Background: Cyclophellitol-derived activity-based probes label the nucleophile of retaining β-glucosidases by exploiting their catalytic mechanism.
Results: Combining two distinct cyclophellitol-derived probes with azide-mediated activity-rescue of mutated retaining β-glucosidases enables acid/base residue identification.
Conclusion: A sensitive, new gel-based method for catalytic residue identification is developed.
Significance: Cyclophellitol-based probes are proposed as useful tools for the identification of catalytic residues in retaining glycosidases.
Keywords: Gaucher Disease, Glycobiology, Glycoconjugate, Glycolipid, Glycosidase, Acid/Base, Activity-based Probe, Labeling, Nucleophile
Abstract
Retaining β-exoglucosidases operate by a mechanism in which the key amino acids driving the glycosidic bond hydrolysis act as catalytic acid/base and nucleophile. Recently we designed two distinct classes of fluorescent cyclophellitol-type activity-based probes (ABPs) that exploit this mechanism to covalently modify the nucleophile of retaining β-glucosidases. Whereas β-epoxide ABPs require a protonated acid/base for irreversible inhibition of retaining β-glucosidases, β-aziridine ABPs do not. Here we describe a novel sensitive method to identify both catalytic residues of retaining β-glucosidases by the combined use of cyclophellitol β-epoxide- and β-aziridine ABPs. In this approach putative catalytic residues are first substituted to noncarboxylic amino acids such as glycine or glutamine through site-directed mutagenesis. Next, the acid/base and nucleophile can be identified via classical sodium azide-mediated rescue of mutants thereof. Selective labeling with fluorescent β-aziridine but not β-epoxide ABPs identifies the acid/base residue in mutagenized enzyme, as only the β-aziridine ABP can bind in its absence. The Absence of the nucleophile abolishes any ABP labeling. We validated the method by using the retaining β-glucosidase GBA (CAZy glycosylhydrolase family GH30) and then applied it to non-homologous (putative) retaining β-glucosidases categorized in GH1 and GH116: GBA2, GBA3, and LPH. The described method is highly sensitive, requiring only femtomoles (nanograms) of ABP-labeled enzymes.
Introduction
Retaining β-glucosidases hydrolyze glycosidic bonds in various endogenous and natural exogenous substrates. In man the documented retaining β-glucosidases fulfill important physiological functions. They belong to different CAZy glycosylhydrolase families (GH1,5 GH30 and GH116; see Fig. 1a), a classification system based on their amino acid sequence, catalytic mechanism, structure, and active site residues (1). Human lysosomal glucocerebrosidase (GBA; categorized in CAZy family GH30) degrades glucosylceramide, and its deficiency causes Gaucher disease, a relatively common lysosomal storage disorder (2–4). Carriers of GBA mutations are at increased risk for developing Parkinsonism (5, 6). Non-lysosomal β-glucosidase (GBA2; GH116) converts glucosylceramide to ceramide at the cytosolic leaflet of membranes (7–9) and has been implicated in ceramide-driven apoptosis (10). GBA2 deficiency was recently also linked to a neurological disorder (11, 12). Cytosolic β-glucosidase GBA3 (GH1) hydrolyzes xenobiotic β-glucosides in the cytosol (13, 14), and GBA3 deficiency is not associated with a health risk (15). Deficiency of lactase-phlorizin hydrolase (LPH; GH1), an intestinal enzyme containing a functional β-glucosidase pocket next to a β-galactosidase pocket, causes lactose intolerance (16–19). Finally, klotho, βklotho, and KLPH (γklotho) share homology with the amino acid sequence of LPH and GBA3 and are also categorized in GH1, but all lack functional β-glucosidase activity (20–25). Klotho has been reported to have β-glucuronidase activity toward artificial substrates when fused with an extracellular domain (24, 25), whereas sialidase activity has also been reported (26). The klotho proteins interact with FGF and FGF receptors, modulating endocrine axes that regulate various metabolic processes (27). Klotho is involved in aging processes (20), whereas βklotho is essential in bile acid homeostasis and the activity of brown adipose tissue (21).
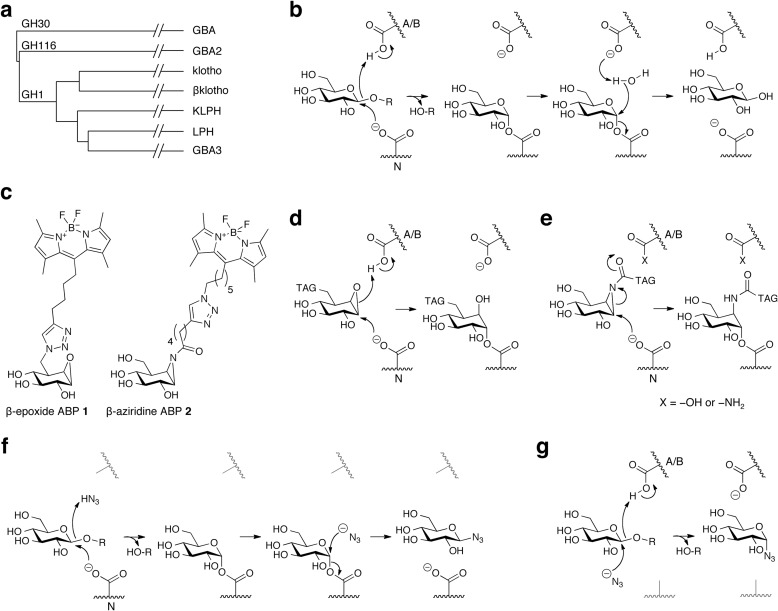
FIGURE 1.
Background. a, protein sequence homology of all retaining β-glucosidases expressed in man, categorized in glycosylhydrolase families GH1, -30, and -116. b, double displacement mechanism of retaining β-glucosidases, with distinct acid/base (A/B, top) and nucleophile (N, bottom). c, structure formulas of cyclophellitol-based ABP 1 (MDW933 (29)) and aziridine containing ABP 2 (MDW1044 (30)) and their nucleophile binding mechanisms (d and e, respectively). Azide-mediated rescue of hydrolysis in case of absent acid/base (f) or nucleophile (g).
Retaining β-glucosidases hydrolyze glucosidic substrates through a two-step double-displacement mechanism driven by a discriminate nucleophile and acid/base residue (Fig. 1, a and b) (18). In the first step the nucleophile attacks the anomeric carbon of the glucose moiety, whereas the acid/base residue protonates the interglucosidic oxygen, resulting in aglycone release and concomitant nucleophile glucosylation (28). In the second step enzyme deglucosylation is promoted by activation of an incoming water nucleophile by the deprotonated acid/base, completing the hydrolysis event with overall retention of configuration at the anomeric carbon of the released glucose molecule.
Recently we reported the development of two different activity-based probe (ABP) classes for retaining β-glucosidases (29, 30). The first class concerns cyclophellitol (β-epoxide) derivatives functionalized with a fluorescent BODIPY moiety grafted to the C8 position (MDW933, hereafter named ABP 1; see Fig. 1c) (29). They bind irreversibly to the nucleophile Glu-340 of GBA in an acid/base-dependent manner; the acid/base residue protonates the β-epoxide, which is concomitantly attacked by the nucleophile; thereby the oxirane opens and forms a stable ester bond with the nucleophile residue (Fig. 1d) (29, 30). Aziridine analogs of cyclophellitol functionalized with a BODIPY group grafted to the β-aziridine nitrogen comprise the other ABP class (MDW1044, hereafter named ABP 2; see Fig. 1c) (30). These bind nucleophiles of various β-glucosidases through a similar mechanism but that can occur independently of the acid/base status (Fig. 1e) (30).
An elegant method has been developed in the past to identify the nucleophile and acid/base residues of retaining glycosidases, namely by using (sodium) azide as an exogenous anion that can function as a substitute for the acid/base or nucleophile residue, allowing the rescue of substrate hydrolysis by enzyme molecules with a mutated catalytic residue (Fig. 1, f and g) (31–33). In this approach, definite identification of the acid/base and nucleophile can be achieved by determining the azido-product stereochemistry via NMR. In retaining β-glucosidases, sodium azide-mediated rescue results in retention of stereochemistry in the case of acid/base absence (β-azidoglucoside; Fig. 1f) and inversion of stereochemistry when the nucleophile is lacking (α-azidoglucoside; Fig. 1g) (31–33). Success of this method hinges on the ability to isolate and identify the nature of the glucosazide adduct formed, which essentially means that the approach works best when executed on isolated, soluble mutant glycosidases.
In a series of related studies, Withers et al. (28, 34–37) have employed various 2-deoxy-2-fluoroglycosides to identify the nucleophile of retaining β-glycosidases by trapping the nucleophile. Covalent and (semi)permanent modification of the nucleophilic residues in retaining β-glucosidases occurs due to the substitution of the C2-hydroxyl by a fluorine, which markedly destabilizes the positive charge developed during the oxocarbenium ion-like transition states flanking the covalent glycosyl-enzyme intermediate, thereby effectively reducing the catalytic rates involved in the double-displacement mechanism by up to 107-fold. Identification of the thus covalently and irreversibly modified nucleophile in this setting is achieved by mass spectrometry analysis of trypsin-digested protein, and the method thus relies on labeling of pure, recombinant enzyme with 2-deoxy-2-fluoroglycosides.
We here report a new and complementary method for the identification of the acid/base and nucleophile residues in the known human retaining β-glucosidases. Our approach entails a combination of site-directed mutagenesis, subsequent identification of putative catalytic residues by sodium azide-mediated rescue of enzymatic activity, and finally discrimination between the acid/base and nucleophile by detection of ABP 2-labeled β-glucosidases on slab-gels. The ABP labeling of retaining β-glucosidases is characterized by high sensitivity and selectivity, allowing visualization of femtomoles, i.e. nanograms, of enzymes within complex mixtures such as cell extracts.
As model we first used GBA (CAZy family GH30) with known nucleophile residue Glu-340 and acid/base residue Glu-235 to validate our method (39). Next, we studied GBA3 (CAZy GH1) for which crystal data predicted Glu-165 and Glu-373 as acid/base and nucleophile, respectively (40). In addition, LPH (GH1), with known catalytic residues in its two pockets (41), was investigated. Finally we studied the enzyme GBA2. Recently, the acid/base and nucleophile of a distant GBA2 homologue in Sulfolobus solfataricus (GH116) have been identified via azide rescue and covalent labeling of the nucleophile with 2-deoxy-2-fluoroglucoside (42). Based on homology alignment, the acid/base and nucleophile in human GBA2 were tentatively appointed previously (42) and by our own work, and these are in line with the residues predicted from the GBA2-homologue in S. solfataricus. We also demonstrate through application of our ABP methodology that none of the proteins klotho, βklotho, and KLPH (GH1) possesses β-glucosidase activity.
EXPERIMENTAL PROCEDURES
General Methods
ABP 1 and ABP 2 were synthesized as described earlier (29, 30). Chemicals were obtained from Sigma if not otherwise indicated. Recombinant GBA (imiglucerase) was purchased from Genzyme. COS-7 cells were cultured in Ham's F-12-DMEM (Invitrogen) supplied with 10% (v/v) FCS. Amino acid sequence alignments were performed in ClustalOmega 1.2.0, and (putative) catalytic residues were identified through entries in UniProt P04062 (GBA), Q9HCG7 (GBA2), Q9H227 (GBA3), P09848 (LPH), Q9UEF7 (klotho), Q86Z14 (βklotho), and Q6UWM7 (KLPH).
Molecular Cloning and Site-directed Mutagenesis
The design of cloning primers for mutation introduction was based on NCBI reference sequences NM_000157.3 (GBA), NM_020944.2 (GBA2), NM_020973.3 (GBA3), NG_008104.2 (LPH), NM_004795.3 (klotho), NM_175737.3 (βklotho), and NM_207338.2 (KLPH). All full-length cDNA sequences were cloned into pcDNA3.1 in-frame with the myc/His vector (Invitrogen). Site-directed mutagenesis was performed using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) with primers shown in Table 1.
TABLE 1.
Primers employed for the site-directed mutagenesis of GBA, GBA2, GBA3, and LPH
| Coding sequence | Substitution | Direction | Primer sequence (5′ → 3′) |
|---|---|---|---|
| GBA | E235G | Forward | GACAGCTGAAAATGGGCCTTCTGCTGGGC |
| Reverse | GCCCAGCAGAAGGCCCATTTTCAGCTGTC | ||
| E235Q | Forward | GACAGCTGAAAATCAGCCTTCTGCTGGGC | |
| Reverse | GCCCAGCAGAAGGCTGATTTTCAGCTGTC | ||
| E340G | Forward | GCTCTTTGCCTCAGGGGCCTGTGTGGGCTCC | |
| Reverse | GGAGCCCACACAGGCCCCTGAGGCAAAGAGC | ||
| E340Q | Forward | GCTCTTTGCCTCACAGGCCTGTGTGGGCTCC | |
| Reverse | GGAGCCCACACAGGCCTGTGAGGCAAAGAGC | ||
| GBA2 | E527G | Forward | GATTTGGCTACCTTGGGGGCCAGGAGTACCG |
| Reverse | CGGTACTCCTGGCCCCCAAGGTAGCCAAATC | ||
| E659G | Forward | CTGAAATGAAGTTTGGCAAGGACCATGATGGAC | |
| Reverse | GTCCATCATGGTCCTTGCCAAACTTCATTTCAG | ||
| D633G | Forward | GTTTGACAAGGACCATGGTGGACTCATTGAAAATGG | |
| Reverse | CCATTTTCAATGAGTCCACCATGGTCCTTGTCAAAC | ||
| E667G | Forward | CATGATGGACTCATTGGAAATGGAGGCTATGCAG | |
| Reverse | CTGCATAGCCTCCATTTCCAATGAGTCCATCATG | ||
| D673G | Forward | GGAGGCTATGCAGGCCAGACCTATGATGG | |
| Reverse | CCATCATAGGTCTGGCCTGCATAGCCTCC | ||
| D677G | Forward | GACCAGACCTATGGTGGATGGGTGACCAC | |
| Reverse | GTGGTCACCCATCCACCATAGGTCTGGTC | ||
| GBA3 | E165G | Forward | CAGTGGATCACCATAAATGGAGCTAATGTTCTTTCTGTG |
| Reverse | CACAGAAAGAACATTAGCTCCATTTATGGTGATCCACTG | ||
| E373G | Forward | CTGTAATTTACATCACTGGGAATGGGTTTCCCCAG | |
| Reverse | CTGGGGAAACCCATTCCCAGTGATGTAAATTACAG | ||
| LPH | E1065G | Forward | GGATGACTTTTAATGGGCCCATGTACCTGGC |
| Reverse | GCCAGGTACATGGGCCCATTAAAAGTCATCC | ||
| E1273G | Forward | CCATTTACATCACCGGAAACGGAGTGGGGC | |
| Reverse | GCCCCACTCCGTTTCCGGTGATGTAAATGG | ||
| E1538G | Forward | GGATCACGCTGAATGGGCCCTTTGTCATTGC | |
| Reverse | GCAATGACAAAGGGCCCATTCAGCGTGATCC | ||
| E1749G | Forward | CCAATTTATGTCACAGGGAATGGAGTGTCCCAGC | |
| Reverse | GCTGGGACACTCCATTCCCTGTGACATAAATTGG |
Protein Expression and Isolation
Confluent COS-7 cells were transfected with empty pcDNA3.1 vector (Mock) or the vector with the described insert, in conjunction with FuGENE (Roche Applied Science) and harvested after 72 h by scraping in 25 mm potassium phosphate buffer (pH 6.5, supplemented with 0.1% (v/v) Triton X-100 and protease inhibitor mixture (Roche Applied Science)). After determination of the protein concentration (BCA kit, Pierce), lysates were aliquoted and frozen at −80 °C.
Enzyme Activity Assays
All assays were performed at 37 °C. Cellular extracts were preincubated for 30 min with inhibitors of GBA (1 mm conduritol β-epoxide; IC50 9.49 mm) (29, 30) and/or GBA2 (20 nm N-(5-adamantane-1-yl-methoxy)pentyl)-deoxy-nojirimycin (AMP-DNM), IC50 2 nm) (43, 44) to distinguish the activity of the enzyme of interest. GBA activity was measured in 150 mm McIlvaine buffer (pH 5.2) supplemented with 0.2% (w/v) sodium taurocholate, 0.1% (v/v) Triton X-100, protease inhibitor mixture (Roche Applied Science), and 0.1% (w/v) BSA. β-Glucosidase activities of GBA2, GBA3, and LPH were measured in McIlvaine buffer without detergents at pH 5.8, 6.5, and 6.0, respectively. Where specified, enzyme assays were performed with 3.75 mm fluorescent 4-methylumbelliferyl-β-d-glucopyranoside (4MU-β-d-Glc), chromogenic 20 mm 4-nitrophenyl β-d-glucopyranoside (4NP-β-d-Glc), or 20 mm 2,4-dinitrophenyl β-d-glucopyranoside (2,4DNP-β-d-Glc). Lactase β-galactosidase activity was quantified by measuring liberated d-glucose from 100 mm lactose (38). Measurements of ABP 1 and 2 IC50 for overexpressed LPH were performed by preincubating cell lysates with an appropriate range of inhibitor dilutions for 30 min at 37 °C, after which residual β-galactosidase and β-glucosidase activities were measured with 100 mm lactose (38) and 3.75 mm 4MU-β-d-Glc (29, 30), respectively. After 90 min, all enzymatic reactions were stopped with 3 m NaOH-glycine (pH 10.8), and liberated 4MU was fluorimetrically determined at λEX 365 nm, λEM 425 nm (29, 30). Reactions with 4NP-β-d-Glc and 2,4DNP-β-d-Glc were quenched through the addition of excess 1 m ice-cold Na2CO3 (pH 10.2), and absorption of 4NP and 2,4DNP was determined at λABS 405 nm (molar extinction coefficients ϵ4NP 8.94 m−1 cm−1 and ϵ2,4DNP 9.27 m−1 cm−1, respectively).
In Vitro ABP Labeling
Cell extracts (10 μg of total protein) were labeled with ABP 1 or 2 in appropriate McIlvaine buffer without BSA at 37 °C in 40-μl total volume: GBA, GBA2 were labeled with 1 μm ABP for 1 h; GBA3, LPH with 10 μm ABP for 1 h; klotho, βklotho and KLPH with 1 mm for 18 h. After labeling and when applicable, His-tagged proteins were purified with TALON beads following manufacturer's instructions (Clontech). Samples were then denatured with 5× Laemmli buffer (50% (v/v), 1 m Tris-HCl (pH 6.8), 50% (v/v) 100% glycerol, 10% (w/v) DTT, 10% (w/v) SDS, 0.01% (w/v) bromphenol blue), boiled for 4 min at 100 °C, and separated by electrophoresis on 7.5% (w/v) SDS-PAGE gel running continuously at 90 V (29). Wet slab-gels were scanned for fluorescence using a Typhoon Variable Mode Imager (Amersham Biosciences) using λEX 488 nm and λEM 520 nm (band pass filter 40 nm) for green fluorescent ABPs 1/2. Western blotting for C-terminally, myc-tagged recombinant proteins was accomplished by running identically treated samples of 100 μg of total protein and transfer of the samples onto a nitrocellulose membrane for 1 h at 12 V followed by blocking the membrane with 2% (w/v) BSA in TBST buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% (v/v) Tween 20). Next, the blots were incubated overnight with 1:2,000 diluted primary mouse α-myc mAb (Cell Signaling, b118, 2% (w/v) BSA in TBST) at 4 °C, washing with TBST for 20 min (repeated 6 times), subsequently incubated with 1:10,000 diluted secondary rabbit α-mouse IRD680 (Cell Signaling, 2% (w/v) BSA in TBST) at room temperature, washed with TBST for 20 min (repeated 6 times), and finally, read-out occurred on an Odyssey Infrared Scanner (Li-Cor).
Sodium Azide-mediated Rescue of Enzymatic Activity
Samples were incubated with ABP (with 10 μg of total protein) or artificial substrate (with 100 μg of total protein) in the presence of 0–5 m sodium azide (solubilized in 10 mm Tris-HCl, adjusted to the optimal pH for each enzyme). As elegantly described earlier (see Ref 33), the presence of (sodium) azide can rescue the enzymatic activity in the absence of the nucleophile or that of the acid/base residue. After substrate reactions, tubes were centrifuged (3000 rpm for 3 min at room temperature) after which the supernatant was collected and vigorously mixed, and liberated chromogenic/fluorogenic product was measured. After ABP labeling reactions, proteins were precipitated with 10% (w/v) trichloroacetic acid for 10 min on ice and centrifuged at 10,000 rpm for 10 min at 4 °C, and the resulting pellet was washed 3 times with cold 50% (v/v) acetone in nanopure water before further use for His-tag pulldown or directly solubilized in 1× Laemmli buffer and applied to SDS-PAGE. Rescue of ABP labeling by the addition of acetic acid (10 m) or formic acid (15 m) followed exactly the same procedure.
RESULTS
Elucidation of the Acid/Base and Nucleophile in GBA
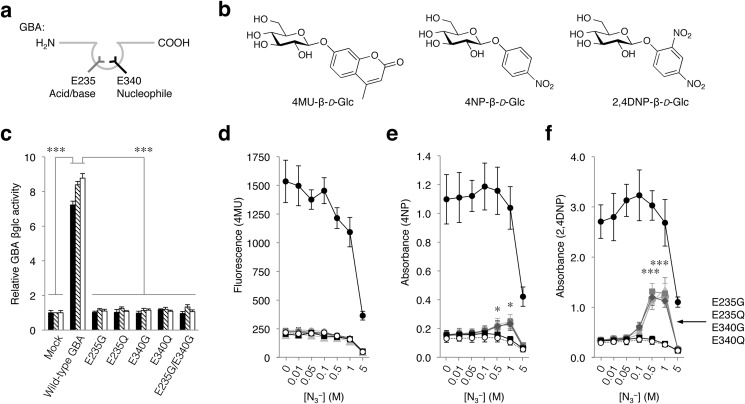
The nucleophile (Glu-340) and acid/base (Glu-235) of GBA have been identified unequivocally (Fig. 2a) (29, 39), and we therefore used this enzyme to validate our envisioned method where nucleophile-modifying cyclophellitol ABPs can distinguish the location of the acid/base and nucleophile in retaining β-glucosidases. Accordingly, GBA isoforms with substituted nucleophile and/or acid/base residues were generated. COS-7 cells were transfected with constructs expressing C-terminally myc/His-tagged human GBA or variants with E235G, E235Q, E340G, E340Q, or E235G/E340G substitutions. All mutagenized GBA proteins were deficient in enzymatic activity toward the artificial substrates 4MU-, 4NP-, and 2,4DNP-β-d-Glc (Fig. 2b).
FIGURE 2.
Proof-of-principle; rescue of substrate hydrolysis. a, schematic depicting GBA with active-site pocket presenting acid/base Glu-235 (left, gray) and nucleophile Glu-340 (right, black). b, structure formulas of 4MU-, 4NP-, and 4MU-β-d-Glc. c, catalytic activity of GBA variants toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc relative to mock (black, hatched, white columns, respectively). Shown is sodium azide-mediated rescue of hydrolysis of 4MU (d)-, 4NP (e)-, and 2,4DNP-β-d-Glc (f) by mock (empty plasmid, open circles), wild-type GBA (filled circles), E235G (gray triangles), E235Q (gray circles), E340G (gray squares), E340Q (gray diamonds), and E235G/E340G (black squares). Data are the average of triplicates ± S.D. with one-way analysis of variance significance; *, p < 0.05; ***, p < 0.001.
Next, we attempted to rescue the enzymatic activity of the aforementioned GBA mutants by co-incubating a fixed concentration of artificial substrate together with a range of sodium azide concentrations. In this context, azide acts as exogenous anion and is at certain concentrations able to substitute the absent acid/base or nucleophilic residue (Fig. 1, f and g) (31, 33, 42). The use of sodium azide has become an important staple in the laboratory toolkit available for catalytic residue discovery and has been thoroughly reviewed by Ly and Withers (32), and its use is elegantly described in manuscripts such as Bravman et al. (33).
Titration of the various GBA mutants with sodium azide did not result in an enhanced hydrolysis rate (i.e. rescue) of the acid/base and nucleophile mutants in combination with fluorogenic 4MU-β-d-Glc (Fig. 2c). Partial, although significant rescue was observed with 4NP-β-d-Glc except for the double E235G/E340G GBA mutant, which lacks both the acid/base and nucleophile (Fig. 2b). The most pronounced rescue was achieved with 2,4DNP-β-d-Glc (Fig. 2e). This can readily be explained by considering that 4-methylumbelliferone (4MU) and 4-nitrophenol (4NP) are inferior leaving groups to 2,4-dinitrophenol (2,4DNP), and all require activation by protonation by the acid/base in the glucosylation step (31–33). At high concentrations, sodium azide severely impaired activity of GBA toward all artificial substrates tested (Fig. 2, d–f).
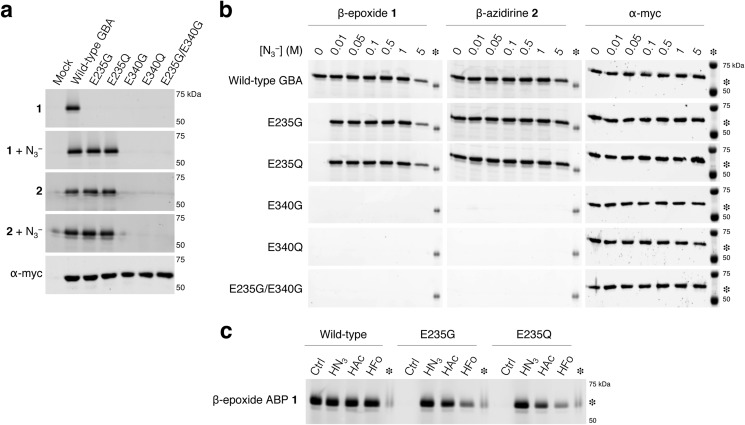
A final step the classic approach to determine the definite location of the acid/base and nucleophile is determination of the stereochemistry of the reaction products, formed during the presence of sodium azide in combination with the various mutants (31–33, 42). This methodology hinges on the feasibility of isolation/purification of, first, the mutagenized glycosidase and, second, of the various azido products. We propose the use of specific, fluorescent cyclophellitol-type ABPs. The ABPs described are highly specific, only bind to the nucleophilic residue, and enable the sensitive detection of attomoles of ABP-labeled retaining β-glucosidases on slab gels (29, 30). In addition, mutagenized proteins can be labeled directly within complex lysates and living cells (29, 30) and can be, when required, purified before gel electrophoresis. To illustrate this ABP application, complete lysates of COS-7 cells expressing recombinant wild-type or mutant GBA were incubated with β-epoxide 1 or β-aziridine 2 and thereafter purified via their His tag with TALON beads. Thereafter, the labeled GBA was visualized by SDS-PAGE and subsequent fluorescence scanning (Fig. 3a).
FIGURE 3.
Proof-of-principle; ABP labeling of GBA. a, overexpressed myc/His-tagged GBA variants in total COS-7 cells with excess ABP 1 or ABP 2 and subsequently purified via His-tag pulldown. Labeling with ABP 1 in the absence/presence of sodium azide (first, second gel), idem with ABP 2 (third and fourth gel), and GBA detected with α-myc (fifth gel). b, β-epoxide 1 and β-aziridine 2 labeling at different sodium azide concentrations. c, rescue of labeling with small acids. Labeling of wild-type GBA and acid/base mutants E235G and E235Q at pH 5.2 with β-epoxide 1 in the absence or presence of 1 m sodium azide (forms hydrazoic acid (HN3)), 10 m acetic acid (HAc), or 15 m formic acid (HFo), and subsequently purification via His-tag pulldown is shown. Inter-gel comparisons facilitated by excess imiglucerase labeled with 50 fm ABP 1 (asterisk) as positive control.
The use of 10 μg of total protein as the source for ABP labeling and subsequent purification step enabled evident detection of ∼12 ng (i.e. 200 fmol) of recombinant and ABP-labeled GBA molecules on gel by using a Typhoon Variable Mode Imager (Amersham Biosciences) with medium sensitivity settings. In contrast, the limit of detection of the GBA loading control via Western blotting for the myc epitope required at least 100 μg of total protein to be loaded on the gel (Fig. 3a).
β-Epoxide 1 requires a priori a protonated acid/base and thus did not label E235G and E235Q GBA. Sodium azide completely restored the ability of E235G and E235Q GBA variants to label with ABP 1 by acting as an external acid/base in the form of hydrazoic acid (HN3; Fig. 3b). Labeling of acid/base mutants E235G and E235Q with ABP 1 reached its maximal intensity when using sodium azide (Fig. 3b) at concentrations where no rescue in catalytic activity could be identified when using 2,4DNP-β-d-Glc (Fig. 2, d–f). The combination of sodium azide with latter substrate did not reach wild-type activity levels, even at optimal conditions. Complete restoration of ABP 1 labeling was also obtained when acetic acid (HAc) or formic acid (HFo) were added instead of sodium azide (Fig. 3c).
β-Aziridine 2 reacts with the nucleophile Glu-340 regardless of the acid/base status in GBA molecules and independently of the presence of sodium azide (Fig. 3, a and b) (30). Substitutions of nucleophile Glu-340 in GBA to Glu-340 or Gln-340 inherently prevented labeling by both ABP classes (Fig. 3b). Having established that ABPs 1 and 2 indeed can be employed to sensitively differentiate the acid/base from nucleophilic residue as compared with the classic sodium azide rescue approach, we next applied the developed method to the other known human retaining β-glucosidases.
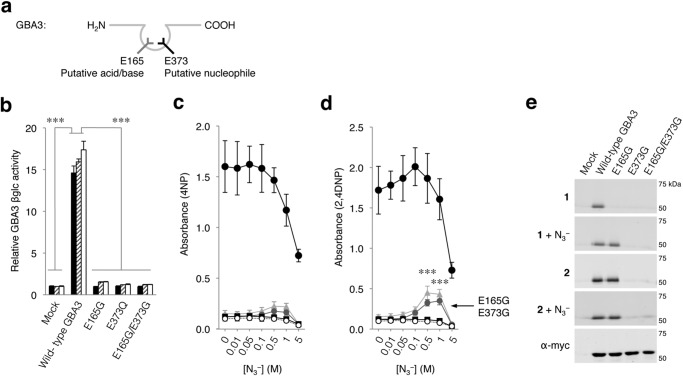
Elucidation of the Acid/Base and Nucleophile in GBA3 from CaZY Family GH1
We next analyzed the applicability of the ABP-labeling approach for the cytosolic retaining β-glucosidase GBA3, which is categorized in CAZy family GH1 and which is non-homologous to GBA (GH30, see also Fig. 1a). Crystallography studies of GBA3 strongly indicate that residues Glu-165 and Glu-373 function as its acid/base and nucleophile, respectively (Fig. 4a) (40). Mutated GBA3 containing E165G and/or E373G displayed no activity toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc (Fig. 4b). Titration with sodium azide resulted in a minor but significant activity rescue for E165G, whereas the E373G mutant did not hydrolyze 4NP-β-d-Glc, indicating their involvement in substrate catalysis (Fig. 4c). A more evident rescue of activity was observed by using 2,4DNP-β-d-Glc as substrate (Fig. 4d).
FIGURE 4.
Cytosolic β-glucosidase GBA3. a, schematic depicting GBA3 with predicted acid/base (left, gray) and nucleophile (right, black). b, catalytic activity of GBA3 variants toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc relative to mock (black, hatched, white columns, respectively). Sodium azide-mediated rescue of 4NP (c)- and 2,4DNP-β-d-Glc (d) hydrolysis by mock (empty plasmid, open circles), wild-type GBA3 (filled circles), E165G (gray triangles), E373Q (gray circles), and E165G/E373G (black squares). e, overexpressed myc/His-tagged GBA3 variants in total COS-7 cells with excess ABP 1 or ABP 2 and subsequently purified via His-tag pulldown. Labeling with ABP 1 in the absence/presence of sodium azide (first and second gels), idem with ABP 2 (third and fourth gel), and GBA3 detected with α-myc (fifth gel). Data are the average of triplicates ± S.D., with one-way analysis of variance significance; ***, p < 0.001.
Substitution of Glu-373 prevented GBA3 labeling by both ABP 1 and 2, whereas substitution of Glu-165 allowed labeling by β-aziridine ABP 2 but not by β-epoxide 1, indicating Glu-165 functions as acid/base. This role was further confirmed by rescue of β-epoxide 1 labeling with sodium azide (Fig. 4e). Our results confirm the prior indications that indeed Glu-373 is the nucleophile and Glu-165 the acid/base residue in GBA3. More importantly, the results illustrate the developed method can be applied to retaining β-glucosidases non-homologous to GBA (GH30), categorized in other CaZY families such as GH1.
Elucidation of the Acid/Base and Nucleophile in LPH, Another Member of GH1
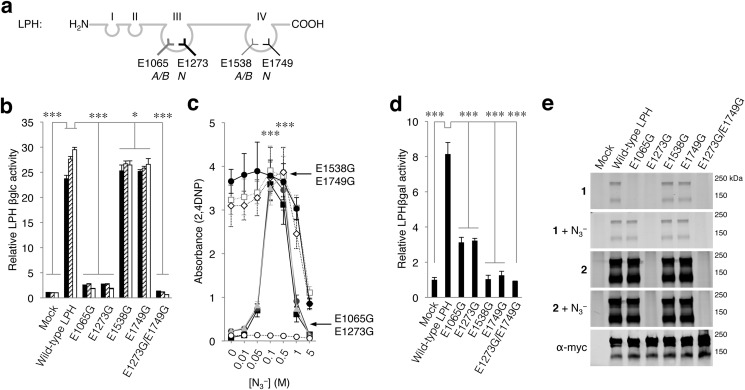
Lactase/phlorizin hydrolase contains four domains with high homology with GBA3 and other GH1 family members (16–18). Two LPH domains, coded III and IV, have functional catalytic pockets with acid/bases Glu-1065 and Glu-1538 and nucleophiles Glu-1273 and Glu-1749, respectively (Fig. 5a) (16–18, 41). Pocket III possesses predominantly β-glucosidase activity, whereas pocket IV catalyzes the hydrolysis of β-galactosides including lactose. We noted earlier that LPH can be labeled by both β-epoxide 1 and β-aziridine 2, but the site of modification was not established (29, 30).
FIGURE 5.
Lactase/phlorizin hydrolase LPH. a, schematic depicting LPH with predicted acid/base (Glu-1065, Glu-1538) and nucleophile (Glu-1273, Glu-1749) for both pockets III and IV. b, catalytic activity of LPH variants toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc relative to mock (black, hatched, white columns, respectively). c, sodium azide-mediated rescue of 2,4DNP-β-d-Glc hydrolysis by mock (empty plasmid, open circles), wild-type LPH (closed circles), E1065G (gray triangles), E1273G (gray circles), E1538G (gray squares), E1749G (gray diamonds), and E1273G/E1749G (black squares). d, glucose liberation from lactose by LPH variants, relative to mock. e, overexpressed myc/His-tagged LPH variants in total COS-7 cells with excess ABP 1 or ABP 2 and subsequently purified via His-tag pulldown. Labeling with ABP 1 in the absence/presence of sodium azide (first and second gel), idem with ABP 2 (third and fourth gel), and LPH detected with α-myc (fifth gel). Data are the average of triplicates ± S.D., with one-way analysis of variance significance; *, p < 0.05; ***, p < 0.001.
Substitution of the acid/base Glu-1065 or nucleophile Glu-1273, both residing in pocket III, to a glycine residue decreased the hydrolysis rate of 4MU-, 4NP-, and 2,4DNP-β-d-Glc substrates by 89–93% (Fig. 5b). The addition of sodium azide restored activity of Glu-1065- and Glu-1273-substituted LPH toward 2,4DNP-β-d-Glc (Fig. 5c) and to a lesser extent to 4NP-β-d-Glc. Release of glucose from lactose is 92–96% decreased by the E1538G and E1749G substitutions in pocket IV (Fig. 5d). Substitution of catalytic residues in pocket III, E1065G and E1273G, also affect the hydrolysis of lactose in pocket IV (reduction by 63–67%), similar to previous published data (Fig. 5d) (41). In contrast, LPH molecules with substitutions in pocket IV (E1538G and E1749G) show normal hydrolysis of 2,4DNP-β-d-Glc in pocket III (Fig. 5, b and c). Similarly, LPH molecules with substitutions in both pockets III and IV are completely restored by sodium azide to wild-type β-glucosidase activity (Fig. 5c).
Labeling of G1065 LPH with β-epoxide 1 only occurred in the presence of sodium azide, whereas labeling of this mutant with β-aziridine 2 was independent of the presence of sodium azide, confirming Glu-1065 acts as an acid/base residue (Fig. 5e). The Gly-1273 LPH variant did not bind ABP 1 or ABP 2 in the absence or presence of sodium azide (Fig. 5e), whereas its enzymatic activity was rescued (2,4DNP-β-d-Glc; Fig. 5c), indicating that Glu-1273 is indeed the nucleophile of pocket III.
Pocket IV of LPH appears not targeted by ABPs 1 or 2 as mutagenesis of its nucleophile and acid/base residue (Glu-1538 and Glu-1749, respectively) did not influence ABP labeling (Fig. 5e) nor did it result in a significantly reduced β-glucosidase activity (Fig. 5b). It seems, therefore, that substitutions in pocket IV have little impact on pocket III but, intriguingly, not vice versa. Wild-type LPH is irreversibly inhibited by ABPs 1 and 2 in its hydrolysis of 2,4DNP-β-d-Glc, and relatively low IC50 values were observed (1.3–1.4 and 0.19–0.21 μm for ABP 1 and 2, respectively). In contrast, ABP 1 and ABP 2 are not potent irreversible inhibitors of lactose hydrolysis mediated by β-galactosidase pocket IV, again indicating that the activity-based probes bind exclusively to the β-glucosidase pocket III of LPH.
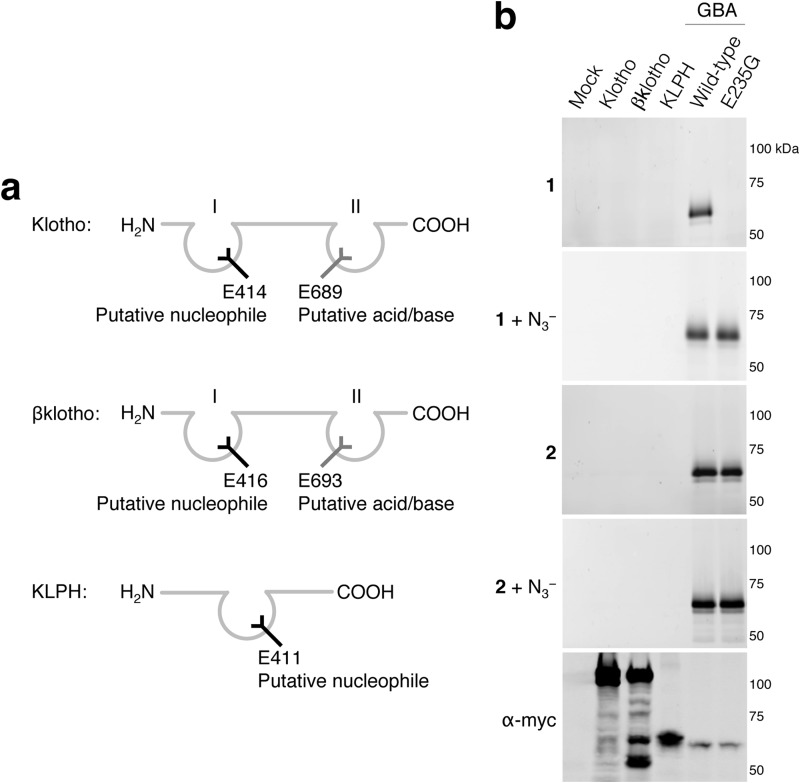
Klotho, βKlotho, and KLPH
Sequence analysis indicates that KLPH possesses one pocket, and klotho and βklotho have two pockets. Moreover, each is predicted to lack either an acid/base or nucleophile residue (Fig. 6a). The absence of β-glucosidase activity toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc was confirmed by no demonstrable ABP labeling (Fig. 6b). Consistently, even after incubations of 18 h with an excess of either ABP (1 mm) and the presence of sodium azide, no labeling was observed.
FIGURE 6.
Klotho, βklotho, and KLPH. a, schematic depicting klotho, βklotho and KLPH with putative acid/bases (gray) and nucleophiles (black) in predicted pockets. b, overexpressed myc/His-tagged klotho, βklotho, KLPH, GBA wild-type, and acid/base-lacking E235G GBA in total COS-7 cells with excess ABP 1 or ABP 2 and purified via His-tag pulldown. Labeling with ABP 1 in the absence/presence of sodium azide (first and second gel), idem with ABP 2 (third and fourth gel), and myc-tagged proteins detected with α-myc (fifth gel).
Elucidation of the Acid/Base and Nucleophile in GBA2, a Member of GH116
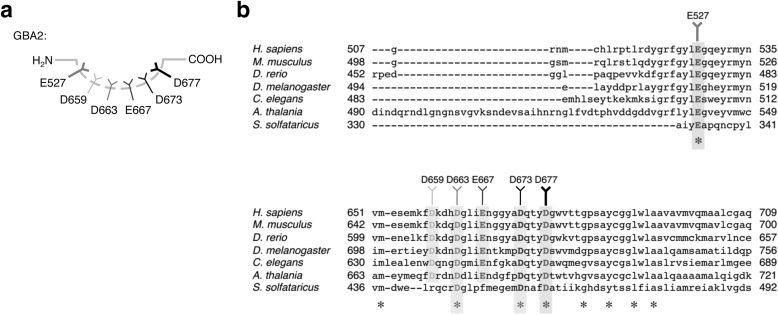
The non-lysosomal β-glucosidase GBA2 has recently received considerable attention, but its catalytic residues currently remain elusive (7–12). Recently, Cobucci-Ponzano et al. (42) identified the acid/base and nucleophile of the GBA2 homologue SSO1353 (GH116) from S. solfataricus via sodium azide rescue of mutagenized SSO1353 proteins and labeling with 2-deoxy-2-fluoroglucoside. Albeit SSO1353 shares low homology with human GBA2, its nucleophile (Glu-335) and acid/base (Asp-462) appear conserved between species, and the authors, therefore, suggest these catalytic residues are located at Glu-527 and Asp-677 in human GBA2 (42).
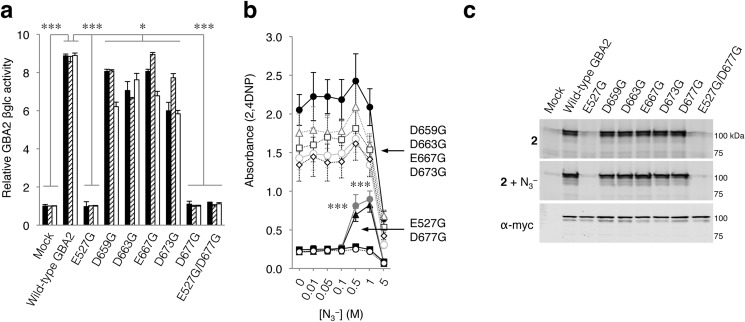
Amino acid alignments of GBA2 homologues expressed by various species revealed, besides Glu-335 and Asp-462, additional conserved aspartate and glutamate residues that may have a role in the catalytic mechanism (Fig. 7, a and b). We, therefore, substituted the conserved aspartate and glutamate residues individually for glycine (E527G, D659G, D663G, E667G, D673G, and D677G; see Fig. 7a). Cells were then transfected with C-terminally myc/His-tagged human wild-type GBA2 or the aforementioned isoforms. Each isoform exhibited enzymatic activity toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc substrates, except Glu-527- and Asp-677-substituted GBA2 (Fig. 8a). Titration with sodium azide subsequently revealed activity-rescue toward 2,4DNP-β-d-Glc only in the case of Gly-527 and Gly-677 GBA2, consistent with a catalytic role (Fig. 8b). Use of 4NP-β-d-Glc gave similar results.
FIGURE 7.
Mapping of catalytic residues in GBA2. a, schematic depicting GBA2 putative catalytic residues (shades of gray), with possible acid/base D667 (left, gray) and nucleophile Glu-527 (black). b, sequence alignment of GBA2 homologues of various species, with highly conserved aspartic (D) and glutamic acids (E).
FIGURE 8.
Non-lysosomal β-glucosidase GBA2. a, catalytic activity of LPH variants toward 4MU-, 4NP-, and 2,4DNP-β-d-Glc relative to mock (black, hatched, white columns, respectively). b, sodium azide-mediated rescue of 2,4DNP-β-d-Glc hydrolysis by mock (empty plasmid, open circles), wild-type GBA2 (filled circles), E527G (filled triangles), D677G (dark gray circles), D659G (dark gray squares), D663G (dark gray diamonds), E667G (light gray triangles), D673G (light gray circles), and E527G/D677G (filled squares). c, overexpressed myc/His-tagged GBA2 variants in total COS-7 cells with excess ABP 1 or ABP 2 and subsequently purified via His-tag pulldown. Labeling with ABP 1 in the absence/presence of sodium azide (first, second gel), idem with ABP 2 (third, fourth gel), and GBA2 detected with α-myc (fifth gel). Data are the average of triplicates ± S.D. with one-way analysis of variance significance p < 0.05*, p < 0.001***.
Finally, the acid/base and nucleophile were thereafter identified by labeling total cell lysates containing Gly-527 and Gly-677 GBA2 with β-epoxide 1 or β-aziridine 2 and subsequent fluorescence scanning of SDS-PAGE slab-gels (Fig. 8c). β-Epoxide 1 did not bind covalently to GBA2 as previously reported (29, 30). β-Aziridine 2 labeled all GBA2 isoforms, except GBA2, carrying the E527G substitution, indicating Glu-527 is the putative nucleophile of GBA2. Labeling of G677 GBA2 by β-aziridine ABP 2 together with its susceptibility for activity-rescue by sodium azide in combination with 4NP- and 2,4DNP-β-d-Glc substrate indicates that Asp-677 is the acid/base in GBA2.
The designation of Glu-527 as the nucleophile and Asp-677 as the acid/base in human GBA2 overlaps with the location of these residues identified in SSO1353 of S. solfataricus (see Fig. 8a) and at their suggested locations in human GBA2 as extrapolated from the data from S. solfataricus (42).
DISCUSSION
Retaining β-glucosidases fulfill important functions in the human body as is perhaps illustrated best by the deleterious consequences of GBA deficiency causing Gaucher disease and the emerging implication of GBA2 in cancer and neuronal pathologies. Better understanding of human β-glucosidases requires identification of their crucial catalytic amino acids for catalysis, the nucleophile, and acid/base residues.
Elegant methodologies have been developed in the past to identify these key residues, such as the use of sodium azide as an external acid/base and/or nucleophile, allowing catalytic activity rescue of enzyme molecules with a mutant catalytic residue and NMR analysis of the resultant product (31–33), or trapping the nucleophile with a 2-deoxy-2-fluoroglycoside and identification via mass-spectrometric detection. These methodologies hinge on the ability to isolate the wild-type or mutagenized enzyme before analysis, a feat usually reserved for at least partly soluble enzymes, and the feasibility to purify sufficient 2-deoxy-2-fluoroglycoside-labeled peptide or azido-glycoside from the treated samples.
We have described here a complementary method that combines the previously reported sodium azide-mediated rescue of enzyme activity to identify the catalytic residues with ABP-labeling in complex lysates to directly discriminate between the acid/base and the nucleophile. ABP labeling can occur directly with high specificity within living cells (data not shown) or in total lysate (29, 30), after which, if required, the recombinant retaining β-glucosidases can be purified via for instance an implemented His tag.
We capitalize on the unique features of epoxide-containing ABP 1 and aziridine-containing ABP 2, which fundamentally differ in their ability to label retaining β-glucosidases. Both ABPs bind covalently to the nucleophile, but β-epoxide 1 requires a functional acid/base, whereas β-aziridine 2 does not. Upon labeling of the nucleophile with the fluorescent ABP, the protein can be detected by highly sensitive fluorescence scanning of SDS-PAGE slab-gels.
The high sensitivity of the presented method warrants discussion. Emitted fluorescence from ABP-labeled proteins on slab-gels can be detected in the attomole range (29), and here 50 femtomoles (i.e. 3 ng) of recombinant GBA, labeled with either ABPs, were clearly visualized. This is in sharp contrast to the at least 10-fold higher protein input minimally required for detection by Western blot and in the enzymatic assays. Therefore, relatively small amounts of recombinant enzymes expressed by mammalian cells after transfection are sufficient for accurate detection with fluorescent ABPs (29, 30).
As we demonstrated, β-aziridine 2 reacts with the nucleophile of GBA, GBA2, GBA3, and the β-glucosidase pocket III of LPH to form a stable covalent adduct through a mechanism independent of the presence of a functional acid/base residue. In contrast, labeling by β-epoxide 1 of GBA, GBA3, and the β-glucosidase pocket III of LPH is prohibited by mutation of the acid/base, coinciding with loss of enzymatic activity that is the result of such mutations. Both enzyme activity and β-epoxide 1 labeling in these mutants can be restored by the addition of sodium azide as external acid/base. Binding of ABP 1 to the nucleophile in absence of the acid/base is completely rescued by employing several orders of magnitude less azide than required for substrate hydrolysis rescue.
Combining the outcome of fluorescent labeling with ABP 1 and 2 of wild-type and site-directed mutagenized β-glucosidases with the sodium azide-mediated rescue of their enzymatic activity and labeling allowed us to identify the acid/base and nucleophile in all human β-glucosidases studied: GBA (Glu-235/Glu-340), GBA3 (Glu-165/Glu-373), LPH (pocket III: Glu-1065/Glu-1273), and GBA2 (Asp-677/Glu-527). The catalytic residues designated in human GBA2 overlap with the recently identified acid/base and nucleophile in the GBA2-homologue SSO1353 from S. solfataricus (42) and their suggested locations in human GBA2. We noted that the proteins klotho, βklotho, and KLPH, although evolved from β-glucosidases given the close homology to members of GH1, are not labeled by either ABP, consistent with their lack of β-glucosidase activity, due to the absence of either a nucleophile or acid/base residue in their proposed structure.
In conclusion, our study demonstrates the versatility of combined use of cyclophellitol-based ABPs 1 and 2 in the highly sensitive identification of the acid/base and nucleophile residues via direct visualization on slab-gels. Rescue of enzyme activity with sodium azide in combination with selective ABP labeling of the nucleophile with β-aziridine 2 in the absence of the acid/base residue may serve as a general approach to discriminate between the catalytic residues of retaining β-glucosidases using fluorescence scanning of gels as a read-out. β-Epoxide 1 labeling in the presence or absence of sodium azide may be used to validate the results obtained with β-aziridine 2 in enzymes that tolerate labeling by this ABP. Broadening of this approach beyond β-glucosidases to other classes of retaining glycosidases should be feasible through variation of the cyclophellitol core structure (30).
Acknowledgment
We thank Syamirah Sulchan for technical assistance.
This work was supported by The Netherlands Organization for Scientific Research (NWO-CW, Top Grant (to H. S. O. and J. M. F. G. A.) and the European Research Council (ERC AdG; to H. S. O.).
- GH
- glycosylhydrolase
- GBA
- glucocerebrosidase
- LPH
- lactase-phlorizin hydrolase
- γklotho
- KLPH
- 4MU-β-d-Glc
- 4-methylumbelliferyl-β-d-glucopyranoside
- 4NP-β-d-Glc
- chromogenic 20 mm 4-nitrophenyl β-d-glucopyranoside
- 2,4DNP-β-d-Glc
- 20 mm 2,4-dinitrophenyl β-d-glucopyranoside
- ABP
- activity-based probe.
REFERENCES
- 1. Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009) The carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37, D233–D238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brady R. O., Kanfer J. N., Bradley R. M., Shapiro D. (1966) Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher's disease. J. Clin. Invest. 45, 1112–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grabowski G. A. (2008) Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet 372, 1263–1271 [DOI] [PubMed] [Google Scholar]
- 4. Grabowski G. A. (2012) Gaucher disease and other storage disorders. Hematology Am. Soc. Hematol. Educ. Program. 2012, 13–18 [DOI] [PubMed] [Google Scholar]
- 5. Tayebi N., Callahan M., Madike V., Stubblefield B. K., Orvisky E., Krasnewich D., Fillano J. J., Sidransky E. (2001) Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol. Genet. Metab. 73, 313–321 [DOI] [PubMed] [Google Scholar]
- 6. Bultron G., Kacena K., Pearson D., Boxer M., Yang R., Sathe S., Pastores G., Mistry P. K. (2010) The risk of Parkinson's disease in type 1 Gaucher disease. J. Inherited Metab. Dis. 33, 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Weely S., Brandsma M., Strijland A., Tager J. M., Aerts J. M. (1993) Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim. Biophys. Acta. 1181, 55–62 [DOI] [PubMed] [Google Scholar]
- 8. Boot R. G., Verhoek M., Donker-Koopman W., Strijland A., van Marle J., Overkleeft H.S., Wennekes T., Aerts J. M. (2007) Identification of the non-lysosomal glucosylceramidase as β-glucosidase 2. J. Biol. Chem. 282, 1305–1312 [DOI] [PubMed] [Google Scholar]
- 9. Yildiz Y., Matern H., Thompson B., Allegood J. C., Warren R. L., Ramirez D. M., Hammer R. E., Hamra F. K., Matern S., Russell D. W. (2006) Mutation of β-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J. Clin. Invest. 116, 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorli S. C., Colié S., Albinet V., Dubrac A., Touriol C., Guilbaud N., Bedia C., Fabriàs G., Casas J., Ségui B., Levade T., Andrieu-Abadie N. (2013. The nonlysosomal β-glucosidase GBA2 promotes endoplasmic reticulum stress and impairs tumorigenicity of human melanoma cells. FASEB J. 27, 489–498 [DOI] [PubMed] [Google Scholar]
- 11. Hammer M. B., Eleuch-Fayache G., Schottlaender L. V., Nehdi H., Gibbs J. R., Arepalli S. K., Chong S. B., Hernandez D. G., Sailer A., Liu G., Mistry P. K., Cai H., Shrader G., Sassi C., Bouhlal Y., Houlden H., Hentati F., Amouri R., Singleton A. B. (2013) Mutations in GBA2 cause autosomal-recessive cerebellar ataxia with spasticity. Am. J. Hum. Genet. 92, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin E., Schüle R., Smets K., Rastetter A., Boukhris A., Loureiro J. L., Gonzalez M. A., Mundwiller E., Deconinck T., Wessner M., Jornea L., Oteyza A. C., Durr A., Martin J. J., Schöls L., Mhiri C., Lamari F., Züchner S., De Jonghe P., Kabashi E., Brice A., Stevanin G. (2013) Loss of function of glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 92, 238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopalan V., Pastuszyn A., Galey W. R., Jr., Glew R. H. (1992) Exolytic hydrolysis of toxic plant glucosides by guinea pig liver cytosolic β-glucosidase. J. Biol. Chem. 267, 14027–14032 [PubMed] [Google Scholar]
- 14. Yahata K., Mori K., Arai H., Koide S., Ogawa Y., Mukoyama M., Sugawara A., Ozaki S., Tanaka I., Nabeshima Y., Nakao K. (2000) Molecular cloning and expression of a novel klotho-related protein. J. Mol. Med. 78, 389–394 [DOI] [PubMed] [Google Scholar]
- 15. Dekker N., Voorn-Brouwer T., Verhoek M., Wennekes T., Narayan R. S., Speijer D., Hollak C. E., Overkleeft H. S., Boot R. G., Aerts J. M. (2011) The cytosolic β-glucosidase GBA3 does not influence type 1 Gaucher disease manifestation. Blood Cells Mol. Dis. 46, 19–26 [DOI] [PubMed] [Google Scholar]
- 16. Skovbjerg H., Sjöström H., Norén O. (1981) Purification and characterisation of amphiphilic lactase/phlorizin hydrolase from human small intestine. Eur. J. Biochem. 114, 653–661 [DOI] [PubMed] [Google Scholar]
- 17. Wacker H., Keller P., Falchetto R., Legler G., Semenza G. (1992) Location of the two catalytic sites in intestinal lactase-phlorizin hydrolase. Comparison with sucrase-isomaltase and with other glycosidases, the membrane anchor of lactase-phlorizin hydrolase. J. Biol. Chem. 267, 18744–18752 [PubMed] [Google Scholar]
- 18. Zecca L., Mesonero J. E., Stutz A., Poirée J. C., Giudicelli J., Cursio R., Gloor S. M., Semenza G. (1998) Intestinal lactase-phlorizin hydrolase (LPH): the two catalytic sites; the role of the pancreas in pro-LPH maturation. FEBS Lett. 435, 225–228 [DOI] [PubMed] [Google Scholar]
- 19. Lloyd M., Mevissen G., Fischer M., Olsen W., Goodspeed D., Genini M., Boll W., Semenza G., Mantei N. (1992) Regulation of intestinal lactase in adult hypolactasia. J. Clin. Invest. 89, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y. I. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 [DOI] [PubMed] [Google Scholar]
- 21. Ito S., Kinoshita S., Shiraishi N., Nakagawa S., Sekine S., Fujimori T., Nabeshima Y. I. (2000) Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech. Dev. 98, 115–119 [DOI] [PubMed] [Google Scholar]
- 22. Ito S., Fujimori T., Hayashizaki Y., Nabeshima Y. (2002) Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim. Biophys. Acta 1576, 341–345 [DOI] [PubMed] [Google Scholar]
- 23. Ito S., Fujimori T., Furuya A., Satoh J., Nabeshima Y., Nabeshima Y. (2005) Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J. Clin. Invest. 115, 2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tohyama O., Imura A., Iwano A., Freund J. N., Henrissat B., Fujimori T., Nabeshima Y. (2004) Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J. Biol. Chem. 279, 9777–9784 [DOI] [PubMed] [Google Scholar]
- 25. Chang Q., Hoefs S., van der Kemp A. W., Topala C. N., Bindels R. J., Hoenderop J. G. (2005) The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 [DOI] [PubMed] [Google Scholar]
- 26. Cha S. K., Ortega B., Kurosu H., Rosenblatt K. P., Kuro-O M., Huang C. L. (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. U.S.A. 105, 9805–9810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetz R., Mohammadi M. (2013) Exploring mechanisms of FGF signaling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14, 166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rempel B. P., Withers S. G. (2008) Covalent inhibitors of glycosidases and their applications in biochemistry and biology. Glycobiology 18, 570–586 [DOI] [PubMed] [Google Scholar]
- 29. Witte M. D., Kallemeijn W. W., Aten J., Li K. Y., Strijland A., Donker-Koopman W. E., van den Nieuwendijk A. M., Bleijlevens B., Kramer G., Florea B. I., Hooibrink B., Hollak C. E., Ottenhoff R., Boot R. G., van der Marel G. A., Overkleeft H. S., Aerts J. M. (2010) Ultrasensitive in situ visualization of active glucocerebrosidase molecules. Nat. Chem. Biol. 6, 907–913 [DOI] [PubMed] [Google Scholar]
- 30. Kallemeijn W. W., Li K. Y., Witte M. D., Marques A. R., Aten J., Scheij S., Jiang J., Willems L. I., Voorn-Brouwer T. M., van Roomen C. P., Ottenhoff R., Boot R. G., van den Elst H., Walvoort M. T., Florea B. I., Codée J. D., van der Marel G. A., Aerts J. M., Overkleeft H. S. (2012) Novel activity-based probes for broad-spectrum profiling of retaining β-exoglucosidases in situ and in vivo. Angew Chem. Int. Ed. Engl. 51, 12529–12533 [DOI] [PubMed] [Google Scholar]
- 31. MacLeod A. M., Tull D., Rupitz K., Warren R. A., Withers S. G. (1996) Mechanistic consequences of mutation of active site carboxylates in a retaining β-1,4-glycanase from Cellulomonas fimi. Biochemistry 35, 13165–13172 [DOI] [PubMed] [Google Scholar]
- 32. Ly H. D., Withers S. G. (1999) Mutagenesis of glycosidases. Annu. Rev. Biochem. 68, 487–522 [DOI] [PubMed] [Google Scholar]
- 33. Bravman T., Belakhov V., Solomon D., Shoham G., Henrissat B., Baasov T., Shoham Y. (2003) Identification of the catalytic residues in family 52 glycoside hydrolase, a β-xylosidase from Geobacillus stearothermophilus T-6. J. Biol. Chem. 278, 26742–26749 [DOI] [PubMed] [Google Scholar]
- 34. Withers S. G., Street I. P., Bird P., Dolphin D. H. (1987) 2-Deoxy-2-fluoro-glucosides: a novel class of mechanism-based glucosidase inhibitors. J. Am. Chem. Soc. 109, 7530–7531 [Google Scholar]
- 35. Withers S. G., Rupitz K., Street I. P. (1988) 2-Deoxy-2-fluoro-d-glycosyl fluorides. A new class of specific mechanism-based glycosidase inhibitors. J. Biol. Chem. 263, 7929–7932 [PubMed] [Google Scholar]
- 36. Williams S. J., Withers S. G. (2000) Glycosyl fluorides in enzymatic reactions. Carbohydr. Res. 327, 27–46 [DOI] [PubMed] [Google Scholar]
- 37. Tull D., Withers S. G., Gilkes N. R., Kilburn D. G., Warren R. A., Aebersold R. (1991) Glutamic acid 274 is the nucleophile in the active site of a “retaining” exoglucanase from Cellulomonas fimi. J. Biol. Chem. 266, 15621–15625 [PubMed] [Google Scholar]
- 38. Andersson U., Butters T. D., Dwek R. A., Platt F. M. (2000) N-Butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo. Biochem. Pharmacol. 59, 821–829 [DOI] [PubMed] [Google Scholar]
- 39. Dvir H., Harel M., McCarthy A. A., Toker L., Silman I., Futerman A. H., Sussman J. L. (2003) X-ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 4, 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noguchi J., Hayashi Y., Baba Y., Okino N., Kimura M., Ito M., Kakuta Y. (2008) Crystal structure of the covalent intermediate of human cytosolic β-glucosidase. Biochem. Biophys. Res. Commun. 374, 549–552 [DOI] [PubMed] [Google Scholar]
- 41. Arribas J. C., Herrero A. G., Martín-Lomas M., Cañada F. J., He S., Withers S. G. (2000) Differential mechanism-based labeling and unequivocal activity assignment of the two active sites of intestinal lactase/phlorizin hydrolase. Eur. J. Biochem. 267, 6996–7005 [DOI] [PubMed] [Google Scholar]
- 42. Cobucci-Ponzano B., Aurilia V., Riccio G., Henrissat B., Coutinho P. M., Strazzulli A., Padula A., Corsaro M. M., Pieretti G., Pocsfalvi G., Fiume I., Cannio R., Rossi M., Moracci M. (2010) A new archaeal β-glycosidase from Sulfolobus solfataricus: seeding a novel retaining β-glycan-specific glycoside hydrolase family along with the human non-lysosomal glucosylceramidase GBA2. J. Biol. Chem. 285, 20691–20703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Overkleeft H. S., Renkema G. H., Neele J., Vianello P., Hung I. O., Strijland A., van der Burg A. M., Koomen G. J., Pandit U. K., Aerts J. M. (1998) Generation of specific deoxynojirimycin-type inhibitors of the non-lysosomal glucosylceramidase. J. Biol. Chem. 273, 26522–26527 [DOI] [PubMed] [Google Scholar]
- 44. Wennekes T., van den Berg R. J., Donker W., van der Marel G. A., Strijland A., Aerts J. M., Overkleeft H. S. (2007) Development of adamantan-1-yl-methoxy-functionalized 1-deoxynojirimycin derivatives as selective inhibitors of glucosylceramide metabolism in man. J. Org. Chem. 72, 1088–1097 [DOI] [PubMed] [Google Scholar]