs A, et al. Prospective 5-year study of peripheral blood CD4, CD8, and CD19/CD20 lymphocytes and serum Igs in children born to HIV-1 women. The P2C2 HIV Study Group. J Allergy Clin Immunol. 2000;106:559–566. doi: 10.1067/mai.2000.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinen J, Easley KA, Mendez H, Shearer WT. Decline of CD3-positive T-cell counts by 6 months of age is associated with rapid disease progression in HIV-1-infected infants. J Allergy Clin Immunol. 2001;108:265–268. doi: 10.1067/mai.2001.116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, et al. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol. 2005;115:848–855. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 8.Zijenah LS, Katzenstein DA, Nathoo KJ, Rusakaniko S, Tobaiwa O, Gwanzura C, et al. T lymphocytes among HIV-infected and -uninfected infants: CD4/CD8 ratio as a potential tool in diagnosis of infection in infants under the age of 2 years. J Transl Med. 2005;3:6. doi: 10.1186/1479-5876-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read JS, Pahwa S, Yin W, Matthews Y, Shearer WT, Diaz C, et al. CD4/CD8 Ratio for diagnosis of HIV-1 infection in infants: the Women and Infants Transmission Study; Program and Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; February 25–28, 2007; Los Angeles, Calif. (abstract No. 686). [Google Scholar]
- 10.Shearer W, Read J, Chen J, Wijayawardana S, Easley K, Palumbo P, et al. CD4/CD8 T cell ratio predicts HIV infection in infants: the NHLBI P2C2 HIV Study. Clin Immunol. 2007;123(suppl):S46. doi: 10.1016/j.jaci.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus (P2C2 HIV) Infection Study: design and methods. The P2C2 HIV Study Group. J Clin Epidemiol. 1996;49:1285–1294. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitt J, Goldfarb J, Schluchter M, Kovacs A, Cooper E, Hodes D, et al. HIV vertical transmission rate determinations are subject to differing definitions and therefore different rates. The Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. J Clin Epidemiol. 1998;51:159–164. doi: 10.1016/s0895-4356(97)00239-4. [DOI] [PubMed] [Google Scholar]
- 13.Simonds RJ, Steketee R, Nesheim S, Matheson P, Palumbo P, Alger L, et al. Impact of zidovudine use on risk and risk factors for perinatal transmission of HIV. Perinatal AIDS Collaborative Transmission Studies. AIDS. 1998;12:301–308. doi: 10.1097/00002030-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo PE, Kwok S, Waters S, Wesley Y, Lewis D, McKinney N, et al. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J Pediatr. 1995;126:592–595. doi: 10.1016/s0022-3476(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 16.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modeling. Appl Stat. 1994;43:429–467. [Google Scholar]
- 17.Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417–1436. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Baum LL, Crowe S, Landay AL. Advances in CD4 cell enumeration in resource-poor countries. Curr Opin HIV/AIDS. 2007;2:234–240. doi: 10.1097/COH.0b013e3280ef6909. [DOI] [PubMed] [Google Scholar]
- 22.Mofenson LM, Harris DR, Moye J, Bethel J, Korelitz J, Read JS, et al. Alternatives to HIV-1 RNA concentration and CD4 count to predict mortality in HIV-1-infected children in resource-poor settings. Lancet. 2003;362:1625–1627. doi: 10.1016/s0140-6736(03)14825-8. [DOI] [PubMed] [Google Scholar]
- 23.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 24.Demmler GJ. Cytomegalovirus. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, editors. Textbook of pediatric infectious diseases. 5th ed. Philadelphia: Saunders; 2004. pp. 1912–1932. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.