Background: Molecular mechanism by which MUC1 promotes cancer cell invasion is unclear.
Results: MUC1 forms a complex with NF-κB p65 transcription factor and binds to urokinase-type plasminogen activator (uPA) promoter, and thereby induces uPA expression.
Conclusion: uPA induced by MUC1 promotes cancer cell invasion.
Significance: uPA is a useful target for inhibiting invasion by MUC1-expressing cancer cells.
Keywords: Cancer; Cell Invasion; Mucin 1, Cell Surface Associated (MUC1); NF-κB Transcription Factor; Transcription Regulation; uPA
Abstract
Mucin 1 (MUC1) is overexpressed in various human malignant tumors and its expression is correlated with a poor prognosis. MUC1 engages in signal transduction by interacting with receptors for growth and differentiation factors, which contributes to the growth and survival of cancer cells. However, the mechanism by which MUC1 promotes cancer cell invasion remains unclear. Microarray analysis revealed that expression of urokinase-type plasminogen activator (uPA) was elevated in MUC1-overexpressing cells. Furthermore, up- and down-modulation of MUC1 expression was clearly correlated with the change of uPA expression. An immunochemical study showed that the distribution of uPA coincided with that of MUC1 in various human cancer tissues. The MUC1 C-terminal domain (MUC1-CD) was associated with nuclear factor-κB (NF-κB) p65 in MUC1-expressing cells. Chromatin immunoprecipitation (ChIP) assays demonstrated that MUC1-CD existed with NF-κB p65 on the uPA promoter. Luciferase assays indicated that the uPA transcriptional activity was correlated with the level of MUC1 expression and that this MUC1-enhancing effect on the uPA transcription was abolished by introduction of mutations into the NF-κB binding sites on the uPA promoter. These results indicate that formation of the MUC1-CD and NF-κB p65 complex enhanced nuclear translocation of NF-κB p65 and subsequent occupancy of NF-κB binding region on the uPA promoter, leading to elevated transcription of uPA. We also demonstrated that uPA induced by MUC1 enhanced the matrix metalloproteinase (MMP)-2 and -9 activities, and consequently promoted cancer cell invasion. Thus, a MUC1 co-operating NF-κB signaling pathway plays a critical role in cancer cell invasion in MUC1-expressing cells.
Introduction
Mucin 1 (MUC1),2 a type I transmembrane glycoprotein, is aberrantly overexpressed in various malignant tumors including colon, breast, and pancreas, and its expression level is correlated with a poor prognosis (1–4). MUC1 is translated as a single polypeptide that undergoes autocleavage in the endoplasmic reticulum, yielding N- and C-terminal subunits that form a heterodimeric complex bound through non-covalent interactions (5, 6). The N-terminal domain (MUC1-ND) is an extracellular domain containing variable numbers of 20-amino acid (AA) tandem repeats (7, 8), and the C-terminal domain (MUC1-CD) is comprised of a 58-AA extracellular domain, a 28-AA transmembrane domain and a 72-AA cytoplasmic tail (9). MUC1-CD also interacts with cytoplasmic proteins related to signal transduction such as β-catenin (10), STAT3 (11), and nuclear factor-κB (NF-κB) (12), which are, thereafter, translocated with MUC1-CD to the nucleus. The Cys-Gln-Cys (CQC) motif in MUC1-CD is required for its homodimerization and interaction with nuclear translocation-related proteins; importin β and nucleoporin p62 (13, 14). Since MUC1 is an extremely huge membrane-bound glycoprotein protruding from the cell surface, cell-cell and cell-extracellular matrix (ECM) interactions are sterically hindered by masking of adhesion molecules such as E-cadherin (15), cancer cell invasion and metastasis being thereby facilitated. Furthermore, degradation of the ECM by proteolytic enzymes is a crucial step in cancer metastasis. Among these enzymes, urokinase-type plasminogen activator (uPA) and matrix metalloproteinases (MMPs) play a key role in degrading the ECM.
uPA, a serine protease, is involved in cell invasion and metastasis (16, 17). uPA is produced by cancer cells and/or surrounding stromal cells as a proenzyme uPA (pro-uPA), and the pro-uPA secreted into the tumor microenvironment is converted to an active form through its binding to the uPA receptor (uPAR). uPA activates plasminogen through proteolytic cleavage to form plasmin. The plasmin can degrade components of the ECM directly and/or indirectly through MMPs, thereby facilitating cell invasiveness (18, 19). uPA also cleaves its own inhibitor, plasminogen activator inhibitor-1 (PAI-1) (20), and activates hepatocyte growth factor (HGF) (21) and platelet-derived growth factor-D (PDGF-D) (22). Additionally, the uPA/uPAR complex interacts with vitronectin and integrins (18, 23–25), which modulates the uPAR-mediated interaction between cells and the ECM and/or activates signaling pathways such as PI3K/AKT (26) and focal adhesion kinase (FAK)/c-Src signaling (27). The level of uPA expression is correlated with the invasive potential and a poor prognosis in various malignant tumors including colon, breast, and stomach (16, 28). The promoter of uPA is highly conserved and contains multiple functional elements including the NF-κB element (29–31).
NF-κB is composed of two subunits (RelA/p65 and p50), and forms a complex with inhibitor of IκB in the cytoplasm. NF-κB activation is induced by degradation of IκB by the IκB kinase (IKK) complex, and subsequently the activated NF-κB p65 subunit is translocated to the nucleus (32). It has been reported that MUC1-CD modulates the activity of the NF-κB pathway in breast cancer cells by interacting with and activating IKK family members and NF-κB p65 (12, 33).
In this study, we found that expression of uPA changed in parallel with the level of MUC1. To reveal the mechanism by which uPA is induced, we investigated whether or not MUC1-CD plays a role in the transcriptional regulation of uPA. Expectedly, formation of the MUC1-CD/NF-κB p65 complex promoted nuclear translocation of NF-κB p65 and occupied the uPA promoter. Thus, MUC1 contributes to NF-κB-mediated uPA transcriptional activation, this being consistent with the fact that MUC1 promotes the invasion and metastasis of cancer cells.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Goat anti-uPA, Armenian (Arm.) hamster anti-MUC1-CD, mouse anti-MUC1-ND, and rabbit anti-heat shock protein 90 β (HSP90 β) (monoclonal) antibodies were purchased from R&D Systems (Minneapolis, MN), Lab Vision (Fremont, CA), BD Biosciences (San Jose, CA), and Bioss (Woburn, MA), respectively. Rabbit anti-NF-κB p65 and goat anti-lamin B antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-β-actin monoclonal antibodies and amiloride, a uPA inhibitor, were from Sigma-Aldrich. GM 6001, a MMPs inhibitor, and JSH-23, an NF-κB inhibitor, were from Enzo Life Sciences (Farmingdale, NY) and Symansis (Auckland, New Zealand), respectively.
Cell Culture
Human HCT116 colon cancer, A549 lung cancer and SKOV3 ovary cancer cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). HCT116 and SKOV3 cells were maintained in DMEM containing 10% heat-inactivated FBS (HI-FBS), 4 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. A549 cells were cultured in F-12K medium (ATCC) containing 10% HI-FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Cell Transfection
MUC1 gene transfectants (HCT116/MUC1 and A549/MUC1) and control cells (HCT116/Mock and A549/Mock) were generated as described previously (34). MUC1 gene knockdown transfectants (SKOV3/Si-1 and -2) and control cells (SKOV3/Scr) were generated by introducing human MUC1 shRNA and scrambled shRNA vectors (OriGene, Rockville, MD), respectively, into SKOV3 cells using Fugene® HD transfection reagent (Promega, Madison, WI) according to the manufacturer's protocol. Stable transfectants were obtained by selection with puromycin (1 μg/ml).
Preparation of RNA and Microarray Analysis
Total RNA was isolated from HCT116/Mock and HCT116/MUC1 cells using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol for RNA extraction.
Total RNA was labeled with either cyanine-3 or cyanine-5 using a Low Input Quick Amp Labeling Kit (Agilent Technologies, Palo Alto, CA) according to the manufacturer's protocol, followed by purification on an RNeasy column (Qiagen, Hilden, Germany). Labeled RNAs were fragmented at 60 °C for 30 min and hybridized to Human Gene Expression 4 × 44K v2 Microarray (Agilent Technologies) at 65 °C for 17 h. Thereafter, the arrays were washed with GE Wash buffer 1 and GE Wash buffer 2 (Agilent Technologies), and dried by centrifugation, followed by scanning with an Agilent DNA Microarray Scanner G2565CA.
Preparation of Cell Lysates and Subcellular Fractionation
Cells were solubilized with cell lysis buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100 (Tx-100), and a Protease Inhibitor Mixture (Nacalai Tesque, Kyoto, Japan)), and then sonicated on ice for 1 min. Lysates were centrifuged at 15,000 × g at 4 °C for 10 min to remove cell debris.
Proteins in cytoplasmic and nuclear fractions were prepared using NE-PRE® Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific, Rockford, IL) according to the manufacturer's protocol. Protein was determined using the DC protein assay (Bio-Rad).
Immunoprecipitation (IP)
HCT116/MUC1 cells were solubilized with cell lysis buffer as described above. MUC1-CD and NF-κB p65 were immunoprecipitated from the lysates by successive incubation with anti-MUC1-CD or anti-NF-κB p65 antibodies, or the respective control IgG and PureProteomeTM Protein A or G Magnetic Beads (Millipore, Billerica, MA).
Immunoblotting (IB)
Proteins and immunoprecipitates were subjected to SDS-PAGE, followed by immunoblotting and incubation with anti-uPA, anti-MUC1-CD, anit-NF-κB p65, anti-HSP90 β, anti-lamin B, or anti-β-actin antibodies. Immune complexes were detected with HRP-conjugated secondary antibodies and chemiluminescence.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde in PBS at room temperature for 20 min and then washed with PBS. Thereafter, the cells were blocked, and permeabilized with 5% BSA and 0.1% Tx-100 in PBS at room temperature for 30 min, and then incubated overnight at 4 °C with anti-MUC1-ND, anti-uPA, anti-NF-κB p65, or anti-MUC1-CD antibodies. The cells, after washing with PBS, were stained with fluorescence-labeled secondary antibodies and DAPI. Images were obtained by confocal fluorescence microscopy (Leica, Mannheim, Germany).
Immunochemical and H&E Staining
Sections of paraffin-embedded tumor and nonmalignant tissues were deparaffinized with xylene and ethanol. Antigen retrieval was performed by treatment of the sections with 0.01 m citric acid buffer, pH 6.0, at 100 °C for 15 min. After washing with PBS, the sections were blocked with 5% BSA in PBS at room temperature for 1 h, and then incubated overnight at 4 °C with anti-MUC1-ND and anti-uPA antibodies. After washing with PBS, the sections were stained with fluorescence-labeled secondary antibodies and DAPI. Images were obtained by fluorescence microscopy (Nikon, Melville, NY). The tissues described above, thereafter, were also subjected to H&E staining. Specimens of tumor and adjacent nonmalignant tissues were obtained from cancer patients in accordance with the protocol approved by Osaka City University.
ChIP and re-ChIP Assays
These assays were performed basically according to Shang et al. (35). Subconfluent cells were cross-linked with 1% formaldehyde in DMEM at room temperature for 10 min, and then the cross-linking reaction was quenched with 0.125 m glycine in PBS at room temperature for 5 min. Thereafter, the cells were washed with cold PBS and collected with a cell scraper. After centrifugation at 2,500 rpm at 4 °C for 10 min, the pellets were resuspended in SDS lysis buffer (50 mm Tris-HCl, pH 8.0, 10 mm EDTA, 0.5% SDS, and a protease inhibitor) and incubated on ice for 15 min. After sonication for 10 s six times, the lysates were centrifuged at 15,000 rpm at 4 °C for 10 min. The supernatants were diluted 5-fold with ChIP dilution buffer (50 mm Tris-HCl, pH 8.0, 167 mm NaCl, 1.1% Tx-100 and 0.11% sodium deoxycholate), and then incubated overnight at 4 °C with anti-NF-κB p65 or anti-MUC1-CD antibodies, or the respective control IgG. Immune complexes were precipitated with Dynabeads® protein A or G (Invitrogen), and subsequently the beads were washed successively with the following buffers; low salt buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1 mm EDTA, 0.1% SDS, 1% Tx-100, and 0.1% sodium deoxycholate), high salt buffer (50 mm Tris-HCl, pH 8.0, 500 mm NaCl, 1 mm EDTA, 0.1% SDS, 1% Tx-100, and 0.1% sodium deoxycholate), LiCl buffer (10 mm Tris-HCl, pH 8.0, 250 mm LiCl, 1 mm EDTA, 0.5% Nonidet P-40, and 0.5% sodium deoxycholate), and Tris-EDTA buffer (10 mm Tris-HCl, pH 8.0, and 1 mm EDTA). The washed beads were resuspended in ChIP elution buffer (10 mm Tris-HCl, pH 8.0, 300 mm NaCl, 5 mm EDTA, and 0.5% SDS) and then incubated overnight at 65 °C to reverse the cross-linking. The eluates were collected and treated with RNase (100 μg/ml) at 37 °C for 30 min, and with proteinase K (100 μg/ml) at 55 °C for 2 h. Then, DNA was purified using a High Pure PCR Cleanup Micro Kit (Roche Applied Science, Indianapolis, IN) and subjected to PCR. For re-ChIP assays, MUC1-CD associated complexes obtained by primary ChIP were treated with re-ChIP elution buffer (50 mm Tris-HCl, pH 8.0, 2 mm EDTA, 1% SDS, 10 mm DTT, and a protease inhibitor) at 37 °C for 30 min. After centrifugation, the supernatant was diluted 20-fold with re-ChIP buffer (50 mm Tris-HCl, pH 8.0, 167 mm NaCl, 2 mm EDTA, and 1.1% Tx-100), from which MUC1-CD-associated complexes NF-κB p65 was immunoprecipitated with anti-NF-κB p65 antibodies. PCR was performed to amplify a distinct region of the uPA promoter with putative NF-κB binding sites (NF-κB BR) (forward primer, 5′-CTC TCA GCA ATC AGC ATG AC-3′; reverse primer, 5′-TCC TCT AGA AGA CTG TGG TCA G-3′), and a uPA non-promoter region (negative control region; CR) (forward primer, 5′-ACT TGG AGA ATG GAG CCT TG-3′; reverse primer, 5′-AGG TCT GCT GGT CGC TTA TC-3′). DNA was identified by 30–35 cycles of PCR, and the intensities of bands were determined with Image J software (National Institutes of Health).
Luciferase Constructs and Luciferase Assays
Genomic DNA was prepared from HCT116/MUC1 cells using NucleoSpin® Tissue (Macherey-Nagel, Düren, Germany). To amplify the uPA promoter region (−1933 to +58), PCR was performed using the following primers: forward primer, 5′-GGC AGA TCT CCT CCA GCC AAG TAA TCT GG-3′; reverse primer, 5′-AAT CCA TGG CTG CGG GGA CAG GTG GAC CC-3′. A BglII/NcoI-blunt-ended PCR product was cloned into the pCR®-Blunt II-TOPO® vector (Invitrogen) according to the manufacturer's protocol, sequenced, and then subcloned into PicaGene Basic Vector 2 (Toyo Ink, Tokyo, Japan: WT/uPA vector). The uPA promoter mutated at the NF-κB and TCF4 (T-cell factor 4) binding site was generated by site-directed mutagenesis using a KOD-Plus-Mutagenesis Kit (TOYOBO, Tokyo, Japan: MutNF-κB/uPA and MutTCF4/uPA vectors, respectively). Mutations from GGG to ACC in the two NF-κB consensus sequences (nucleotide positions; −1867 to −1865 and −1844 to −1842) and ones from TT to CG in the two TCF4 consensus sequences (nucleotide positions; −739 to −738 and −564 to −563) were introduced.
To determine uPA promoter activity, cells were co-transfected with the pRL-TK vector (Toyo Ink) plus the WT/uPA vector, the MutNF-κB/uPA vector, the MutTCF4/uPA vector or an empty vector using Fugene® HD transfection reagent, and after 24 h, the promoter activity was assayed by using a PicaGene Dual Sea Pansy Luminescence Kit (Toyo Ink) according to the manufacturer's protocol. Promoter activity (i.e. Firefly luciferase) was normalized as to transfection efficiency by using Renilla luciferase activity (pRL-TK).
ELISA
Subconfluent cells were washed with PBS and then cultured in serum-free medium for 24 h. uPA in the conditioned medium was measured using an ELISA kit, an AssayMax Human Urokinase (uPA) ELISA Kit (Assaypro, St. Charles, MO), according to the manufacturer's protocol.
Gelatin and Casein Zymography Assays
These assays were performed basically according to He et al. (36) and Toupance et al. (37). The conditioned medium was electrophoresed on an SDS-PAGE gel containing gelatin (1 mg/ml) or casein (0.1% (w/v)) and plasminogen (5 μg/ml) under non-reducing conditions. The gels were washed with 2.5% Tx-100 in 10 mm Tris-HCl buffer, pH 7.5, at room temperature for 1 h to remove SDS, and subsequently incubated with 10 mm Tris-HCl buffer, pH 7.5, at room temperature for 30 min. Thereafter, the gels for casein zymography were soaked in 50 mm Tris-HCl buffer, pH 7.5, containing 150 mm NaCl and 10 mm CaCl2 at 37 °C for 14 h. The gels for gelatin zymography were soaked in the above solution plus 2 μm ZnCl2 at 37 °C for 36 h. The gels were stained with Coomassie Blue R250.
Treatment with Chemical Inhibitors
HCT116/MUC1 cells were treated with JSH-23 (10 μm) or DMSO for 30 h to examine their effects on the formation and nuclear translocation of the MUC1-CD/NF-κB p65 complex and uPA expression. HCT116/MUC1 cells were incubated with GM6001 (20 μm), amiloride (25 μm), JSH-23 (10 μm), or DMSO to examine the effect on cell invasion.
Cell Migration and Invasion Assays
In vitro cell migration and invasion assays were performed using transwell chambers (24-well culture plates) with polycarbonate membranes (pore size, 8.0 μm; Corning Inc, Corning, NY). The membrane was pre-coated with fibronectin (1 μg, Asahi Glass, Tokyo, Japan) and Matrigel (5 μg, BD Biosciences) for migration and invasion assays, respectively. The cells were suspended in serum-free medium containing 0.1% BSA and then seeded into the upper chambers. In some cases, the cells were treated with chemical inhibitors as described above. The lower wells were filled with medium containing 10% HI-FBS. After incubation for 20 h, the membranes were fixed with methanol and stained with a H&E solution, and then non-migrating or non-invading cells were removed with a cotton swab. The numbers of migrating or invading cells were determined in five random fields per membrane and the ratio defined by the following formula is referred as percent invasion. Percent invasion = mean number of invading cells/mean number of migrating cells × 100.
Statistical Analysis
Differences between two groups were assessed using Student's t test. Differences among three or more groups were evaluated by analysis of variance (ANOVA), followed by Tukey-Kramer or Dunnett test.
RESULTS
uPA Expression Is Correlated with the Level of MUC1 in Various Tumors
Aberrant overexpression of MUC1 in various malignant tumors is correlated with a poor prognosis. To determine how MUC1 induces tumor progression, we generated MUC1-overexpressing cells by introducing human MUC1cDNA into a human colon cancer cell line, HCT116 cells (HCT116/MUC1), and performed microarray analysis of mRNA using HCT116/MUC1 and HCT116/Mock cells to identify the differently expressed mRNA in these cells. Among some genes that were differently expressed in the two types of cells, we focused on that of urokinase-type plasminogen activator (uPA), the expression of which was elevated about 2.4-fold in HCT116/MUC1 cells as compared with in HCT116/Mock cells (Fig. 1A), because uPA plays an essential role in tumor invasion. uPA expression in some cells expressing different levels of MUC1 was further investigated using a human lung epithelial cancer cell line, A549 cells, and a human ovary cancer cell line, SKOV3 cells. Since A549 cells express a low level of MUC1, MUC1-overexpressing cells (A549/MUC1) were prepared by introduction of human MUC1cDNA. Conversely, since SKOV3 cells endogenously express MUC1, MUC1 knockdown cells (SKOV3/Si-1 and -2) were obtained by MUC1 shRNA treatment. The cell lysates were subjected to SDS-PAGE, followed by immunoblotting and detection with anti-uPA and anti-MUC1-CD antibodies. The level of uPA was increased in MUC1-overexpressing cells (HCT116/MUC1 and A549/MUC1) and reduced in MUC1 knockdown cells (SKOV3/Si-1 and -2) as compared with in the respective control cells (Fig. 1B). Increased expression of uPA in HCT116/MUC1 cells was also confirmed by immunostaining (Fig. 1C).
FIGURE 1.

Related expression of uPA with MUC1 in various cancer cells. A, expression of MUC1 and uPA mRNA in HCT116/Mock and HCT116/MUC1 cells was examined by microarray analysis. B, cell lysates prepared from HCT116/Mock, HCT116/MUC1, A549/Mock, A549/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were subjected to SDS-PAGE, followed by immunoblotting (IB), and detection with anti-uPA, anti-MUC1-CD, and anti-β-actin antibodies. β-Actin served as a loading control. C, expression of MUC1 and uPA in HCT116/Mock and HCT116/MUC1 cells was detected immunocytochemically using the combinations of mouse anti-MUC1-ND and Alexa Fluor 488-labeled rabbit anti-mouse IgG antibodies (green), and goat anti-uPA and Alexa Fluor 594-labeled rabbit anti-goat IgG antibodies (red). Nuclei were stained with DAPI (blue). Images were taken at ×630 magnification. Scale bar: 25 μm.
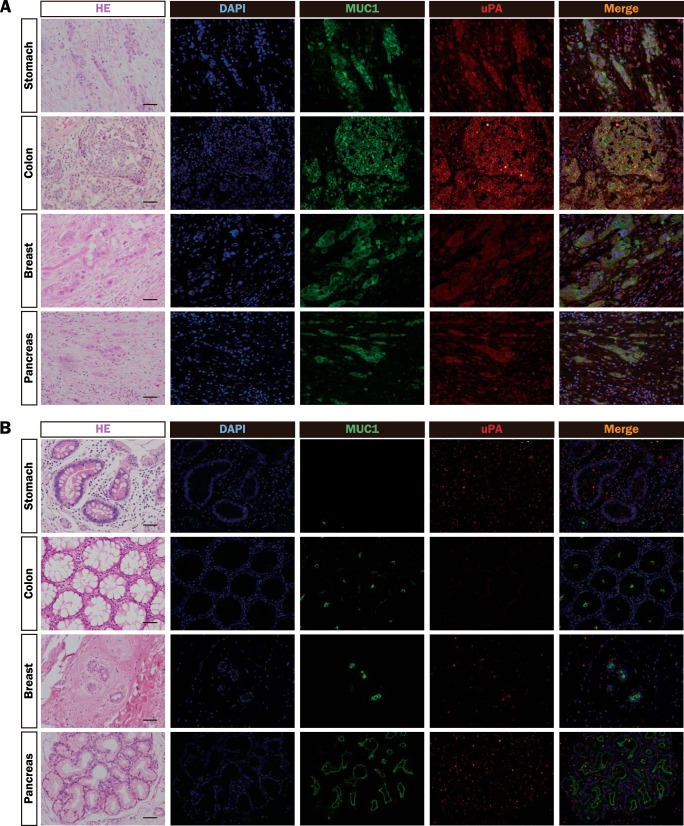
To further investigate the distributions of uPA and MUC1, human tumor tissues (Fig. 2A) and nonmalignant tissues (Fig. 2B) were immunostained with anti-MUC1-ND and anti-uPA antibodies. Expectedly, the distribution of uPA coincided with that of MUC1 in tumor tissues. Thus, these results suggest that the expression of uPA is strongly correlated with that of MUC1.
FIGURE 2.
Distributions of MUC1 and uPA in various tumor and nonmalignant tissues. A and B, sections of paraffin-embedded human tumor (A) and nonmalignant (B) tissues (stomach, colon, breast, and pancreas) were stained with H&E, DAPI (blue), and the combinations of mouse anti-MUC1-ND and Alexa Fluor 488-labeled rabbit anti-mouse IgG antibodies (green), and goat anti-uPA and Alexa Fluor 594-labeled rabbit anti-goat IgG antibodies (red), and then observed by microscopy. Images were taken at ×200 magnification. Scale bar: 100 μm.
Interaction of MUC1-CD with the NF-κB p65 Subunit Promotes Translocation of Their Complex to the Nucleus
The transcription of uPA is regulated by nuclear factor-κB (NF-κB) (30, 31), and the MUC1 C-terminal domain (MUC1-CD) interacts directly with NF-κB (12). MUC1-CD was immunoprecipitated from HCT116/MUC1 cell lysates and subjected to SDS-PAGE, followed by immunoblotting. NF-κB p65 was co-immunoprecipitated with MUC1-CD (Fig. 3A, lane b). In a reciprocal experiment, MUC1-CD was detected in the immunoprecipitates with anti-NF-κB p65 antibodies (Fig. 3A, lane d). Thus, formation of the MUC1-CD/NF-κB p65 complex was confirmed in HCT116/MUC1 cells. Next, we examined whether or not its complex formation promotes the nuclear translocation of NF-κB. Lysates of HCT116 and SKOV3, and the respective MUC1-overexpressing and knockdown cells prepared as described under “Experimental Procedures” were fractionated into cytoplasmic and nuclear fractions. The fractions were directly subjected to SDS-PAGE, followed by immunoblotting and detection with anti-NF-κB p65 and anti-MUC1-CD antibodies. In addition to the cytoplasmic fraction, MUC1-CD was also detected in the nuclear fractions of HCT116/MUC1 (Fig. 3B, lane d) and SKOV3/Scr (Fig. 3B, lane h) cells. Although similar levels of NF-κB p65 were detected in the cytoplasmic fractions of these cells irrespective of the different levels of MUC1 (Fig. 3B, lanes a, b, e, f, g), NF-κB p65 was increased (Fig. 3B, lanes c, d), and decreased (Fig. 3B, lanes h, i, j) in the nuclear fractions of HCT116/MUC1 cells, and SKOV3/Si-1 and -2 cells, respectively. These results indicate that NF-κB p65 in the nuclear fractions increased in parallel with the level of MUC1. Using confocal imaging, the distributions of MUC1-CD and NF-κB p65 in the nucleus were examined immunochemically. As shown in Fig. 3C, NF-κB p65 was completely co-localized with MUC1-CD in the nucleus, suggesting that nuclear translocation of NF-κB p65 was promoted by formation of the complex with MUC1-CD.
FIGURE 3.

Translocation of the MUC1-CD/NF-κB p65 complex to the nucleus. A, MUC1-CD and NF-κB p65 were immunoprecipitated (IP) from lysates of HCT116/MUC1 cells with anti-MUC1-CD (lane b) or anti-NF-κB p65 (lane d) antibodies, or the respective control IgG (lanes a, c), and then subjected to SDS-PAGE, followed by immunoblotting (IB), and detection with anti-NF-κB p65 and anti-MUC1-CD antibodies. B, proteins contained in cytoplasmic and nuclear fractions prepared from HCT116/Mock, HCT116/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were subjected to SDS-PAGE, followed by immunoblotting (IB), and detection with anti-NF-κB p65, anti-MUC1-CD, anti-HSP90 β, and anti-lamin B antibodies. HSP90 β and lamin B served as cytoplasmic and nuclear fraction markers, respectively. C, HCT116/Mock and HCT116/MUC1 cells were immunostained with the combinations of rabbit anti-NF-κB p65 and Alexa Fluor 488-labeled goat anti-rabbit IgG antibodies (green), and Arm. hamster anti-MUC1-CD and Alexa Fluor 594-labeled goat anti-Arm. hamster IgG antibodies (red), and with DAPI (blue). The arrow indicates the MUC1-CD/NF-κB p65 complex in the nucleus.
The MUC1-CD/NF-κB p65 Complex Is Recruited to the uPA Promoter
The uPA promoter contains two potential NF-κB binding sites (−1867 to −1858 and −1844 to −1835), and transcription of uPA is regulated by the binding of NF-κB to its promoter. To determine whether or not the MUC1/NF-κB complex is responsible for transcriptional regulation of uPA, ChIP assays were performed using anti-NF-κB p65 antibodies. Chromatin was immunoprecipitated from the cells expressing different levels of MUC1 as described above with anti-NF-κB p65 antibodies. The uPA promoter region containing two consensus NF-κB binding sites (NF-κB BR; −1955 to −1729) and an other region as a control (CR; +3348 to +3531) were amplified by PCR, and the intensities of the bands were determined as described under “Experimental Procedures.” Occupancy of NF-κB p65 at NF-κB BR in HCT116/MUC1 cells was enhanced about 4-fold as compared with in HCT116/Mock cells (Fig. 4, A and C). Similarly, silencing of MUC1 in SKOV3 cells remarkably decreased the occupancy of NF-κB p65 in SKOV3/Si-1 and -2 cells (Fig. 4, B and D). Furthermore, re-ChIP assays were performed to verify that NF-κB p65 associated with the uPA promoter was simultaneously associated with MUC1-CD. The uPA promoter was co-occupied by NF-κB p65 and MUC1-CD in HCT116/MUC1 cells (Fig. 4, E and G) and SKOV3/Scr cells (Fig. 4, F and H). These findings suggest that MUC1-CD promotes recruitment of NF-κB p65 to the uPA promoter.
FIGURE 4.
Enhanced occupancy of NF-κB p65 on the uPA promoter in a MUC1-CD-dependent manner. A and B, NF-κB p65 containing complexes were obtained from cell lysates of HCT116/Mock, HCT116/MUC1 (A), SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 (B) cells with anti-NF-κB p65 antibodies or a control IgG. The DNA eluted from the immunoprecipitates was amplified by PCR with pairs of primers corresponding to the NF-κB binding region (NF-κB BR; −1955 to −1729) or a negative control region (CR; +3348 to +3531), and then subjected to agarose gel electrophoresis, followed by staining by ethidium bromide (EtBr). C and D, intensities of the bands in Fig. 4, A and B were determined with Image J software. The value obtained from the intensity of input DNA was taken as 100% (means ± S.D., n = 3, **, p < 0.01). E and F, MUC1-CD containing complexes were obtained from cell lysates of HCT116/MUC1 (E) and SKOV3/Scr (F) cells with anti-MUC1-CD antibodies or a control IgG. In re-ChIP assays, the MUC1-CD containing complexes described above were dissociated, and then re-immunoprecipitated with anti-NF-κB p65 antibodies. The DNA eluted from the (re)-immunoprecipitates was amplified by PCR, and analyzed as described in Fig. 4, A and B. G and H, intensities of the bands in Fig. 4, E and F were determined as described in Fig. 4, C and D (means ± S.D., n = 3).
The MUC1-CD/NF-κB p65 Complex Enhances uPA Transcriptional Activity through Its Binding to the uPA Promoter
To further determine whether or not uPA transcriptional activity is regulated through the mechanism described above, luciferase assays were performed. As shown in Fig. 5A, the uPA transcriptional activity was enhanced in MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr) as compared with in the respective control cells. Furthermore, to assess in more detail whether or not this transcriptional activation is induced through the formation of the MUC1-CD/NF-κB p65 complex on the uPA promoter, we constructed plasmids in which mutations (GGG to ACC; −1867 to −1865 and −1844 to −1842) were introduced into the two NF-κB binding consensus sequences, and then the plasmids were transfected into the cells. Expectedly, the uPA transcriptional activity was reduced to the level in control cells by the mutations of the NF-κB binding sites in the uPA promoter region in MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr) (Fig. 5A), indicating that binding of the MUC1-CD/NF-κB complex to the uPA promoter actually induces transcription of uPA. In this context, expression of uPA is known to be regulated by multiple elements including NF-κB. For instance, previous reports have suggested that binding of β-catenin to the uPA promoter also up-regulates the expression of uPA in colorectal cancers (38). MUC1-CD forms a complex with β-catenin and thereby β-catenin is stabilized (10, 34, 39–41). Thus, the MUC1-CD/β-catenin complex is also expected to regulate the uPA expression. It is well known that TCF/LEF (T-cell factor/lymphoid enhancer factor) transcription factor is necessary for the MUC1-CD/β-catenin complex to bind to the promoters of β-catenin/TCF signaling target genes. Therefore, if uPA expression is regulated by the MUC1-CD/β-catenin complex, it is predicted that uPA transcriptional activity is altered by mutation of the TCF binding sites on its promoter. To investigate this possibility, we constructed a plasmid in which mutations (TT to CG; −739 to −738 and −564 to −563) were introduced into the TCF4 binding consensus sequence, and then the plasmids were transfected into MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr). The transcriptional activity was determined as described in Fig. 5A, it being revealed that uPA transcriptional activity was not altered by the mutation of two TCF4 binding sites in the uPA promoter region (Fig. 5B). Thus, these findings suggest that transcription of uPA is regulated by the MUC1-CD/NF-κB p65 complex but not by MUC1-CD/β-catenin complex in HCT116 and SKOV3 cells.
FIGURE 5.
Enhancement of uPA transcriptional activity in relation to the elevated occupancy of NF-κB p65 on the uPA promoter. A, HCT116/Mock, HCT116/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were transiently co-transfected with a control reporter vector, the pRL-TK vector, plus an empty vector, the WT/uPA vector or the MutNF-κB/uPA vector. B, HCT116/MUC1 and SKOV3/Scr cells were transiently co-transfected with a control reporter vector, the pRL-TK vector, plus an empty vector, the WT/uPA vector or the MutTCF4/uPA vector. The respective cells co-transfected as described above were cultured for 24 h, and then uPA promoter activities of each cell were analyzed by means of luciferase assays. The values obtained for each reporter vector transfectants were normalized as to an internal control, Renilla luciferase, and the promoter activity of empty vector transfectants was taken as 1 (means ± S.D., n = 3, **, p < 0.01; NS, not significant). WT, wildtype uPA promoter vector transfectants; MutNF-κB, NF-κB binding site-mutated uPA promoter vector transfectants; MutTCF4, TCF4 binding site-mutated uPA promoter vector transfectants.
MUC1 Promotes Cell Invasiveness through Elevation of the uPA Level
we next investigated whether or not the MUC1 level is closely related to the ability of cancer cell invasion as a phenotype. uPA is secreted into the extracellular space, and plays a critical role directly and/or indirectly in cancer cell invasion. Thus, we measured the amounts of secreted uPA in the conditioned medium of the cells expressing different levels of MUC1 as described above by ELISA. The level of secreted uPA increased prominently in HCT116/MUC1 cells, and decreased moderately in SKOV3/Si-1 and -2 cells (Fig. 6A). Since uPA is involved in matrix metalloproteinases (MMPs) activation, we also measured uPA and MMPs activities in the conditioned medium by means of zymography assays. Consistently, higher levels of uPA and MMP-2/9 activities were detected in MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr) as compared with in the respective control cells (Fig. 6B). The uPA and MMPs activities are well-known to be involved in cell invasion. Thus, we performed invasion and migration assays using Matrigel- and fibronectin-coated chambers, respectively, and calculated the ratio of invading cells versus migrating cells to exclude the possibility that other cell properties such as cell proliferation affect the invasion assay. The invasive ability was enhanced in MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr) as compared with in the respective control cells (Fig. 7, A and B), this being consistent with the levels of enzymatic activities of uPA and MMPs.
FIGURE 6.

Activities of uPA and MMP-2/9 in the conditioned media of various cells expressing different levels of MUC1. A, levels of uPA secreted into the conditioned medium of HCT116/Mock, HCT116/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were analyzed by ELISA (means ± S.D., n = 3, **, p < 0.01). B, MMP-2/9 and uPA activities in the conditioned medium of the cells described above were analyzed by gelatin zymography and casein zymography. Equal amounts of protein from the conditioned medium were subjected to SDS-PAGE and the gels were treated as described under “Experimental Procedures.”
FIGURE 7.
Cell invasiveness of various cells expressing different levels of MUC1. A, cell invasiveness of HCT116/Mock, HCT116/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells was evaluated by means of Boyden chamber cell migration and invasion assays, which were performed by using fibronectin (FN)- and Matrigel (MG)-coated chambers, respectively. The cells (5 × 104 cells) were seeded into the upper chambers, and after 20 h, the membranes were fixed as described under “Experimental Procedures,” and images of migrating or invading cells were taken (×100 magnification, scale bar: 200 μm). B, numbers of cells that passed through fibronectin-coated membranes (migrating cells) or Matrigel-coated membranes (invading cells) were determined in five random fields. Histographs show the ratios of invading cells versus migrating cells as percent invasion. Percent invasion = mean number of invading cells (MG)/mean number of migrating cells (FN) × 100 (means ± S.D., n = 3, *, p < 0.05 and **, p < 0.01).
Furthermore, to determine whether or not up-regulation of the cell invasion is really caused by uPA induced by MUC1, we performed the invasion assays using cells treated with GM6001, amiloride and JSH-23, which are inhibitors of MMPs, uPA and NF-κB, respectively. As shown in Fig. 8, A and B, cell invasion was clearly inhibited by all these inhibitors. It should also be noted that inhibition of NF-κB had a similar inhibitory effect on cell invasion as that of MMPs and uPA. In this context, we examined the effect of JSH-23 on the formation of the MUC1-CD/NF-κB p65 complex, and subsequent nuclear translocation and uPA expression. To examine the formation of the MUC1-CD/NF-κB p65 complex, co-immunoprecipitation assays were performed using HCT116/MUC1 cells treated with or without JSH-23. NF-κB p65 was co-immunoprecipitated with MUC1-CD from the lysates of JSH-23-non-treated cells but not from those of JSH-23-treated cells (Fig. 9A). Furthermore, nuclear translocation of the MUC1-CD/NF-κB p65 complex and uPA expression were also eventually down-regulated (Fig. 9, B and C). In addition, by using MTT assays, we also confirmed that these inhibitors had no effect on cell viability under the same conditions as those used for the invasion assays (data not shown). Thus, these findings suggest that uPA induced by MUC1 enhances the invasive ability of MUC1-expressing cancer cells.
FIGURE 8.

Inhibitory effects of GM6001, amiloride and JSH-23 on the invasiveness of HCT116/MUC1 cells. A, cell invasion assay was performed in the presence of GM6001 (20 μm), amiloride (25 μm), JSH-23 (10 μm), or DMSO (vehicle) in HCT116/MUC1 cells as described under “Experimental Procedures.” Images of the cells that passed through Matrigel-coated membranes were taken as described in Fig. 7A. B, degree of invasion was assessed, the value obtained in the control experiment in which the cells were treated with DMSO being taken as 100% (means ± S.D., n = 3, **, p < 0.01).
FIGURE 9.

Effect of JSH-23 on the formation of the MUC1-CD/NF-κB p65 complex. A, MUC1-CD and NF-κB p65 were immunoprecipitated (IP) from lysates of HCT116/MUC1 cells treated with JSH-23 (10 μm) or DMSO (vehicle) and analyzed as described in Fig. 3A. B, NF-κB p65, MUC1-CD, HSP90 β, and lamin B contained in cytoplasmic and nuclear fractions prepared from HCT116/MUC1 cells treated with JSH-23 (10 μm) or DMSO (vehicle) were analyzed as described in Fig. 3B. C, cell lysates prepared from HCT116/MUC1 cells treated with JSH-23 (10 μm) or DMSO (vehicle) were subjected to SDS-PAGE, followed by immunoblotting (IB), and detection with anti-uPA, anti-NF-κB p65, anti-MUC1-CD, and anti-β-actin antibodies. β-Actin served as a loading control.
DISCUSSION
Overexpression of MUC1 contributes to constitutive activation of growth and survival pathways as well as cell invasion and metastasis (1–4). MUC1 engages in signal transduction through MUC1-ND-mediated ligand-binding (39, 42, 43) or by interacting with receptors for growth (34, 40) and differentiation factors (10–12), which contributes to the growth and survival of cancer cells. With respect to invasion and metastasis, its molecular role has not been clarified. MUC1 plays a role in the physical barrier of cell-cell and cell-ECM interactions, which enhance cell invasion. From another point of view, MUC1 may be relevant to cell invasion more directly through the signaling events associated with MUC1. For instance, expression of PDGF-A is elevated by interaction of MUC1 with hypoxia-inducible factor 1-α (HIF1-α) in pancreatic cancer cells, leading to cell invasion (44). Singh et al. have also shown that PDGF receptor-β (PDGFR-β)-mediated phosphorylation of MUC1 enhances invasion by pancreatic adenocarcinoma cells (45). However, the MUC1-mediated events that enhance cell invasion have not been elucidated in detail. By microarray analysis using MUC1-expressing cells, we demonstrated that uPA may be one of the factors regulated by MUC1 (Fig. 1A). uPA expression seems to parallel the level of MUC1 in various cancer cells (Fig. 1B). Consistently, the distribution of uPA in various human tumor tissues precisely coincided with that of MUC1 (Fig. 2A). In addition, the levels of both MUC1 and uPA in various human tumor tissues seem to be higher than that of nonmalignant tissues (Fig. 2, A and B). Based on these results, we postulated that uPA expression may play a pivotal role in promoting cell invasion in MUC1-expressing cancer cells, because it is generally agreed that uPA is involved in the invasive potential and a poor prognosis in various malignant tumors (16, 17). Previous reports have also demonstrated that high levels of MUC1 could be detected in invasive and metastatic carcinomas among various types of tumors (1–3). Thus, the relationship between MUC1 and the invasive potential of cancer cells suggests that MUC1 may be related with uPA-mediated events.
MUC1 is associated with various transcription factors through its cytoplasmic region; one of which is NF-κB. MUC1-CD cannot only interact directly with NF-κB p65 through a GGSSLSY motif, but also decrease the interaction of NF-κB with IκBα by enhancing the phosphorylation and degradation of IκBα, and consequently promotes transcription of the NF-κB target genes (12, 33). In addition, uPA has been revealed to be included among the NF-κB target genes. Thus, we speculated that the underlying molecular mechanism of MUC1-enhanced invasiveness could be explained by the function of uPA, the expression of which may be promoted by association of MUC1-CD with NF-κB. Enhanced translocation of the MUC1-CD/NF-κB p65 complex (Fig. 3B) and the merged image of the nucleus (Fig. 3C) clearly indicate that its complex promotes nuclear translocation of NF-κB p65 in MUC1-expressing cells. Notably, the MUC1-CD/NF-κB p65 complex was revealed to be associated directly with the NF-κB binding region on the uPA promoter (Fig. 4), leading to NF-κB-mediated uPA transcriptional activation (Fig. 5A). Interestingly, it has been reported that gene transcription of zing finger E-box binding homeobox 1 (ZEB1), which triggers epithelial-mesenchymal transition (EMT), is regulated similarly by formation of the MUC1-CD/NF-κB p65 complex in breast cancer cells (46).
uPA is secreted from cancer cells and/or surrounding stromal cells into the extracellular space, and subsequently activates MMPs. As shown in Fig. 6, the levels of secreted uPA and MMP-2/9 as well as their enzyme activities were elevated in MUC1-expressing cells. Activation of uPA is initiated by its binding to uPAR and then the uPA/uPAR complex activates signaling pathways. It has been reported that uPAR is expressed in various human cancers, and its expression is frequently correlated with a poor prognosis, and in some cases is predictive of invasion and metastasis (47–49). Furthermore, uPAR expression is regulated by β-catenin signaling as well as uPA expression (50, 51). Therefore, there is a possibility that uPAR may also be expressed in a MUC1-dependent manner. To investigate this possibility, we examined the expression of uPAR in HCT116/Mock and HCT116/MUC1 cells by using flow cytometry and SDS-PAGE. No significant differences in uPAR expression were detected between the two types of cells (data not shown). In contrast, since expression of MMPs, especially that of MMP-9, is regulated by the NF-κB signaling pathway (52), enhancement of MMPs activities in MUC1-expressing cells may not be wholly due to the elevated expression of uPA. Thus, the levels of MMP-2/9 in HCT116/Mock and HCT116/MUC1 cells were also compared by SDS-PAGE. No significant difference in MMP-2/9 expression was detected between the two types of cells (data not shown). Therefore, changes in MMPs activities in various cells expressing different levels of MUC1 are solely dependent on the level of uPA expression regulated by MUC1, indicating a significant role of MUC1.
uPA and MMP-2/9 activation is responsible for degradation of the ECM, thereby facilitating cell invasion. Indeed, percent invasion was increased for MUC1-expressing cells, and correlated with the uPA and MMP-2/9 enzymatic activities (Fig. 7). It has been reported that MUC1 itself regulates cellular functions such as cell proliferation, cell viability and migration (4). Thus, it is important to discriminate the function of MUC1-induced molecules from that of MUC1 itself. We performed both invasion and migration assays to evaluate the contributions of uPA and MMP-2/9 more precisely. As shown in Fig. 8, enhancement of cell invasion found in HCT116/MUC1 cells was inhibited by treatment with MMPs, uPA and NF-κB inhibitors. In particular, it should be noted that JSH-23, an NF-κB inhibitor, exhibited a similar inhibitory effect on cell invasion as that of MMPs and uPA inhibitors. These results strongly suggest that cancer cell invasion is regulated by the MUC1 co-operating cascade of the MUC1-CD/NF-κB-uPA-MMPs signaling pathway (Fig. 10).
FIGURE 10.

Cell invasion-enhancing cascade in MUC1-expressing cancer cells. Formation of the MUC1-CD/NF-κB p65 complex promotes the nuclear translocation of NF-κB p65 and subsequently enhances the uPA transcriptional activity, resulting in enhancement of uPA expression and subsequent cell invasion by MUC1-expressing cancer cells.
Including our present study, in most studies on MUC1-related transcriptional regulation, MUC1-CD but not MUC1-ND has been focused. Recently, we reported that siglec-9, a sialic acid-binding Ig-like lectin, and galectin-3, a β-galactoside-binding lectin, induce the formation of the MUC1-CD/β-catenin complex through the binding of these lectins to MUC1-ND (39, 53). Since we also found that HCT116/MUC1 cells produced galectin-3 endogenously and a part of it associated on the cell surface, and that galectin-3 bound to MUC1-ND (data not shown), we speculated that the formation of the MUC1-CD/NF-κB p65 complex might be enhanced by the binding of galectin-3 to MUC1-ND. To examine this possibility, after excluding the cell surface-bound galectin-3 by washing with lactose-containing medium, HCT116/MUC1 cells were treated with recombinant galectin-3 and then the level of NF-κB p65 co-immunoprecipitated with MUC1-CD was determined. When HCT116/MUC1 cells were incubated with recombinant galectin-3, NF-κB p65 co-immunoprecipitated with MUC1-CD increased, suggesting that the binding of galectin-3 to MUC1-ND enhanced the formation of the MUC1-CD/NF-κB p65 complex (data not shown). Detailed studies on this issue are currently underway.
In conclusion, our results demonstrate that MUC1 enhances the invasiveness of cancer cells through activation of the NF-κB signaling pathway by the formation of the MUC1-CD/NF-κB p65 complex, and subsequent production of uPA and activation of MMP-2/9. This cascade is a novel mechanism by which MUC1 promotes cell invasion, and inhibition of this cascade may be one of the useful targets for treating cancer. Indeed, in one instance, it has been demonstrated that GO-203 cell-penetrating peptide, which blocks MUC1-CD dimerization, inhibits the proliferation of MUC1-expressing cancer cells, and has entered phase I evaluation for patients with refractory tumors (13, 54). Our results also suggest that not only MUC1 but also uPA may be a useful target for inhibiting invasion by MUC1-expressing cancer cells.
This work was supported by the Private University Strategic Research Foundation Support Program.
- MUC1
- mucin 1
- MUC1-ND
- MUC1 N-terminal domain
- MUC1-CD
- MUC1 C-terminal domain
- uPA
- urokinase-type plasminogen activator
- NF-κB
- nuclear factor-κB
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Nakamori S., Ota D. M., Cleary K. R., Shirotani K., Irimura T. (1994) MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 106, 353–361 [DOI] [PubMed] [Google Scholar]
- 2. Kufe D., Inghirami G., Abe M., Hayes D., Justi-Wheeler H., Schlom J. (1984) Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma 3, 223–232 [DOI] [PubMed] [Google Scholar]
- 3. Qu C. F., Li Y., Song Y. J., Rizvi S. M., Raja C., Zhang D., Samra J., Smith R., Perkins A. C., Apostolidis C., Allen B. J. (2004) MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br. J. Cancer 91, 2086–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kufe D. (2009) Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V., Hilkens J. (1992) Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 267, 6171–6177 [PubMed] [Google Scholar]
- 6. Parry S., Silverman H. S., McDermott K., Willis A., Hollingsworth M. A., Harris A. (2001) Identification of MUC1 proteolytic cleavage sites in vivo. Biochem. Biophys. Res. Commun. 283, 715–720 [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui J., Abe M., Hayes D., Shani E., Yunis E., Kufe D. (1988) Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc. Natl. Acad. Sci. U.S.A. 85, 2320–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. (1988) A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J. Biol. Chem. 263, 12820–12823 [PubMed] [Google Scholar]
- 9. Merlo G. R., Siddiqui J., Cropp C. S., Liscia D. S., Lidereau R., Callahan R., Kufe D. (1989) Frequent alteration of the DF3 tumor-associated antigen gene in primary human breast carcinomas. Cancer Res. 49, 6966–6971 [PubMed] [Google Scholar]
- 10. Yamamoto M., Bharti A., Li Y., Kufe D. (1997) Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 272, 12492–12494 [DOI] [PubMed] [Google Scholar]
- 11. Ahmad R., Rajabi H., Kosugi M., Joshi M. D., Alam M., Vasir B., Kawano T., Kharbanda S., Kufe D. (2011) MUC1-C oncoprotein promotes STAT3 activation in an autoinductive regulatory loop. Sci. Signal. 4, ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad R., Raina D., Joshi M. D., Kawano T., Ren J., Kharbanda S., Kufe D. (2009) MUC1-C oncoprotein functions as a direct activator of the nuclear factor-κB p65 transcription factor. Cancer Res. 69, 7013–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raina D., Ahmad R., Rajabi H., Panchamoorthy G., Kharbanda S., Kufe D. (2012) Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. Int. J. Oncol. 40, 1643–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leng Y., Cao C., Ren J., Huang L., Chen D., Ito M., Kufe D. (2007) Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J. Biol. Chem. 282, 19321–19330 [DOI] [PubMed] [Google Scholar]
- 15. Wesseling J., van der Valk S. W., Hilkens J. (1996) A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol. Biol. Cell 7, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreasen P. A., Kjøller L., Christensen L., Duffy M. J. (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int. J. Cancer 72, 1–22 [DOI] [PubMed] [Google Scholar]
- 17. Danø K., Behrendt N., Høyer-Hansen G., Johnsen M., Lund L. R., Ploug M., Rømer J. (2005) Plasminogen activation and cancer. Thromb. Haemost. 93, 676–681 [DOI] [PubMed] [Google Scholar]
- 18. Blasi F., Carmeliet P. (2002) uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 3, 932–943 [DOI] [PubMed] [Google Scholar]
- 19. Mason S. D., Joyce J. A. (2011) Proteolytic networks in cancer. Trends Cell Biol. 21, 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen L. S., Andreasen P. A., Grøndahl-Hansen J., Skriver L., Danø K. (1986) Plasminogen activators catalyse conversion of inhibitor from fibrosarcoma cells to an inactive form with a lower apparent molecular mass. FEBS Lett. 196, 269–273 [DOI] [PubMed] [Google Scholar]
- 21. Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. (1992) Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 11, 4825–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ustach C. V., Kim H. R. (2005) Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol. Cell Biol. 25, 6279–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waltz D. A., Chapman H. A. (1994) Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J. Biol. Chem. 269, 14746–14750 [PubMed] [Google Scholar]
- 24. Wei Y., Czekay R. P., Robillard L., Kugler M. C., Zhang F., Kim K. K., Xiong J. P., Humphries M. J., Chapman H. A. (2005) Regulation of α5β1 integrin conformation and function by urokinase receptor binding. J. Cell Biol. 168, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei Y., Eble J. A., Wang Z., Kreidberg J. A., Chapman H. A. (2001) Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol. Biol. Cell 12, 2975–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gondi C. S., Kandhukuri N., Dinh D. H., Gujrati M., Rao J. S. (2007) Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int. J. Oncol. 31, 19–27 [PMC free article] [PubMed] [Google Scholar]
- 27. Aguirre Ghiso J. A. (2002) Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 21, 2513–2524 [DOI] [PubMed] [Google Scholar]
- 28. Sidenius N., Blasi F. (2003) The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 22, 205–222 [DOI] [PubMed] [Google Scholar]
- 29. Irigoyen J. P., Muñoz-Cánoves P., Montero L., Koziczak M., Nagamine Y. (1999) The plasminogen activator system: biology and regulation. Cell Mol. Life Sci. 56, 104–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novak U., Cocks B. G., Hamilton J. A. (1991) A labile repressor acts through the NFkB-like binding sites of the human urokinase gene. Nucleic Acids Res. 19, 3389–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cicek M., Fukuyama R., Cicek M. S., Sizemore S., Welch D. R., Sizemore N., Casey G. (2009) BRMS1 contributes to the negative regulation of uPA gene expression through recruitment of HDAC1 to the NF-κB binding site of the uPA promoter. Clin. Exp. Metastasis 26, 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayden M. S., Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 33. Ahmad R., Raina D., Trivedi V., Ren J., Rajabi H., Kharbanda S., Kufe D. (2007) MUC1 oncoprotein activates the IκB kinase beta complex and constitutive NF-κB signalling. Nat. Cell Biol. 9, 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y., Ren J., Yu W., Li Q., Kuwahara H., Yin L., Carraway K. L., 3rd, Kufe D. (2001) The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J. Biol. Chem. 276, 35239–35242 [DOI] [PubMed] [Google Scholar]
- 35. Shang Y., Hu X., DiRenzo J., Lazar M. A., Brown M. (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 [DOI] [PubMed] [Google Scholar]
- 36. He Y., Liu X. D., Chen Z. Y., Zhu J., Xiong Y., Li K., Dong J. H., Li X. (2007) Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin. Cancer Res. 13, 3115–3124 [DOI] [PubMed] [Google Scholar]
- 37. Toupance S., Brassart B., Rabenoelina F., Ghoneim C., Vallar L., Polette M., Debelle L., Birembaut P. (2012) Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin. Exp. Metastasis 29, 511–522 [DOI] [PubMed] [Google Scholar]
- 38. Hiendlmeyer E., Regus S., Wassermann S., Hlubek F., Haynl A., Dimmler A., Koch C., Knoll C., van Beest M., Reuning U., Brabletz T., Kirchner T., Jung A. (2004) Beta-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 64, 1209–1214 [DOI] [PubMed] [Google Scholar]
- 39. Tanida S., Akita K., Ishida A., Mori Y., Toda M., Inoue M., Ohta M., Yashiro M., Sawada T., Hirakawa K., Nakada H. (2013) Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J. Biol. Chem. 288, 31842–31852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ren J., Raina D., Chen W., Li G., Huang L., Kufe D. (2006) MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol. Cancer Res. 4, 873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang L., Chen D., Liu D., Yin L., Kharbanda S., Kufe D. (2005) MUC1 oncoprotein blocks glycogen synthase kinase 3β-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 65, 10413–10422 [DOI] [PubMed] [Google Scholar]
- 42. Rahn J. J., Shen Q., Mah B. K., Hugh J. C. (2004) MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J. Biol. Chem. 279, 29386–29390 [DOI] [PubMed] [Google Scholar]
- 43. Shen Q., Rahn J. J., Zhang J., Gunasekera N., Sun X., Shaw A. R., Hendzel M. J., Hoffman P., Bernier A., Hugh J. C. (2008) MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol. Cancer Res. 6, 555–567 [DOI] [PubMed] [Google Scholar]
- 44. Sahraei M., Roy L. D., Curry J. M., Teresa T. L., Nath S., Besmer D., Kidiyoor A., Dalia R., Gendler S. J., Mukherjee P. (2012) MUC1 regulates PDGFA expression during pancreatic cancer progression. Oncogene 31, 4935–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh P. K., Wen Y., Swanson B. J., Shanmugam K., Kazlauskas A., Cerny R. L., Gendler S. J., Hollingsworth M. A. (2007) Platelet-derived growth factor receptor β-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 67, 5201–5210 [DOI] [PubMed] [Google Scholar]
- 46. Rajabi H., Alam M., Takahashi H., Kharbanda A., Guha M., Ahmad R., Kufe D. (2014) MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 33, 1680–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cantero D., Friess H., Deflorin J., Zimmermann A., Bründler M. A., Riesle E., Korc M., Büchler M. W. (1997) Enhanced expression of urokinase plasminogen activator and its receptor in pancreatic carcinoma. Br. J. Cancer 75, 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Memarzadeh S., Kozak K. R., Chang L., Natarajan S., Shintaku P., Reddy S. T., Farias-Eisner R. (2002) Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc. Natl. Acad. Sci. U.S.A. 99, 10647–10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hemsen A., Riethdorf L., Brünner N., Berger J., Ebel S., Thomssen C., Jänicke F., Pantel K. (2003) Comparative evaluation of urokinase-type plasminogen activator receptor expression in primary breast carcinomas and on metastatic tumor cells. Int. J. Cancer 107, 903–909 [DOI] [PubMed] [Google Scholar]
- 50. Mann B., Gelos M., Siedow A., Hanski M. L., Gratchev A., Ilyas M., Bodmer W. F., Moyer M. P., Riecken E. O., Buhr H. J., Hanski C. (1999) Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. U.S.A. 96, 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Q., Sun Z. X., Allgayer H., Yang H. S. (2010) Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene 29, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farina A. R., Tacconelli A., Vacca A., Maroder M., Gulino A., Mackay A. R. (1999) Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor κB elements. Cell Growth Differ. 10, 353–367 [PubMed] [Google Scholar]
- 53. Tanida S., Mori Y., Ishida A., Akita K., Nakada H. (2014) Galectin-3 binds to MUC1-N-terminal domain and triggers recruitment of β-catenin in MUC1-expressing mouse 3T3 cells. Biochim. Biophys. Acta 1840, 1790–1797 [DOI] [PubMed] [Google Scholar]
- 54. Raina D., Kosugi M., Ahmad R., Panchamoorthy G., Rajabi H., Alam M., Shimamura T., Shapiro G. I., Supko J., Kharbanda S., Kufe D. (2011) Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol. Cancer Ther. 10, 806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]