Background: Flavonoids are natural compounds capable of modulating signaling pathways in a variety of biological processes.
Results: Isoquercitrin inhibits canonical Wnt signaling in Xenopus embryos downstream of β-catenin and impairs the growth in colon cancer cells in vitro, without cytotoxic effects.
Conclusion: Isoquercitrin inhibits Wnt/β-catenin pathway and the growth of colon cancer cells in vitro.
Significance: Isoquercitrin should be further investigated as a potential anti-tumoral agent targeting Wnt/β-catenin signaling.
Keywords: β-Catenin (B-Catenin), Colon Cancer, Flavonoid, Wnt Signaling, Xenopus, Quercetin
Abstract
Flavonoids are plant-derived polyphenolic molecules that have potential biological effects including anti-oxidative, anti-inflammatory, anti-viral, and anti-tumoral effects. These effects are related to the ability of flavonoids to modulate signaling pathways, such as the canonical Wnt signaling pathway. This pathway controls many aspects of embryonic development and tissue maintenance and has been found to be deregulated in a range of human cancers. We performed several in vivo assays in Xenopus embryos, a functional model of canonical Wnt signaling studies, and also used in vitro models, to investigate whether isoquercitrin affects Wnt/β-catenin signaling. Our data provide strong support for an inhibitory effect of isoquercitrin on Wnt/β-catenin, where the flavonoid acts downstream of β-catenin translocation to the nuclei. Isoquercitrin affects Xenopus axis establishment, reverses double axes and the LiCl hyperdorsalization phenotype, and reduces Xnr3 expression. In addition, this flavonoid shows anti-tumoral effects on colon cancer cells (SW480, DLD-1, and HCT116), whereas exerting no significant effect on non-tumor colon cell (IEC-18), suggesting a specific effect in tumor cells in vitro. Taken together, our data indicate that isoquercitrin is an inhibitor of Wnt/β-catenin and should be further investigated as a potential novel anti-tumoral agent.
Introduction
Flavonoids are plant-derived polyphenolic molecules. They have potential biological effects including anti-oxidative, anti-inflammatory, anti-viral, and anti-tumoral effects, in addition to prevention of cardiovascular diseases (1). Flavonoids are modulators of several cellular signaling pathways such as the Wnt/β-catenin pathway (canonical Wnt signaling) (2, 3). This signaling pathway controls many aspects of embryonic development as it plays a major role in embryonic axis determination and tissue maintenance (4–6).
In the absence of the Wnt ligand, cytoplasmic β-catenin is targeted for destruction via phosphorylation and proteasomal degradation (7). β-Catenin is a key component of the canonical Wnt signaling pathway. Activation of frizzled receptors and the co-receptor low-density lipoprotein-related receptor 5/6 (LRP5/6) by Wnt ligands disrupts this destruction complex. In this situation, β-catenin is translocated to the nucleus, where it associates with the T-cell factor/lymphoid enhancer factor (TCF/LEF) to activate specific Wnt target genes (7, 8).
Wnt signaling deregulation leads to several developmental defects and has been linked to many types of cancer in humans (9, 10). In the last decade, effects of flavonoids on modulation of Wnt/β-catenin signaling have been the focus of many studies. These molecules act on different components of the Wnt signaling pathway, such as Dkk1 (11), Gsk3-β (12), Dsh (13), and β-catenin/TCF/LEF (3, 14, 15). Recently, we characterized in vivo effects of the flavonoid quercetin as a negative modulator of the Wnt/β-catenin signaling pathway in Xenopus embryos (15). We also observed that quercetin shows high and nonspecific toxicity.
Our previous data have shown that isoquercitrin, which is derived from quercetin, affects the proliferation of glioblastoma cells, with lower toxicity (16). These anti-proliferative effects were accompanied by changes in β-catenin cellular localization, suggesting that Wnt/β-catenin signaling might be altered by this flavonoid (16).
Hence, we conducted a series of in vivo assays in Xenopus embryos to investigate whether isoquercitrin has an effect on Wnt/β-catenin signaling. The use of Xenopus allows a fast and clear functional reading on the role of small molecules in this signaling pathway (11, 15, 16). In addition, we monitored cell growth, death, migration, and toxicity of colon cancer cells upon isoquercitrin treatment. Taken together, our data indicate that isoquercitrin acts as an inhibitor of Wnt/β-catenin in Xenopus embryo experiments (in vivo) and in vitro and thus should be further investigated as a potential anti-tumoral agent.
EXPERIMENTAL PROCEDURES
Embryo Manipulations
Adult frogs (Nasco Inc.) were stimulated with 1000 IU human chorionic gonadotropin (Ferring Pharmaceuticals, Kiel, Germany). Xenopus embryos were obtained by in vitro fertilization and staged according to Nieuwkoop and Farber (17). We treated the embryos with flavonoids and performed the embryo manipulations according to Amado et al. (15).
Histological Analysis
For histological staining, Xenopus embryos were fixed in Bouin's fixative (Sigma-Aldrich), dehydrated, embedded in Paraplast Plus (Sigma-Aldrich), sectioned at 7 μm, dewaxed, and stained with hematoxylin and eosin as described by Reis et al. (18).
In Situ Hybridization
Xenopus embryos were fixed in MEMFA (MOPS, EGTA, MgSO4, and formaldehyde buffer; final concentrations: 100 mm MOPS (pH 7.4), 2 mm EGTA, 1 mm MgSO4, 3.7% (v/v) formaldehyde) at 4 °C overnight and then dehydrated in a methanol series (25, 50, 75, and 100%). Whole-mount in situ hybridization was performed according to Abreu et al. (19) with modifications suggested by Reversade and De Robertis (20) for Xenopus. Next, Xenopus embryos were treated with a bleaching solution (2.5% 20× SSC, 5% formamide, 4% H2O2 in H2O).
Luciferase Assay
Four-cell-stage embryos were injected into the ventral or dorsal marginal zone with 300 pg of luciferase reporter plasmid (S01234-Luc) and 50 pg of TK-Renilla, alone or in combination with 10 pg of xWnt8, 500 pg of LRP6, 200 pg of β-catenin WT, 50 pg of β-catenin S33A, and 500 pg of Lef1 ΔN VP16 mRNA. After microinjection, embryos were treated with flavonoids until sibling control embryos reached stage 10.5. Triplicates of four embryos were lysed according to the manufacturer's protocol (Dual-Luciferase reporter system, Promega), and 20 μl of embryo lysate was used for luciferase detection.
LiCl Treatment
Dorsalized embryos were obtained by LiCl treatment as described previously by Kao et al. (21). Four-cell-stage embryos were treated with flavonoids at 75 and 150 μm, whereas controls were treated with 1% DMSO.2 When embryos reached the 32-cell stage, they were treated with 0.3 m LiCl in 0.1× Barth for 15 min and then thoroughly washed in 0.1× Barth. After LiCl treatment, embryos were treated again with flavonoids, and the control embryos were treated with 1% DMSO until stage 10.5. Then the flavonoids or DMSO were removed and the embryos were cultured until stage 32. Axis phenotypes were scored by the dorsal-anterior index (DAI) (21).
Western Blotting Analysis
Lysate samples from flavonoid HCT-116-treated cells at 75 and 150 μm were harvested in a sample buffer (0.02 mmol/liter dithiothreitol; 1.38 mmol/liter sodium dodecyl sulfate; 125 mmol/liter Tris-HCl, pH 6.8; and 20% glycerol). Protein was quantified by the Lowry method, and 10 mg of the total lysate was loaded in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted and transferred to a polyvinylidene fluoride membrane (Hybond-PTM, Amersham Biosciences, São Paulo, Brazil). Membranes were pre-blocked in 5% nonfat dry milk in Tris-buffered saline, 0.001% Tween 20 for 1 h and then incubated overnight with anti-cyclin D1 (1:2000, Cell Signaling), anti-PCNA (1:2000 Cell Signaling), anti-cleaved caspase-3 (1:500, Cell Signaling), anti-β-catenin (1:1000, Sigma), anti-lamin (1:1000, Cell Signaling), and anti-α-tubulin (1:5000, Sigma) primary antibodies previously diluted in Tris-buffered saline, 0.001% Tween 20, 5% nonfat milk. Secondary antibodies conjugated with horseradish peroxidase were used to probe the membranes, and the reaction was visualized using a Pierce Fast Western blot kit, SuperSignal West Pico chemiluminescent substrate.
MTT Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide (MTT) was used to assay mitochondrial activity in viable cells. Cells were plated at a concentration of 1.0 × 104 cells/well in 96-well tissue culture plates in a medium containing 10% fetal bovine serum and cultured for 24 h. Next, cells were treated for 24 h with different concentrations of flavonoids (12.5, 25, 50, 75, 150, 200 μm). MTT was added to each well at a final concentration of 0.5 mg/ml 2 h before cell harvesting. The formazan reaction product was dissolved with 100% DMSO and quantified spectrophotometrically at 570 nm using a UV-visible system.
Immunocytochemistry
Immunostaining was performed as described by Garcia-Abreu et al. (22). Briefly, cells were fixed in 4% paraformaldehyde in PBS at pH 7.6, washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Samples were then blocked for 1 h with PBS containing 5% bovine serum albumin. A rabbit anti-β-catenin (1:200, Sigma) and anti-α-tubulin (1:500, Sigma) primary antibody were incubated for 1 h at room temperature. Specific secondary antibodies conjugated with Cy3 fluorochrome and FITC fluorochrome (1:10,000, Invitrogen) were incubated for 1 h at room temperature. After PBS washes, slides were mounted and observed in a Nikon TE 2000 inverted microscope (Melville, NY). Images were captured using a CoolSNAP-Pro (Media Cybernetics, Bethesda, MD) digital camera.
Flavonoid Treatment of Cells
In this study, we used human colon cancer HCT-116, SW480, DLD-1, and non-tumor cell IEC-18. Low-passage cells were cultured in Dulbecco's modified Eagle's medium until 70% confluence was reached. HCT-116 cells were treated with flavonoid isoquercitrin for 24 h at concentrations of 75 and 150 μm. DMSO (used as a vehicle to solubilize the flavonoids) was added to control cultures at 1% v/v. To stimulate the Wnt pathway, cells were treated with Wnt3a-conditioned medium (CM) for 6 h at 25% of conditioned medium. Cells were co-treated with Wnt3a-CM and isoquercitrin for 6 h at 75 or 150 μm.
Proliferation Assay
In this study, we used human colon cancer HCT-116, SW480, DLD-1, and non-tumor cell IEC-18. (ATCC). Low-passage cells were cultured in Dulbecco's modified Eagle's medium until 70% confluence was reached. For the proliferation assay, HCT-116 cells were treated with isoquercitrin for 24 h at 150 μm, and a Click-it EdU (Life Technologies) assay was performed according to the manufacturer's protocol. DMSO was used as a vehicle to solubilize the flavonoids and was added to control cultures at 1,5% v/v.
Scratch Assays
Confluent SW480, HCT116, DLD-1, and IEC-18 cell cultures in 12-well plates were treated with 150 μm isoquercitrin. A line down the center of each well was scraped with a p200 pipette tip followed by a wash to remove debris. Images were taken at 0 h, scratch widths were measured, and wound closure was calculated by dividing widths measured after 18 h of incubation by the initial scraped width. Each experiment was carried out in triplicate, and three fields were counted per well by scorers blinded to experimental conditions.
Statistics
The figures show the mean of n = 3 replicates; standard error and significance (p value) were determined by paired Mann-Whitney test (GraphPad Prism Software, version 5.03).
RESULTS
Isoquercitrin Induces Axial Defects in Xenopus Embryos
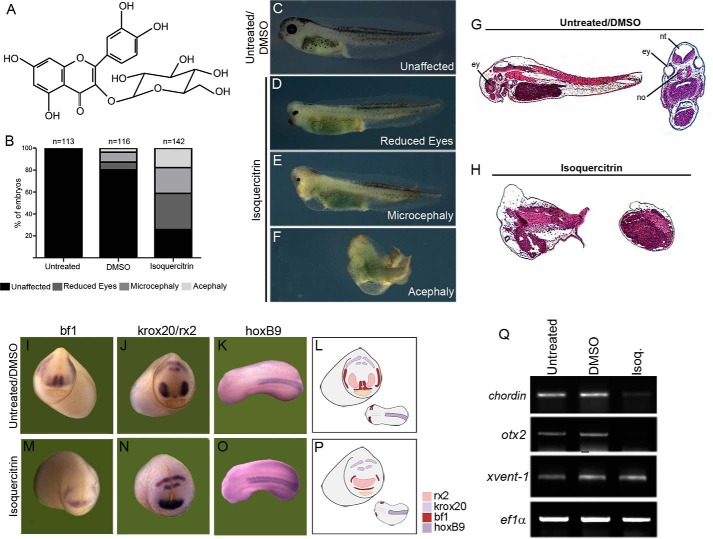
Previous results from our group showed that the flavonoid isoquercitrin changes β-catenin cellular localization in glioblastoma cells (15). Modification of components of the Wnt/β-catenin pathway at a specific time point during frog development can cause axis defects (4). Therefore, to investigate whether isoquercitrin affects Wnt/β-catenin signaling, we performed Xenopus embryo experiments. Four-cell-stage Xenopus embryos were treated with isoquercitrin (Fig. 1A) to investigate its function in affecting axis development. Morphological analysis showed that isoquercitrin treatment induced a strong abnormal phenotype in which embryos lacked dorsal-anterior structures (Fig. 1, C–F). We noted that 26% of embryos were unaffected, 33% displayed reduced eyes, 23% were microcephalic, and 18% were acephalic, where no head structure was observed (Fig. 1, B–F, and Table 1). The MTT assay revealed that isoquercitrin did not affect the viability of treated embryos (data not shown). Histological analysis reinforced the morphological observations because dorsal structures were absent, such as the neural tube and notochord (Fig. 1, G–H). We also analyzed gene expression in anterior and posterior markers by in situ hybridization and PCR. We observed that the anterior markers bf1 (forebrain), rx2 (eyes), and krox20 (rhombomeres 3 and 5) had the expression domains reduced, but the posterior marker HoxB9 (spinal cord) was not affected by flavonoid treatment (Fig. 1, I–P, and Table 2). We also observed through RT-PCR that expression of dorsal-anterior markers Chordin and Otx2 was lower, whereas the ventral marker xvent1 was not affected. Taken together, these results show that isoquercitrin treatment significantly affected Xenopus embryo development, most likely by disturbing axial patterning.
FIGURE 1.
Isoquercitrin induces axial defects in Xenopus embryos. A, molecular structure of isoquercitrin. B, quantification of the phenotypes obtained by isoquercitrin at 150 μm treatment and control (DMSO). C, control embryo. D–F, isoquercitrin leads to reduced eyes (D), microcephalic (E), or acephalic (F) phenotypes. G and H, histological sections show lack of dorsal structures after isoquercitrin treatment. In situ hybridization reveals disturbance in expression domain of bf1 (I and M), krox20/rx2 (J and N), and hoxB9 (K and O). L and P, schematic representation of the alterations in the gene expression of anterior region of the embryo. Q, PCR reveals reduced expression of anterior markers chordin and otx2, whereas xvent-1 is unaffected after isoquercitrin (Isoq.) treatment (lane 3). Ef1-α was used as loading control.
TABLE 1.
Quantification of phenotypes of untreated, DMSO-treated, and isoquercitrin-treated embryos
| Phenotype |
|||||
|---|---|---|---|---|---|
| n | Unaffected | Reduced Eyes | Microcephaly | Acephaly | |
| Untreated | 116 | 116 | 0 | 0 | 0 |
| DMSO | 113 | 91 | 8 | 10 | 4 |
| Isoquercitrin | 142 | 37 | 47 | 33 | 25 |
TABLE 2.
Quantification of in situ hybridization of untreated, DMSO-treated, and isoquercitrin-treated embryos
The reduction of rx2, krox20, n-tubulin, and hox-B9 genes was evaluated. Isoq., isoquercitrin.
| Gene | Untreated |
DMSO |
Isoq. 75 μm |
Isoq. 150 μm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Unaffected | Reduced | n | Unaffected | Reduced | n | Unaffected | Reduced | n | Unaffected | Reduced | |
| % | % | % | % | % | % | % | % | |||||
| rx2 | 23 | 100 | 0 | 29 | 89 | 11 | 28 | 75 | 25 | 28 | 12,5 | 87,5 |
| krox20 | 23 | 100 | 0 | 29 | 89 | 11 | 28 | 75 | 25 | 28 | 12,5 | 87,5 |
| n-tubulin | 25 | 100 | 0 | 31 | 94 | 9 | 31 | 60 | 40 | 29 | 44 | 66 |
| hox-B9 | 24 | 100 | 0 | 31 | 100 | 0 | 27 | 100 | 0 | 24 | 100 | 0 |
Isoquercitrin Inhibits Wnt/β-Catenin
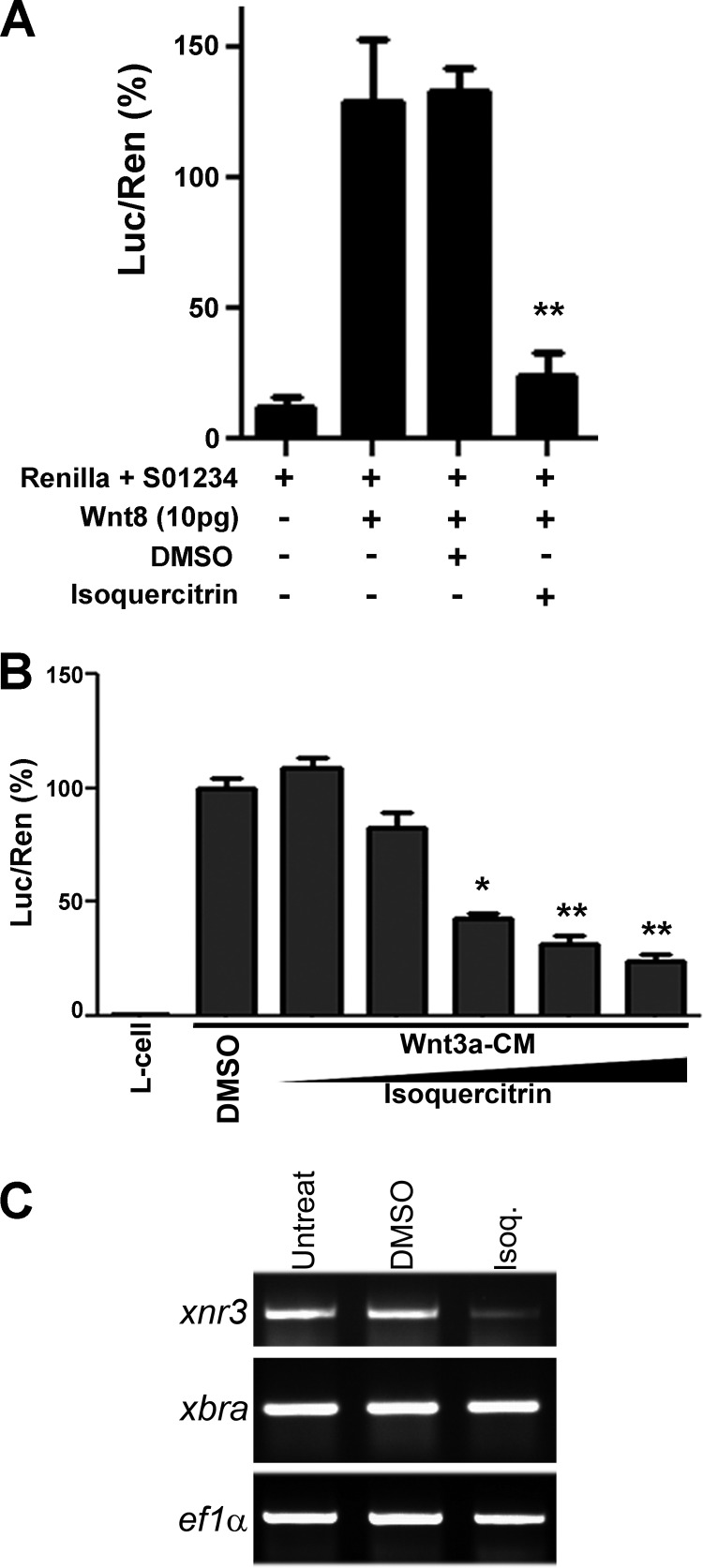
The disturbance of axial patterning of Xenopus embryos suggested modulation of the canonical Wnt signaling pathway. Our previous study suggested that isoquercitrin changes β-catenin cellular localization (16). To test this hypothesis, we conducted a siamois reporter assay (S01234-Lux) for the Wnt/β-catenin pathway in Xenopus embryos (23). To induce canonical Wnt signaling, xWnt8 mRNA was injected and showed almost 10.0-fold induction of luciferase siamois reporter activity (Fig. 2A). The induction of the gene reporter was reduced after treatment with isoquercitrin, reaching 5.0-fold less than the control (Fig. 2A). Similar experiments were performed by using RKO-BAR/Renilla cells. These cells were incubated with Wnt3a-CM and treated with increasing concentrations of isoquercitrin (12.5, 25, 50, 75, 150, 200 μm). The treatment significantly inhibited gene reporter activity in a concentration-dependent manner, reaching 70% reduction in activity when cells were treated with 200 μm of the flavonoid (Fig. 2B). Isoquercitrin also significantly decreased the endogenous expression of xnr3, a specific target of Wnt/β-catenin signaling. However, isoquercitrin was not able to affect expression of xbra, a pan-mesodermal marker that is a nodal target gene (24) (Fig. 2C). These data reinforce the idea that the flavonoid isoquercitrin acts as an inhibitor of Wnt/β-catenin signaling.
FIGURE 2.
Isoquercitrin inhibits Wnt/β-catenin. A, embryos were injected with Renilla + S01234 reporter, xwnt8 (10 pg), followed by DMSO or isoquercitrin treatment (150 μm). The activation of the gene reporter was counteracted by the treatment with isoquercitrin, reaching 5.0-fold less than the control. Luc, luciferase; Ren, Renilla. B, RKO-BAR/Renilla cells incubated with Wnt-3a conditioned medium (Wnt3a-CM) and treated with 12.5, 25, 50, 100, and 200 μm isoquercitrin, showing that reduction of gene reporter activity is concentration-dependent. C, whole-embryo PCR showing endogenous expression of xnr3, Wnt target gene, and xbra, Nodal target gene. *, p < 0.05; **, p < 0.01. Isoq., isoquercitrin.
Isoquercitrin Inhibits Ectopic Axis Formation and Rescues Lithium Chloride-dorsalized Embryos
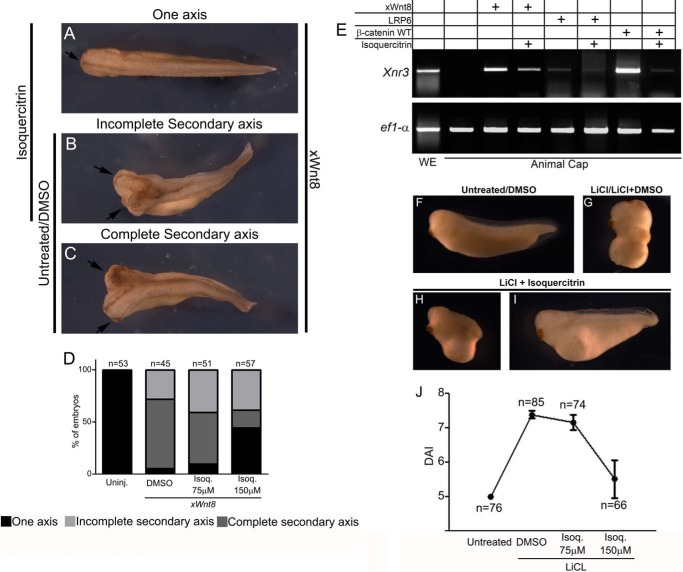
The ventral injection of xWnt8 mRNA in Xenopus embryos induced 94% secondary axis formation (Fig. 3, A–C). With this in mind, we analyzed the capability of isoquercitrin to affect the ectopic axis formation induced by xWnt8. In fact, the presence of isoquercitrin reduced secondary axis formation (Fig. 3, A–C). As shown in Fig. 3D, 55% of embryos displayed an ectopic axis and only 17% displayed a complete double axis. Consistently, RT-PCR analysis of animal cap explants revealed that isoquercitrin treatment reduced the xnr3 mRNA induction by activators of Wnt (Fig. 3E). To substantiate our results, we used another standard method to analyze the canonical Wnt signaling pathway in Xenopus embryos, the LiCl assay. LiCl is an inhibitor of GSK3-β, a key kinase involved in phosphorylation of β-catenin. As a consequence of LiCl treatment, embryos become hyperdorsalized. The LiCl assay was performed in embryos and scored according to the DAI (21). Isoquercitrin was able to counteract the LiCl-dorsalized embryos phenotype and showed a DAI close to 5 (Fig. 3, G–J) whereas LiCl-treated embryos showed a DAI close to 7.4 (Fig. 3, G and J). These data suggest that isoquercitrin is able to inhibit in vivo canonical Wnt signaling stimulation as well as LiCl-induced dorsalization in Xenopus embryos.
FIGURE 3.
Isoquercitrin inhibits ectopic axis formation and rescues lithium chloride-dorsalized embryos. A–C, phenotypes of axis patterning induced by ventral xwnt8 injection. Note that embryos display one axis, or an incomplete or complete secondary axis. D, isoquercitrin (Isoq.) treatment reduced ectopic axis formation. Uninj., uninjected. E, isoquercitrin-treated or untreated animal cap explants were injected with xWnt8, LRP6, or β-catenin. mRNA levels of xnr3 and the loading ef1α were analyzed by RT-PCR. F–I, LiCl assay phenotypes. J, graph displays a DAI index after LiCl and isoquercitrin treatment. Note that isoquercitrin reduces the DAI of lithium chloride-dorsalized embryos from approximately 7.4 to 5. WE, whole embryo.
Isoquercitrin Affects Canonical Wnt Signaling Downstream of β-Catenin Nucleus Translocation
Knowing that isoquercitrin inhibits the in vivo and in vitro Wnt/β-catenin signaling pathway, we asked where in the canonical Wnt pathway the flavonoid exerts its effect. To answer this question, we performed an epistasis analysis using specific activators of different points in the Wnt/β-catenin pathway. A Wnt/β-catenin-specific siamois reporter was co-injected with Wnt activators, followed by isoquercitrin treatment. Treatment with isoquercitrin was able to reduce signaling activation induced by Wnt8, LRP6, β-catenin WT, and β-catenin S33A mRNA, a stabilized form of β-catenin that is constantly translocated to the nuclei (Fig. 4) (25). However, when embryos were injected with a constitutive active Lef1 form (26), Lef1 ΔN VP16 mRNA, isoquercitrin was not able to inhibit Wnt pathway stimulation (Fig. 4). These data suggest that isoquercitrin acts downstream of the β-catenin nuclear translocation.
FIGURE 4.
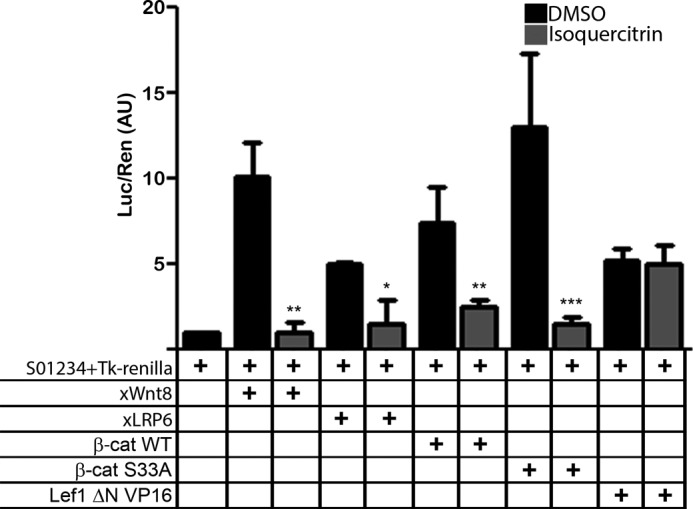
Isoquercitrin affects canonical Wnt signaling downstream to β-catenin nucleus translocation. Isoquercitrin interfered with Wnt signaling activation when Xenopus embryos were injected with siamois reporter gene S01234 and co-injected with Wnt8, LRP6, β-catenin WT, and β-catenin (β-cat) S33A but not with Lef1 ΔN VP16. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Luc, luciferase; Ren, Renilla; AU, arbitrary units.
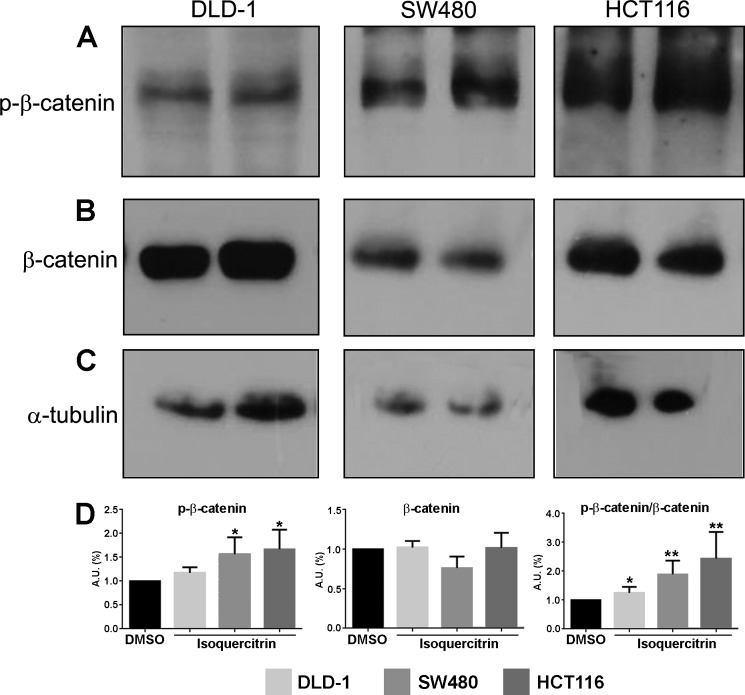
Isoquercitrin Affects β-Catenin Phosphorylation in Colon Cancer Cells
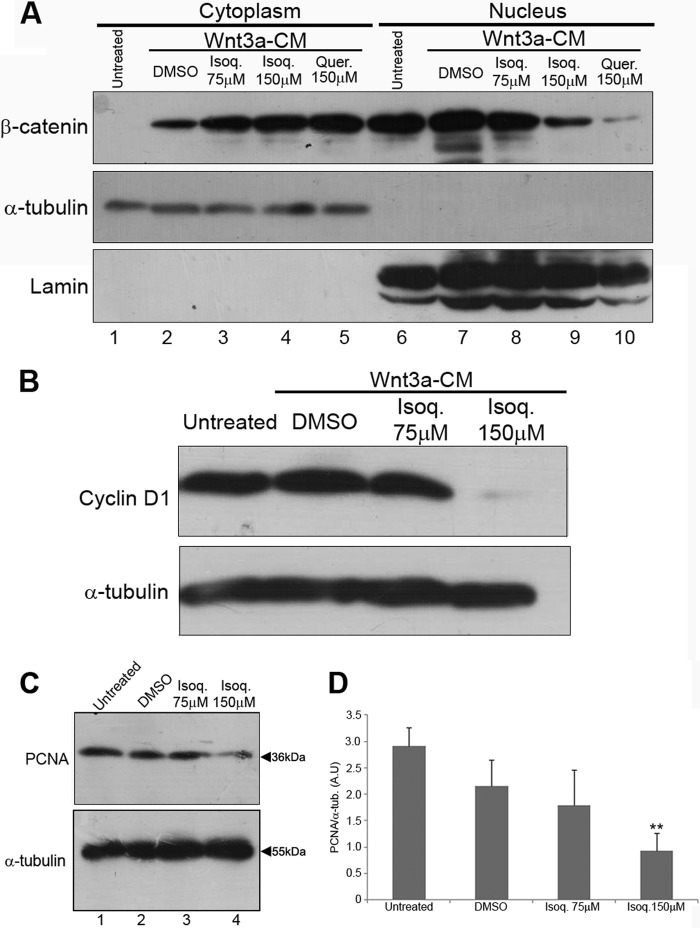
The Wnt/β-catenin pathway is constitutively activated in more than 80% of human colon cancers (27). As isoquercitrin inhibited canonical Wnt signaling in the Xenopus experiments, we asked whether it could change the phosphorylation status of β-catenin in colon cancer cells. Isoquercitrin treatment of colon cancer cell lines DLD-1, HCT116, and SW480 increased the level of phosphorylated β-catenin (Fig. 5A), whereas total β-catenin was less reduced in HCT116 and SW480 or unaffected in DLD-1 (Fig. 5B). Consistently, when we analyzed the ratio of phosphorylated-β-catenin to total β-catenin in each colon cancer cell line, we found that isoquercitrin caused a significant increase in levels of phosphorylated-β-catenin (Fig. 5D). Because the effect on HCT116 cells was remarkable, we used this cell line to analyze β-catenin localization and levels of cyclin D1, a Wnt/β-catenin target gene, in HCT116 (29) (Fig. 6). We showed that isoquercitrin affects β-catenin localization, inducing accumulation of large amounts in the cytoplasm while decreasing β-catenin in the nucleus (Fig. 6A). In addition, isoquercitrin also reduces the levels of cyclin D1 in HCT-116 cells (Fig. 6B). Consistently, in the HCT116 cell line, the PCNA level was decreased after isoquercitrin treatment in a dose-dependent manner (Fig. 6, C and D). These data support an inhibitory effect of isoquercitrin on Wnt/β-catenin signaling in colon cancer cell lines.
FIGURE 5.
Isoquercitrin affects β-catenin phosphorylation in colon cancer cells. Phosphorylation (p) of β-catenin was analyzed after 24 h of isoquercitrin treatment. A, in all colon cancer cell lines, phospho-β-catenin levels increased. B, total β-catenin levels are not significantly affected in colon cancer cell lines. C, α-tubulin was used as loading control. D, protein level graphs of phospho-β-catenin, β-catenin, and the ratio between phospho-β-catenin and total β-catenin. Note that phospho-β-catenin increased after isoquercitrin treatment. *, p < 0.05; **, p < 0.01. A.U., arbitrary units.
FIGURE 6.
Isoquercitrin affects β-catenin cell localization and Wnt target expressions and suppresses cell proliferation in HCT-116 colon cancer cells. A, β-catenin level is increased in the cytoplasm after isoquercitrin (Isoq.) treatment in a dose-dependent manner, whereas it is decreased in the nucleus in the same condition. α-Tubulin and lamin were used as cytoplasm and nucleus loading controls, respectively. The Wnt signaling pathway was stimulated with Wnt-3a conditioned media (Wnt3a-CM). Quercetin (Quer.) was used as a known flavonoid able to inhibit the Wnt signaling pathway. B, cyclin D1 expression was decreased after isoquercitrin treatment in a dose-dependent manner. DMSO was used as control. C, Western blotting analysis PCNA expression. α-Tubulin was used as loading control. D, protein levels graph of PCNA. *, p < 0.05; ***, p < 0.001. A.U., arbitrary units.
Isoquercitrin Showed No Toxic Effects on Colon Cancer Cells
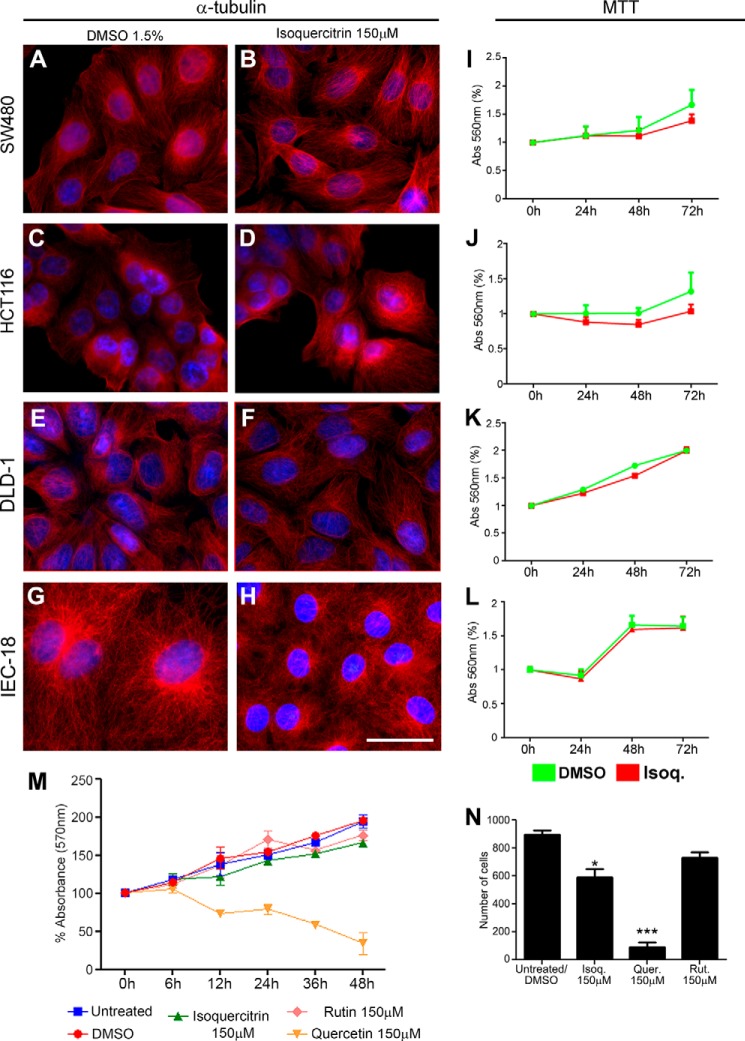
As we observed an inhibition of Wnt/β-catenin in colon cancer cell lines, we next asked what would be the effects of this inhibition on colon cancer cells and on the non-tumoral colon cell line IEC-18. First, we performed immunocytochemistry against α-tubulin and performed an MTT assay to evaluate the morphology and viability, respectively, of these cells after isoquercitrin treatment (Fig. 7). Isoquercitrin did not affect the cell morphology in all cell lines analyzed (Fig. 7, A–H) or cell viability after 24–72 h of isoquercitrin treatment (Fig. 7, I–L). We compared the effects of isoquercitrin, quercetin, and rutin on HCT116 cells. Quercetin is a well known flavonoid with a number of biological effects that have been well described (15, 28). Rutin is a flavonoid that has little effect on tumor cells (3, 16). Quercetin impaired cell viability after 12 h of treatment (Fig. 7M) and dramatically decreased the number of cells in culture after 24 h (Fig. 7N). However, neither isoquercitrin nor rutin affected cell viability after 48 h of treatment (Fig. 7M). The cytotoxic effect of quercetin was accompanied by changes in cell morphology in HCT116, SW480, and DLD-1 (not shown). These data show that exposure of colon cancer cells to isoquercitrin did not show any apparent toxic effect, according to the tests we applied.
FIGURE 7.
Isoquercitrin shows no toxic effects on colon cancer cells and non-tumor colon cells. A–H, immunochemistry against α-tubulin to analyze cell morphology. Cell morphology was not affected by isoquercitrin treatment. I–L, MTT assay measured at 0, 24, 48 and 72 h showed lower toxicity of isoquercitrin (Isoq.). M, MTT assay on HCT116 cells measured at 0, 6, 12, 24, and 48 h showed lower toxicity of isoquercitrin or rutin treatment, whereas quercetin treatment was toxic. N, total cell counts were reduced by isoquercitrin (Isoq.) and quercetin (Quer.) treatments but were not affected by rutin (Rut.) treatment. *, p < 0.05; ***, p < 0.001. Abs, absorbance.
Isoquercitrin Suppressed Cell Proliferation in Colon Cancer Cells but Did Not Affect Non-tumoral Colon Cells
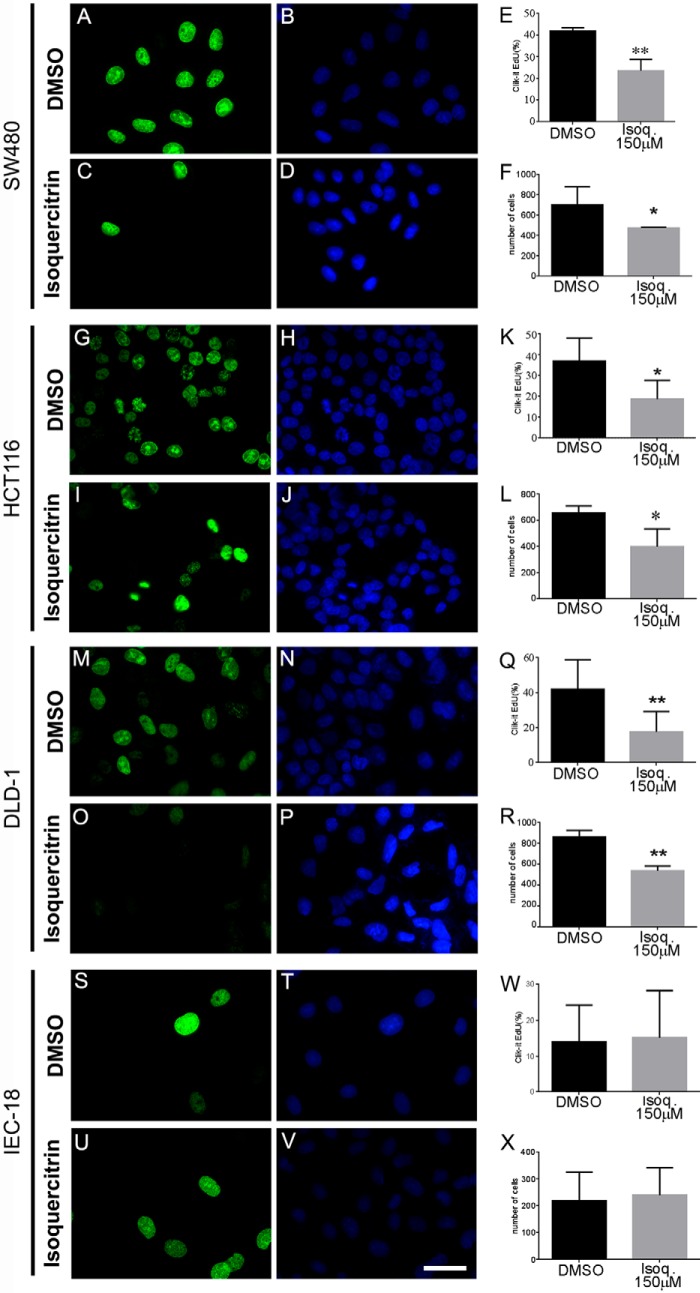
To investigate whether isoquercitrin treatment affects cell proliferation, we performed a cell proliferation assay (Click-it EdU assay). Isoquercitrin significantly decreased cell proliferation in all tumor cell lines analyzed (Fig. 8). The SW480 cell line treated with DMSO showed a 40% proliferation ratio. When these cells were treated with isoquercitrin for 24 h, the proliferation level decreased to ∼24% (Fig. 8, A–E). Consistently, the total number of cells in culture was decreased by nearly 50% (Fig. 8F). Similarly, HCT-116 and DLD-1 cell lines treated with DMSO showed cell proliferation ratios of 39 and 42%, respectively (Fig. 8, G–K and M–Q). After isoquercitrin treatment, the cell proliferation ratio decreased to 18 and 17%, respectively (Fig. 8, K and Q). The total number of HCT116 and DLD1 cells was also reduced after isoquercitrin treatment (Fig. 8, L and R).
FIGURE 8.
Isoquercitrin suppresses cell proliferation in colon cancer cells but does not affect non-tumoral colon cells. Colon cancer cells were analyzed by Click-it EdU assay to measure proliferation. Click-it EdU immunocytochemistry is shown for SW480 (A–D), HCT116 (G–J), DLD-1 (M–P), and IEC-18 (S–V) cells. Isoquercitrin (Isoq.) treatment significantly reduced cell proliferation in all colon cancer cell lines analyzed, but did not affect cell proliferation in non-tumor cell line IEC-18. E, in SW480 cells, the proliferation ratio decreased nearly to 24%. F, L, and R, total number of cells in SW480, HCT116, and DLD-1 also decreased nearly to 50%. K, in HCT116 cells, the proliferation ratio decreased nearly to 18%. Q, in DLD-1 cells, the proliferation ratio decreased nearly to 17%. W and X, in IEC18 cells, the proliferation ratio was not affected (W), and the total number of cells was unaffected (X) by isoquercitrin treatment. *, p < 0.05; **, p < 0.01.
We also observed that cell proliferation ratio of the non-tumoral cell line IEC-18 did not change after DMSO or isoquercitrin treatment (Fig. 8, S–X). Together these data show that isoquercitrin inhibits the growth of three colon cancer cell lines, but has no effect on a non-tumoral colon cell line, suggesting a specific effect on colon tumor cells.
Isoquercitrin Inhibits Cell Migration in Colon Cancer Cells
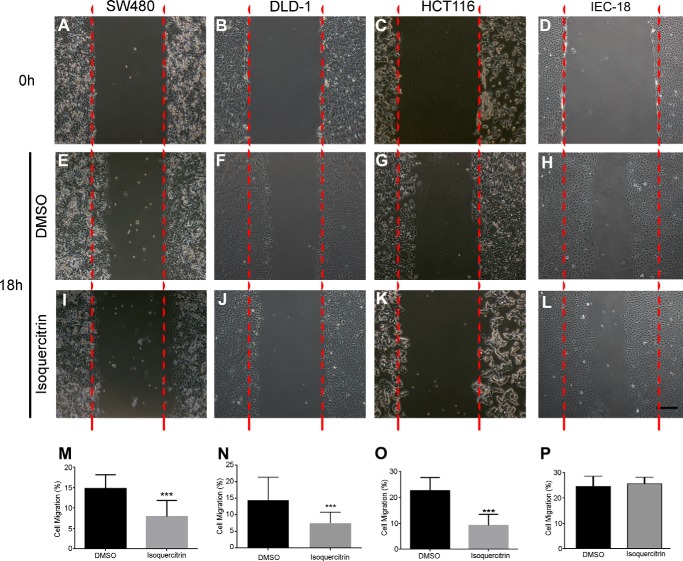
As isoquercitrin inhibited cell growth of colon cancer cell lines, we asked whether cell migration, another fundamental process deregulated in cancer cells, could be affected by the flavonoid treatment. To do this, DLD-1, HCT116, SW480, and IEC-18 cells were treated with isoquercitrin during a scratch assay (Fig. 9). At 18 h after scratch, in DMSO-treated cells, the migration rates were 14, 13, 22 and 25% in SW480, DLD-1, HCT116, and IEC-18, respectively (Fig. 9, A–H). When these cells were treated with isoquercitrin, the migration was significantly reduced, to 8, 7, and 8% in SW480, DLD-1, and HCT116, respectively (Fig. 9, I–K and M–O). However, migration of IEC-18 cells was not affected after isoquercitrin treatment (Fig. 9, L and P). These data indicate that isoquercitrin inhibited migration in these colon cancer cell lines, but had no apparent effect on the non-tumor colon cells.
FIGURE 9.
Isoquercitrin inhibits cell migration in colon cancer cells but does not affect non-tumoral colon cells. Colon cancer cells were investigated for potential to migrate into a cell-free scratch region. A–D, SW480, DLD-1, HCT116, and IEC-18 cells at 0 h of scratch assay. E–H, SW480, DLD-1, HCT116, and IEC-18 cells after DMSO treatment for 18 h of scratch assay. I–L, SW480, DLD-1, HCT116, and IEC-18 cells after isoquercitrin treatment for 18 h of scratch assay. Note that migration was inhibited by isoquercitrin in all colon cancer cell lines used. No effect was observed in non-tumor IEC-18 cells. M–P, graphs representing the cell migration distance from the scratch border after 18 h of treatment. ***, p < 0.001.
DISCUSSION
In this study, we used in vivo and in vitro models to characterize isoquercitrin as an inhibitor of the Wnt/β-catenin signaling pathway, acting downstream of β-catenin translocation to nuclei. Isoquercitrin treatment affected Xenopus axial patterning, reversed the Wnt8-induced double axis and the LiCl hyperdorsalization phenotype, reduced expression of the Wnt8 direct target gene, Xnr3, and increased β-catenin phosphorylation levels in cancer cells. In addition, this flavonoid impaired growth of colon cancer cells (DLD-1, HCT116, and SW480 cell lines) by inhibiting proliferation and migration with no apparent cytotoxic effect. These effects were not observed in non-tumor colon cell line IEC-18.
In the last 10 years, several studies have reported that the anti-tumoral effects of flavonoids are related to their ability to modulate signaling pathways, including canonical Wnt signaling (2). In fact, we observed that isoquercitrin was able to affect axis establishment (Fig. 1) and to rescue the ectopic axis formation induced by xWnt8 (Fig. 3), which are Xenopus phenotypes associated with canonical Wnt signaling modulation (4, 15). These findings are in agreement with an inhibitory action of isoquercitrin on Wnt/β-catenin and provide evidence of an isoquercitrin effect in vivo.
Most colorectal carcinomas harbor genetic alterations that result in stabilization of β-catenin, leading to abnormal stimulation of canonical Wnt signaling (30, 31). We used HCT-116, DLD-1, and SW480 cells that have mutations in components of Wnt/β-catenin to investigate the cell growth inhibitory effects of isoquercitrin. We observed that isoquercitrin reduced cell proliferation and cell migration in all tumor cells tested. However, no effect was observed in non-tumor cells (Figs. 8 and 9). In addition, these effects were not accompanied by changes in cell morphology and showed lower toxicity (Fig. 7).
Isoquercitrin and quercetin share the same structural skeleton and are able to inhibit in vitro Wnt/β-catenin signaling, and both have cell proliferation inhibition effects (15, 16). However, quercetin induces high nonspecific toxicity in these cells (32, 33).
Our data indicate that isoquercitrin is an inhibitor of canonical Wnt signaling, and for the first time we showed at which level in the Wnt pathway the flavonoid exerts its effect. The epistasis analysis showed that isoquercitrin inhibits the signaling downstream of β-catenin nuclear translocation as isoquercitrin reverses the effect of β-catenin S33A, which cannot be phosphorylated and avoids degradation. In that case, β-catenin S33A is constantly translocated to the nuclei, thereby activating the pathway (25). The β-catenin S33A activation was inhibited by isoquercitrin, but not by Lef1ΔNVP16, which activated the pathway independently of β-catenin (26) (Fig. 4). Additionally, isoquercitrin also affected β-catenin phosphorylation in colon cancer cells (Fig. 5). These data reinforce the supposition that the effect on the Wnt/β-catenin signaling pathway was specific because our antibody recognizes two β-catenin phosphorylation sites (Ser-33 and Ser-37) specific for GSK3 phosphorylation (7). Interestingly, previous studies showed that quercetin is also able to negatively modulate canonical Wnt signaling downstream of β-catenin by blocking its association with Tcf-4 (34). Thus, isoquercitrin and quercetin might share the same mechanism for canonical Wnt signaling inhibition despite the toxic effects of quercetin. This toxic effect was reconfirmed by the cell morphology and MTT data obtained in this study. Glycosylated flavonoids are known to be less toxic than their aglycone counterparts (36). One possible reason for this lower toxicity is the different absorption and bioavailability of glycosylated flavonoids as the structure of the flavonoid seems to affect its absorption rate (35–37). However, the mechanisms involved in this absorption are still poorly understood and should be investigated.
Currently, there is an active search for new modulators of the Wnt pathway, which may have a broad impact on biology and medicine. In this study, we observed that isoquercitrin combines two interesting aspects for anti-tumoral research. First, the flavonoid blocks the Wnt signaling, most likely at the nuclear complex, between β-catenin and TCF, which supports its potential to interfere with tumor growth in other cell types as most colon tumors accumulate β-catenin in the nucleus (27, 30, 31). Second, isoquercitrin shows lower toxicity than quercetin, a known Wnt inhibitor already tested in clinical trials (38–40). These two main aspects provide strong support for further investigation of isoquercitrin as a potential novel anti-tumoral flavonoid.
In summary, our study dissected possible specific targets of isoquercitrin in Wnt/β-catenin signaling. It thus supported the tumor cell growth effect in vitro of this glycosylated flavonoid. Additionally, this investigation added to the understanding of the Wnt/β-catenin signaling pathway, which is known to be deregulated in many types of human cancer. Further investigation should be undertaken to understand the role of flavonoids as a potential novel class of anti-tumoral growth agents.
Acknowledgments
We are grateful to Professor Xi He from the Boston Children's Hospital, Harvard Medical School, and Professor Eddy De Robertis from the Department of Biological Chemistry, UCLA for generously providing plasmids. We thank Fabio Zuim and Fernando Lourenço Dutra for providing technical assistance and Simone Rodrigues for care of the frog colony.
This work was supported by CNPq (National Council of Technological and Scientific Development), FAPERJ (Rio de Janeiro State Foundation for Research and Development), and CAPES (Federal Agency for Higher Education).
- DMSO
- dimethyl sulfoxide
- DAI
- dorsal-anterior index
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide
- PCNA
- proliferating cell nuclear antigen
- CM
- conditioned medium.
REFERENCES
- 1. Sies H. (2010) Polyphenols and health: update and perspectives. Arch. Biochem. Biophys. 501, 2–5 [DOI] [PubMed] [Google Scholar]
- 2. Sarkar F. H., Li Y., Wang Z., Kong D. (2009) Cellular signaling perturbation by natural products. Cell. Signal. 21, 1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amado N. G., Fonseca B. F., Cerqueira D. M., Neto V. M., Abreu J. G. (2011) Flavonoids: potential Wnt/β-catenin signaling modulators in cancer. Life Sci. 89, 545–554 [DOI] [PubMed] [Google Scholar]
- 4. McMahon A. P., Moon R. T. (1989) Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58, 1075–1084 [DOI] [PubMed] [Google Scholar]
- 5. Niehrs C. (2010) On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development 137, 845–857 [DOI] [PubMed] [Google Scholar]
- 6. Zhang X., Abreu J. G., Yokota C., MacDonald B. T., Singh S., Coburn K. L. A., Cheong S.-M., Zhang M. M., Ye Q.-Z., Hang H. C., Steen H., He X. (2012) Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 149, 1565–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim S.-E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M. V., MacDonald B. T., Zhang X., Garcia Abreu J., Peng L., He X. (2013) Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 340, 867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destrée O., Clevers H. (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 [DOI] [PubMed] [Google Scholar]
- 9. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 10. Clevers H. (2006) Wnt/β-Catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 11. Tarapore R. S., Siddiqui I. A., Mukhtar H. (2012) Modulation of Wnt/β-catenin signaling pathway by bioactive food components. Carcinogenesis 33, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. (2004) Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79, 727–747 [DOI] [PubMed] [Google Scholar]
- 13. Hallett R. M., Kondratyev M. K., Giacomelli A. O., Nixon A. M., Girgis-Gabardo A., Ilieva D., Hassell J. A. (2012) Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PloS One 7, e33976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J., Lee J., Jung E., Hwang W., Kim Y.-S., Park D. (2010) Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of β-catenin protein. Life Sci. 86, 416–423 [DOI] [PubMed] [Google Scholar]
- 15. Amado N. G., Fonseca B. F., Cerqueira D. M., Reis A. H., Simas A. B., Kuster R. M., Mendes F. A., Abreu J. G. (2012) Effects of natural compounds on Xenopus embryogenesis: a potential read out for functional drug discovery targeting Wnt/β-catenin signaling. Curr. Top. Med. Chem. 12, 2103–2113 [DOI] [PubMed] [Google Scholar]
- 16. Amado N. G., Cerqueira D. M., Menezes F. S., da Silva J. F., Neto V. M., Abreu J. G. (2009) Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes β-catenin cellular localization. Anticancer Drugs 20, 543–552 [DOI] [PubMed] [Google Scholar]
- 17. Nieuwkoop P. D., Faber J. (eds) (1956) Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis, p. 22, Garland Publishing, London [Google Scholar]
- 18. Reis A. H., Almeida-Coburn K. L., Louza M. P., Cerqueira D. M., Aguiar D. P., Silva-Cardoso L., Mendes F. A., Andrade L. R., Einicker-Lamas M., Atella G. C. (2012) Plasma membrane cholesterol depletion disrupts prechordal plate and affects early forebrain patterning. Dev. Biol. 365, 350–362 [DOI] [PubMed] [Google Scholar]
- 19. Abreu J. G., Ketpura N. I., Reversade B., De Robertis E. (2002) Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat. Cell Biol. 4, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reversade B., De Robertis E. (2005) Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123, 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kao K. R., Masui Y., Elinson R. P. (1986) Lithium-induced respecification of pattern in Xenopus laevis embryos. Nature 322, 371–373 [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Abreu J., Moura Neto V., Carvalho S. L., Cavalcante L. A. (1995) Regionally specific properties of midbrain glia: I. Interactions with midbrain neurons. J. Neurosci. Res. 40, 471–477 [DOI] [PubMed] [Google Scholar]
- 23. Brannon M., Gomperts M., Sumoy L., Moon R. T., Kimelman D. (1997) A β-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 11, 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haramoto Y., Takahashi S., Asashima M. (2007) Monomeric mature protein of Nodal-related 3 activates Xbra expression. Dev. Genes Evol. 217, 29–37 [DOI] [PubMed] [Google Scholar]
- 25. Huang P., Senga T., Hamaguchi M. (2007) A novel role of phospho-β-catenin in microtubule regrowth at centrosome. Oncogene 26, 4357–4371 [DOI] [PubMed] [Google Scholar]
- 26. Aoki M., Hecht A., Kruse U., Kemler R., Vogt P. K. (1999) Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. U.S.A. 96, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeulen L., De Sousa E Melo F., van der Heijden M., Cameron K., de Jong J. H., Borovski T., Tuynman J. B., Todaro M., Merz C., Rodermond H., Sprick M. R., Kemper K., Richel D. J., Stassi G., Medema J. P. (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biol. 12, 468–476 [DOI] [PubMed] [Google Scholar]
- 28. Dajas F. (2012) Life or death: neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 143, 383–396 [DOI] [PubMed] [Google Scholar]
- 29. Tetsu O., McCormick F. (1999) β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 30. De Sousa E Melo F., Wang X., Jansen M., Fessler E., Trinh A., de Rooij L. P. M. H., de Jong J. H., de Boer O. J., van Leersum R., Bijlsma M. F., Rodermond H., van der Heijden M., van Noesel C. J., Tuynman J. B., Dekker E., Markowetz F., Medema J. P., Vermeulen L. (2013) Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 19, 614–618 [DOI] [PubMed] [Google Scholar]
- 31. Markowitz S. D., Bertagnolli M. M. (2009) Molecular basis of colorectal cancer. New Engl. J. Med. 361, 2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russo G. L. (2007) Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 74, 533–544 [DOI] [PubMed] [Google Scholar]
- 33. Boots A. W., Li H., Schins R. P., Duffin R., Heemskerk J. W., Bast A., Haenen G. R. (2007) The quercetin paradox. Toxicol. Applied Pharmacol. 222, 89–96 [DOI] [PubMed] [Google Scholar]
- 34. Park C. H., Chang J. Y., Hahm E. R., Park S., Kim H.-K., Yang C. H. (2005) Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Comm. 328, 227–234 [DOI] [PubMed] [Google Scholar]
- 35. Nijveldt R. J., van Nood E., van Hoorn D. E., Boelens P. G., van Norren K., van Leeuwen P. A. (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 74, 418–425 [DOI] [PubMed] [Google Scholar]
- 36. Pietta P.-G. (2000) Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042 [DOI] [PubMed] [Google Scholar]
- 37. Tapiero H., Tew K. D., Ba G. N., Mathé G. (2002) Polyphenols: do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 56, 200–207 [DOI] [PubMed] [Google Scholar]
- 38. Cialdella-Kam L., Nieman D. C., Sha W., Meaney M. P., Knab A. M., Shanely R. A. (2013) Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. Br. J. Nutr. 109, 1923–1933 [DOI] [PubMed] [Google Scholar]
- 39. Ferry D. R., Smith A., Malkhandi J., Fyfe D. W., deTakats P. G., Anderson D., Baker J., Kerr D. J. (1996) Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 2, 659–668 [PubMed] [Google Scholar]
- 40. Okamoto T. (2005) Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 16, 275–278 [PubMed] [Google Scholar]