Background: Epigenetic regulation plays an important role in cancer-associated signaling pathways.
Results: The bromodomain protein inhibitor I-BET151 attenuates Hedgehog signaling downstream of Smoothened.
Conclusion: I-BET151 mediated inhibition of Hedgehog-Gli activity acts downstream of Smoothened.
Significance: Modulation of bromodomain protein activity attenuates the growth of Hedgehog-driven tumors.
Keywords: Bromodomain-containing Protein 4 (BRD4), Drug Screening, Epigenetics, Hedgehog Signaling Pathway, Medulloblastoma, Gli, I-BET151
Abstract
Epigenetic enzymes modulate signal transduction pathways in different biological contexts. We reasoned that epigenetic regulators might modulate the Hedgehog (HH) signaling pathway, a main driver of cell proliferation in various cancers including medulloblastoma. To test this hypothesis, we performed an unbiased small-molecule screen utilizing an HH-dependent reporter cell line (Light2 cells). We incubated Light2 cells with small molecules targeting different epigenetic modulators and identified four histone deacetylase inhibitors and a bromodomain and extra terminal domain (BET) protein inhibitor (I-BET151) that attenuate HH activity. I-BET151 was also able to inhibit the expression of HH target genes in Sufu−/− mouse embryonic fibroblasts, in which constitutive Gli activity is activated in a Smoothened (Smo)-independent fashion, consistent with it acting downstream of Smo. Knockdown of Brd4 (which encodes one of the BET proteins) phenocopies I-BET151 treatment, suggesting that Brd4 is a regulator of the HH signaling pathway. Consistent with this suggestion, Brd4 associates with the proximal promoter region of the Gli1 locus, and does so in a manner that can be reversed by I-BET151. Importantly, I-BET151 also suppressed the HH activity-dependent growth of medulloblastoma cells, in vitro and in vivo. These studies suggest that BET protein modulation may be an attractive therapeutic strategy for attenuating the growth of HH-dependent cancers, such as medulloblastoma.
Introduction
One possible point of therapeutic intervention in HH-driven3 cancers is transcription of Gli proteins, which are mediated by epigenetic enzymes and modulators (1–3). These epigenetic pathway components have emerged as attractive drug targets in several cancers (4). Epigenetic modulators affect gene expression in part by modifying histone acetylation, which alters chromatin compaction and transcription (5). Histone acetyltransferases attach acetyl groups to lysine residues on histones, whereas histone deacetylases remove those acetyl groups. Reader proteins work in conjunction with histone acetyltransferases and histone deacetylases to modulate gene transcription, by binding acetylated histones and recruiting transcriptional complexes (6). The bromodomain and extra terminal domain (BET) proteins have emerged as an important class of reader proteins (7). The BET protein Brd4 is a key transcriptional regulator in multiple cancers (8–11). Other BET bromodomain proteins include Brd2, Brd3, and Brdt (7). Our recent studies, along with those from other laboratories, suggest that small-molecule Brd2/Brd4 inhibitors reduce glioblastoma and medulloblastoma cell growth (8, 11). These small molecules are acetylated histone mimics, which can compete with the Brd2- or Brd4-histone interaction (12) and thus reduce transcription of oncogenes important for glioblastoma cell proliferation (11).
Medulloblastoma is the most common malignant pediatric brain tumor (13). Surgical resection and radiation therapy are the standard of care for patients suffering from the Sonic Hedgehog (SHH) subtype of medulloblastoma (14). However, intellectual and cognitive difficulties are associated with these standard treatments. Small-molecule inhibitors of the HH pathway signaling protein Smoothened (Smo) have been utilized in clinical trials (15), although resistance to these Smo antagonists has been observed (16, 17). This has motivated the search for other points of therapeutic intervention for the treatment of medulloblastoma, preferably ones that act at or downstream of the Gli transcription factors that ultimately regulate canonical HH signaling. Importantly, several recent studies have demonstrated that epigenetic pathways are deregulated in medulloblastoma (18), suggesting that signaling via the HH pathway is dependent on epigenetic modulators. Thus, identifying potentially druggable epigenetic targets may assist in limiting medulloblastoma growth.
As several studies have demonstrated links between epigenetic enzymes and signaling pathways (19), we hypothesized that inhibitors of epigenetic modulators might function to attenuate HH signaling. We performed an unbiased screen to isolate such inhibitors and identified a BET inhibitor (I-BET151) as a specific modulator of HH signaling. We further demonstrate that I-BET151 acts downstream of Smo. I-BET151-mediated inhibition of HH signaling is likely related to Brd4 inhibition because Brd4 depletion also attenuates HH-dependent induction of target genes. Further, Brd4 enriches on the Gli1 locus in a manner that can be regulated by I-BET151. Importantly, I-BET151 attenuated the viability of medulloblastoma cancer stem/progenitor cells (CSCs) in vitro and the growth of an HH activity-dependent medulloblastoma allograft in vivo.
EXPERIMENTAL PROCEDURES
Assays
Light2 cells (20) were cultured in DMEM supplemented with 10% newborn calf serum, 1% penicillin/streptomycin, 0.2 μg/μl G418, and 0.1 μg/μl Zeocin. Sufu−/− mouse embryonic fibroblasts (MEFs) were a gift from Dr. Rune Tofgard and were cultured in DMEM supplemented with 10% newborn calf serum and 1% penicillin/streptomycin. Compound screening was performed in a 96-well plate format. The compounds were titrated by Hanks' balanced salt solution and added to Light2 cells at different concentrations in the presence of the Smo agonist SAG. Twenty-four hours later, cells were lysed in passive lysis buffer, and luciferase activity was determined as described previously (21). All compounds were screened in the presence of 0.5% newborn calf serum. The selected compounds (positive “hits”) were purchased from Tocris and assayed individually. To assay gene expression, RNA was extracted from cells using an RNeasy kit (Qiagen), converted into cDNA (Applied Biosystems), and subsequently analyzed using real-time PCR, utilizing TaqMan probes as per the manufacturer's instructions (Life Technologies). The Brd4 siRNA SMARTpool as well as non-target control siRNA (Dharmacon) was transfected into Sufu−/− MEFs using Lipofectamine 2000 (Life Technologies) for 72 h. ChIP analyses were performed by cross-linking cells with 1% formaldehyde, quenching this reaction with glycine, and then resuspending in an SDS buffer (22). This lysate was then sonicated to yield chromatin fragments of ∼300–800 bp. These chromatin fragments were immunoprecipitated with Brd4 antibodies (Bethyl Laboratories) or IgG control, reverse cross-linked at 65 °C, and treated with RNase A and proteinase K, and then the relevant region of the Gli1 locus was amplified by quantitative PCR. CSC viability was assayed by quantitating trypan blue (Life Technologies) exclusion using the Bio-Rad automatic cell counter TC20TM.
Mice and Drug Administration
All mice were handled in accordance with the policies of the University of Miami Institutional Animal Care and Use Committee. Spontaneous medulloblastomas from Ptch1+/− mice (The Jackson Laboratory) were subcutaneously grafted onto CD-1 nude mice (Charles River Laboratories). A subset of the tumors from these allografted mice was harvested and enzymatically digested using a papain dissociation system (Worthington). The resulting cell suspension was maintained in CSC selection medium containing Neurobasal, GlutaMAX, B27, N2, EGF, FGF, and Pen Strep media (Life Technologies) (23). A subset of the cohort of mice bearing these allografted medulloblastomas was treated with 30 mg/kg of I-BET151 in DMSO, by daily intraperitoneal injection, once the tumors reached a size of ∼200 mm3. Tumor size was measured daily, using the formula: V = π × L × W × W/6, where V is tumor volume, W is tumor width, and L is tumor length (24). Medulloblastoma tumors were subsequently harvested and then processed for H&E staining or HH target gene expression. Statistical analysis was determined by Student's two-tailed t test, unless otherwise stated. p values ≤ 0.05 were considered statistically significant.
RESULTS
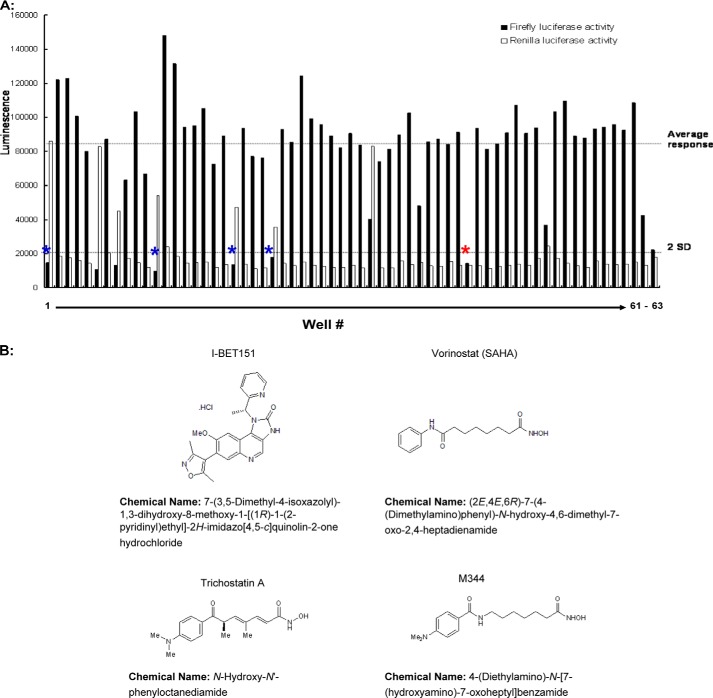
To screen for inhibitors of epigenetic modulators that attenuate HH activity, we used an established fibroblast cell line (Light2 cells) that stably expresses an HH-dependent firefly luciferase reporter gene, as well as a control Renilla luciferase gene driven by the TK promoter (20). These cells were activated with the Smo agonist SAG to induce luciferase activity, along with varying concentrations (0, 0.5, 1, 2, or 10 μm) of 60 distinct, well characterized epigenetic enzyme inhibitors, a number of which are in various stages of clinical evaluation (Table 1). As a positive control, we used the clinically approved Smo antagonist vismodegib (25). DMSO-treated cells, and cells not stimulated with SAG, were used as negative controls. Ideally, relevant inhibitors would significantly attenuate firefly luciferase expression, but have minimal effects on Renilla luciferase expression at equipotent doses. Based on the dose-dependent effects of these inhibitors on Light2 cells (Fig. 1A and data not shown), five inhibitors were selected for further evaluation. Four of these inhibitors were distinct histone deacetylase inhibitors, and one was the BET inhibitor I-BET151. We purchased four of the epigenetic inhibitors identified in our screen (one was not commercially available) and performed more extensive dose-response curves (see Fig. 1B for their structures). All four of these inhibitors attenuated HH signaling, and did so in a dose-dependent manner (Fig. 2). As histone deacetylase inhibitors have been previously implicated in regulating HH activity (2, 26), for the remainder of this study, we focus solely on the BET bromodomain inhibitor I-BET151.
TABLE 1.
Candidate small-molecules evaluated in this screen
SAHA, suberanilohydroxamic acid; CTPB, N-(4-chloro-3-trifluoromethyl-phenyl)-2-ethoxy-6-pentadecyl-benzamide.
| Well No. | Small molecule |
|---|---|
| 1 | Trichostatin A |
| 2 | 2,4-Pyridinedicarboxylic acid |
| 3 | Garcinol |
| 4 | Splitomicin |
| 5 | BML-210 |
| 6 | Apicidin |
| 7 | Suberoyl bis-hydroxamic acid |
| 8 | Scriptaid |
| 9 | Nullscript |
| 10 | 5-Aza-2′-deoxycytidine (Decitabine) |
| 11 | Zebularine |
| 12 | Vorinostat (SAHA) |
| 13 | Isonicotinamide |
| 14 | ITSA-1 |
| 15 | Sodium phenylbutyrate |
| 16 | Tranylcypromine hemisulfate |
| 17 | Valproic acid |
| 18 | EX-527 |
| 19 | Resveratrol |
| 20 | M-344 |
| 21 | Nicotinamide |
| 22 | BML-266 |
| 23 | Piceatannol |
| 24 | Fluoro-SAHA |
| 25 | Valproic acid hydroxamate |
| 26 | AGK2 |
| 27 | Salermide |
| 28 | MC-1293 |
| 29 | Anacardic acid |
| 30 | B2 |
| 31 | BIX-01294·3HCl |
| 32 | Butyrolactone 3 |
| 33 | CTPB |
| 34 | Oxamflatin |
| 35 | Sirtinol |
| 36 | Suramin·6Na |
| 37 | BML-278 |
| 38 | NCH-51 |
| 39 | CI-994 |
| 40 | NSC-3852 |
| 41 | Aminoresveratrol sulfate |
| 42 | BML-281 |
| 43 | Triacetylresveratrol |
| 44 | I-BET151 |
| 45 | GSK126 |
| 46 | SGC0946 |
| 47 | GSK-J1 |
| 48 | GSK-J4 |
| 49 | Daminozide |
| 50 | LSD1-C76 |
| 51 | ACY-1215 (Rocilinostat) |
| 52 | CUDC-907 |
| 53 | CUDC-101 |
| 54 | UNC1215 |
| 55 | UNC669 |
| 56 | AGI-5198 (IDH-C35) |
| 57 | AGI-6780 |
| 58 | MI-2 |
| 59 | IOX2 |
| 60 | WDR5-C47 |
FIGURE 1.
A screen for epigenetic inhibitors of HH signaling. A Light2 reporter cell line, engineered to express a Gli site-driven luciferase reporter gene and TK-driven control luciferase gene, was treated with the Smo activator SAG (100 nm). These cells were subsequently utilized to screen a panel of 60 small-molecule inhibitors targeting various epigenetic regulators. A, four different concentrations of inhibitors were used: 0.5, 1, 2, and 10 μm, and results for 2 μm are shown. The mean responses to these various inhibitors (top dashed line) and two standard deviations of this mean (bottom dashed line) are shown. Columns 1–60 represent individual epigenetic enzyme inhibitors, whereas columns 61–63 represent DMSO, vismodegib (100 nm), and unstimulated activity, respectively. The positive hits from this screen are highlighted by blue asterisks or a red asterisk (I-BET151). B, chemical structures of small-molecule HH inhibitors.
FIGURE 2.
Validation of four distinct epigenetic HH inhibitors. Four of the five inhibitors selected from the screen shown in Fig. 1 were individually purchased and then reanalyzed using SAG-activated Light2 cells. In these dose-response curves, Gli site-driven luciferase activity is normalized to cell number. Error bars represent the S.E. of three independent experiments. TSA, trichostatin A; SAHA, suberanilohydroxamic acid.
A number of key components of the HH signaling pathway have already been identified (Fig. 3A), and their importance in various human pathologies has been underscored by their common mutation frequencies (25). In general, HH binds to its core receptor protein Ptch1, inducing the derepression of the seven-transmembrane protein Smo (27). Activated Smo ultimately regulates the Gli family of transcription factors, via alterations in protein half-life, via an array of post-translational modifications, and through its association with the negative regulator Sufu. The Sufu/Gli association is destabilized via trafficking through the primary cilium, a microtubule-enriched organelle required for canonical HH signaling. To identify the components of the HH signal transduction pathway disrupted by IBET-151, we treated Light2 cells with SAG and different doses of I-BET151 and then quantitated the expression levels of Ptch1, Smo, Gli2, or Gli1 (Fig. 3B). Although the expression levels of these four signaling components were affected by I-BET151, the IC50 of I-BET151 for reducing Gli1 levels was most similar to that observed for attenuating SAG-induced luciferase activity (Fig. 2).
FIGURE 3.
I-BET151 attenuates Gli1 expression. A, a model of the HH signaling pathway, showing pivotal positive (green) and negative (red) regulators. B, Light2 cells stimulated with SAG (100 nm) were treated with the indicated doses of I-BET151, and the expression levels of various HH signaling components were determined. Error bars represent the S.E. of three independent experiments.
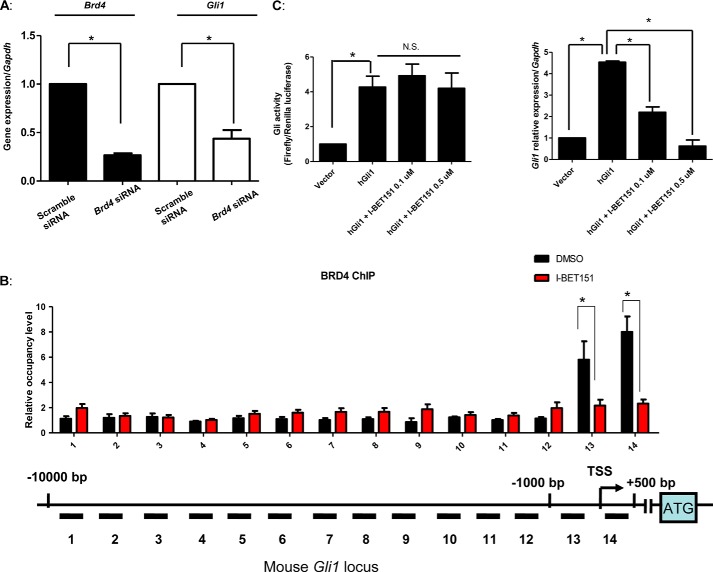
The majority of HH inhibitors described to date bind to and attenuate the activity of Smo, including the Food and Drug Administration-approved small-molecule vismodegib (25). One convenient way to identify those inhibitors that act downstream of Smo is to screen them using Sufu−/− MEFs as loss of Sufu results in the Smo-independent activation of Gli proteins. We therefore treated Sufu−/− MEFs with two different doses of I-BET151 and then quantitated the expression levels of Ptch1, Smo, Gli2, or Gli1. I-BET151 attenuated the expression of these pivotal HH signaling components in Sufu−/− MEFs in a manner similar to its effect in SAG-stimulated Light2 cells (Fig. 4A), and once again the expression of Gli1 was the most sensitive to I-BET151. Consistent with Sufu−/− MEFs harboring a Smo-independent activation of Gli proteins, vismodegib was unable to attenuate Gli activity in these cells. We also examined the expression of several oncogenes and cell cycle-related genes that are commonly attenuated by I-BET151. Consistent with I-BET151 functioning through the attenuation of multiple signaling nodes (28, 29), the expression of c-Myc, P21, and Cdk4 was inhibited in a similar manner to Gli1 (Fig. 4B). We also took advantage of the constitutive Gli1 expression of Sufu−/− MEFs to examine its dependence on the I-BET151 target Brd4. We knocked down Brd4 expression using a validated Brd4 siRNA SMARTpool or a control siRNA. Brd4 knockdown resulted in an ∼60% reduction of Gli1 levels, similar to the reduction in expression of Brd4 itself (Fig. 5A). Thus, consistent with I-BET151 acting specifically in these cells to inhibit Brd4 function, knockdown of Brd4 also attenuated Gli1 expression.
FIGURE 4.
I-BET151 acts downstream of Smo in the HH pathway. A, Sufu−/− MEFs harbor a constitutively active HH signaling pathway, activated downstream of Smo. These MEFS were treated with either I-BET151 (100 nm or 500 nm) or vismodegib (100 nm) for 24 h, and the expression levels of various HH signaling components was determined. B, Sufu−/− MEFs were treated with 0.5 μm I-BET151 for 8, 16, or 24 h. RNA was collected and subjected to quantitative RT-PCR. The expression of the indicated gene products was then normalized to that of Gapdh. Error bars represent the S.E. of three independent experiments. p values ≤ 0.05 are considered statistically significant and indicated by an asterisk.
FIGURE 5.
I-BET151 reduces Brd4 occupancy of the Gli1 locus. A, knockdown of Brd4 inhibits HH target gene expression. 50 nm Brd4 siRNA or scramble siRNA was transfected into Sufu−/− MEFs for 72 h. RNA was subsequently extracted for quantitative PCR to determine the expression of the indicated genes. B, schematic of the mouse Gli1 locus from −10,000 bp to +500 bp, relative to the transcription start sites (TSS) shown. Brd4 occupancy was analyzed by ChIP-quantitative PCR in Sufu−/− MEFs treated with 0.5 μm I-BET151 and then normalized to a control ChIP performed using rabbit IgG. C, a plasmid expressing human GLI1 from a CMV promoter was transfected into Light2 cells and then treated with 0.1 or 0.5 μm I-BET151 48 h later. After 24 h of treatment, firefly luciferase activity was measured and normalized to Renilla luciferase activity, followed by isolation of RNA to measure the expression of the indicated HH target genes. Error bars represent the S.E. of three independent experiments. p values ≤ 0.05 are considered statistically significant and indicated by an asterisk. NS, not significant.
As Brd4 interacts with chromatin-associated acetylated histones to regulate target gene transcription (30), we tested the hypothesis that I-BET151 treatment attenuates Gli1 transcription by reducing Brd4 occupancy of the Gli1 locus. Consistent with this hypothesis, we found that Brd4 associates with the proximal regulatory region of the Gli1 locus, near the transcriptional start site. Interestingly, I-BET151 treatment of cells decreased the occupancy of Brd4 on this region of the Gli1 locus (Fig. 5B). We also noted that the effects of I-BET151 on Gli-driven luciferase activity could be overcome by the constitutive expression of a human GLI1 cDNA, which lacks the proximal regulatory region of the Gli1 locus that Brd4 enriches on (Fig. 5C). As a control, we also show that I-BET151 still attenuated the expression of endogenous mouse Gli1. Together, these results suggest that I-BET151 abrogates Gli1 transcription by reducing the association of Brd4 with the proximal regulatory region of the Gli1 locus.
To test the ability of I-BET151 to attenuate the growth of an HH-driven cancer, we used a Ptch1+/−-derived medulloblastoma mouse model (31). Spontaneous tumors from this well characterized medulloblastoma model were used to isolate CSCs, which were maintained as a non-adherent spheroid culture, and to establish a transplantable allograph model (32). Such CSC lines harbor a constitutively activated HH pathway and considerable Gli1 expression and proliferate in a Smo-dependent manner (32). Treatment of CSCs with I-BET151 reduced their viability within 3 days of treatment and did so in a dose-dependent manner (Fig. 6A). Moreover, the effect of I-BET151 on CSCs was positively associated with decreased Gli1 levels (Fig. 6B). To determine the effect of I-BET151 on cancer growth in vivo, randomized nude mice were implanted with Ptch1+/−-derived medulloblastoma tissue and treated with I-BET151 or vehicle once the tumors were ∼200 mm3 in size. Although the tumors in the vehicle-treated mice grew rapidly, the growth of the tumors in the I-BET151-treated mice was significantly attenuated (Fig. 6, C and D). Importantly, the body weight of the I-BET151-treated mice was not statistically different from that of the vehicle-treated mice (Fig. 6E). Further, consistent with I-BET151 attenuating tumor growth through inhibition of HH signaling, I-BET151 reduced the levels of the HH target gene Gli1 relative to the tumors in the vehicle-treated mice (Fig. 6F).
FIGURE 6.
I-BET151 abrogates HH activity-driven medulloblastoma growth. Medulloblastoma cancer stem/progenitor cells established from a Ptch1+/− medulloblastoma were treated as follows. A, treated with the indicated doses of I-BET151 for 3 days prior to determining cell viability. Cell viability was measured by trypan blue staining and then quantitated. B, twenty-four hours after I-BET151 treatment, Gli1 expression was quantified. Ptch1+/− medulloblastoma tissue was subcutaneously implanted into immunodeficient mice (n = 5 for vehicle group and n = 6 for experimental group). When the tumor size reached ∼200 mm3, the mice were treated with 30 mg/kg of I-BET151 or DMSO. C, tumor volume was measured daily. D, hematoxylin and eosin staining was performed to identify differences in histology between vehicle- and I-BET151-treated tumor tissue. E, body weights of the I-BET151- or vehicle-treated mice were measured at the indicated times. F, Gli1 gene expression in tumor tissue from the I-BET151- or vehicle-treated mice. Error bars represent the S.E. of three independent experiments, unless otherwise indicated. p values ≤ 0.05 are considered statistically significant and indicated by an asterisk.
DISCUSSION
In the present study, we demonstrate that BET protein inhibition attenuates HH signaling. Treatment with the BET inhibitor I-BET151 reduced levels of an HH pathway reporter gene in fibroblasts, as well as the expression of multiple components of the HH pathway, including Gli1 but not Smo. Further, expression of Gli1 was more sensitive to I-BET151 treatment than that of the other components, consistent with this being one of the critical BET targets in the HH pathway. This was also observed in Sufu−/− MEFs, which are not dependent on Smo (33) or primary cilia (34) for HH signaling, placing the action of I-BET151 downstream of Smo. As Brd4 enriches on the Gli1 locus in a manner that can be reversed by I-BET151, and Brd4 depletion in Sufu−/− MEFs reduces Gli1 levels, this effect of I-BET151 is likely through Brd4. Importantly, I-BET151-mediated inhibition of Brd4 activity also occurred in vivo, potently reducing the growth of a Ptch+/−-driven medulloblastoma. These results, along with those of a similar recent study (35), provide evidence for a previously unappreciated role for the BET bromodomain proteins in HH signaling.
The BET protein involved in medulloblastoma cell growth is likely Brd4 because its depletion reduced HH induction of Gli1 in wild type and Sufu−/− MEFs. However, whether other BET bromodomain proteins are linked to medulloblastoma is unknown. BET bromodomain protein regulation of HH signaling may be mediated via direct and indirect transcriptional regulation of Gli1 and Gli2, the primary transcriptional activators of the HH signaling pathway (36). However, whether this is the main mode of controlling HH signaling in medulloblastoma and other cancers has not been addressed. In the case of glioblastoma, we have previously determined that Brd2 or Brd4 depletion reduces tumor cell growth (11). Thus, it is possible that both of these proteins are also required for medulloblastoma progression. Inhibiting Brd2 or Brd4 in medulloblastoma with brain-penetrant versions of BET inhibitors may be therapeutically attractive. JQ1 is a well characterized, brain-penetrant BET bromodomain inhibitor (12) that has shown efficacy in preclinical models of brain cancer (9, 37). However, JQ1 suffers from chemical liabilities that make it unsuitable for clinical testing (28). Thus, there is a need to identify brain-penetrant BET bromodomain inhibitors that are drug-like for the treatment of human medulloblastoma patients.
The majority of HH inhibitors described to date bind to and attenuate the activity of Smo (25). Although such Smo inhibitors will likely have significant clinical utility, many other signaling pathways activate Gli proteins in a non-canonical manner (27). Thus, inhibitors that function downstream of Smo, especially those that function at the level of Gli proteins, are likely to have even greater clinical utility. Collectively, our studies suggest that BET inhibitors may provide a viable mechanism for inhibiting the HH pathway in multiple cancers by directly down-regulating Gli1.
Acknowledgments
We thank members of the Robbins, Capobianco, and Ayad laboratories for providing insights during discussions regarding this manuscript. We are also grateful to Dr. Rune Toftgård (Karolinska Institute) for providing us with Sufu−/− MEF cells.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1CA082628 (to D. J. R.) and RO1NS067289 (to N. G. A.).
- HH
- Hedgehog
- Smo
- Smoothened
- BET
- bromodomain and extra terminal domain
- CSC
- cancer stem/progenitor cell
- MEF
- mouse embryonic fibroblast
- SAG
- Smo agonist
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1. Malatesta M., Steinhauer C., Mohammad F., Pandey D. P., Squatrito M., Helin K. (2013) Histone acetyltransferase PCAF is required for Hedgehog-Gli-dependent transcription and cancer cell proliferation. Cancer Res. 73, 6323–6333 [DOI] [PubMed] [Google Scholar]
- 2. Canettieri G., Di Marcotullio L., Greco A., Coni S., Antonucci L., Infante P., Pietrosanti L., De Smaele E., Ferretti E., Miele E., Pelloni M., De Simone G., Pedone E. M., Gallinari P., Giorgi A., Steinkühler C., Vitagliano L., Pedone C., Schinin M. E., Screpanti I., Gulino A. (2010) Histone deacetylase and Cullin3-RENKCTD11 ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat. Cell Biol. 12, 132–142 [DOI] [PubMed] [Google Scholar]
- 3. Jagani Z., Mora-Blanco E. L., Sansam C. G., McKenna E. S., Wilson B., Chen D., Klekota J., Tamayo P., Nguyen P. T., Tolstorukov M., Park P. J., Cho Y. J., Hsiao K., Buonamici S., Pomeroy S. L., Mesirov J. P., Ruffner H., Bouwmeester T., Luchansky S. J., Murtie J., Kelleher J. F., Warmuth M., Sellers W. R., Roberts C. W., Dorsch M. (2010) Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat. Med. 16, 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dawson M. A., Kouzarides T. (2012) Cancer epigenetics: from mechanism to therapy. Cell 150, 12–27 [DOI] [PubMed] [Google Scholar]
- 5. Grunstein M. (1997) Histone acetylation in chromatin structure and transcription. Nature 389, 349–352 [DOI] [PubMed] [Google Scholar]
- 6. Musselman C. A., Lalonde M. E., Côté J., Kutateladze T. G. (2012) Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 19, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filippakopoulos P., Knapp S. (2014) Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 13, 337–356 [DOI] [PubMed] [Google Scholar]
- 8. Henssen A., Thor T., Odersky A., Heukamp L., El-Hindy N., Beckers A., Speleman F., Althoff K., Schäfers S., Schramm A., Sure U., Fleischhack G., Eggert A., Schulte J. H. (2013) BET bromodomain protein inhibition is a therapeutic option for medulloblastoma. Oncotarget 4, 2080–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandopadhayay P., Bergthold G., Nguyen B., Schubert S., Gholamin S., Tang Y., Bolin S., Schumacher S. E., Zeid R., Masoud S., Yu F., Vue N., Gibson W. J., Paolella B. R., Mitra S. S., Cheshier S. H., Qi J., Liu K. W., Wechsler-Reya R., Weiss W. A., Swartling F. J., Kieran M. W., Bradner J. E., Beroukhim R., Cho Y. J. (2014) BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin. Cancer Res. 20, 912–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venkataraman S., Alimova I., Balakrishnan I., Harris P., Birks D. K., Griesinger A., Amani V., Cristiano B., Remke M., Taylor M. D., Handler M., Foreman N. K., Vibhakar R. (2014) Inhibition of BRD4 attenuates tumor cell self-renewal and suppresses stem cell signaling in MYC driven medulloblastoma. Oncotarget 5, 2355–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pastori C., Daniel M., Penas C., Volmar C. H., Johnstone A. L., Brothers S. P., Graham R. M., Allen B., Sarkaria J. N., Komotar R. J., Wahlestedt C., Ayad N. G. (2014) BET bromodomain proteins are required for glioblastoma cell proliferation. Epigenetics 9, 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W. B., Fedorov O., Morse E. M., Keates T., Hickman T. T., Felletar I., Philpott M., Munro S., McKeown M. R., Wang Y., Christie A. L., West N., Cameron M. J., Schwartz B., Heightman T. D., La Thangue N., French C. A., Wiest O., Kung A. L., Knapp S., Bradner J. E. (2010) Selective inhibition of BET bromodomains. Nature 468, 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostrom Q. T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N. E., Kruchko C., Barnholtz-Sloan J. S. (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-oncology 15, Suppl. 2, ii1–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilbertson R. J. (2004) Medulloblastoma: signalling a change in treatment. Lancet Oncol. 5, 209–218 [DOI] [PubMed] [Google Scholar]
- 15. Lin T. L., Matsui W. (2012) Hedgehog pathway as a drug target: Smoothened inhibitors in development. Onco. Targets Ther. 5, 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yauch R. L., Dijkgraaf G. J., Alicke B., Januario T., Ahn C. P., Holcomb T., Pujara K., Stinson J., Callahan C. A., Tang T., Bazan J. F., Kan Z., Seshagiri S., Hann C. L., Gould S. E., Low J. A., Rudin C. M., de Sauvage F. J. (2009) Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326, 572–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dijkgraaf G. J., Alicke B., Weinmann L., Januario T., West K., Modrusan Z., Burdick D., Goldsmith R., Robarge K., Sutherlin D., Scales S. J., Gould S. E., Yauch R. L., de Sauvage F. J. (2011) Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 71, 435–444 [DOI] [PubMed] [Google Scholar]
- 18. Batora N. V., Sturm D., Jones D. T., Kool M., Pfister S. M., Northcott P. A. (2014) Transitioning from genotypes to epigenotypes: why the time has come for medulloblastoma epigenomics. Neuroscience 264, 171–185 [DOI] [PubMed] [Google Scholar]
- 19. Arzate-Mejía R. G., Valle-García D., Recillas-Targa F. (2011) Signaling epigenetics: novel insights on cell signaling and epigenetic regulation. IUBMB Life 63, 881–895 [DOI] [PubMed] [Google Scholar]
- 20. Taipale J., Chen J. K., Cooper M. K., Wang B., Mann R. K., Milenkovic L., Scott M. P., Beachy P. A. (2000) Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 [DOI] [PubMed] [Google Scholar]
- 21. Singh S., Tokhunts R., Baubet V., Goetz J. A., Huang Z. J., Schilling N. S., Black K. E., MacKenzie T. A., Dahmane N., Robbins D. J. (2009) Sonic Hedgehog mutations identified in holoprosencephaly patients can act in a dominant negative manner. Hum. Genet. 125, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weaver K. L., Alves-Guerra M. C., Jin K., Wang Z., Han X., Ranganathan P., Zhu X., DaSilva T., Liu W., Ratti F., Demarest R. M., Tzimas C., Rice M., Vasquez-Del Carpio R., Dahmane N., Robbins D. J., Capobianco A. J. (2014) NACK is an integral component of the Notch transcriptional activation complex and is critical for development and tumorigenesis. Cancer Res. 74, 4741–4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh S. K., Hawkins C., Clarke I. D., Squire J. A., Bayani J., Hide T., Henkelman R. M., Cusimano M. D., Dirks P. B. (2004) Identification of human brain tumour initiating cells. Nature 432, 396–401 [DOI] [PubMed] [Google Scholar]
- 24. Tomayko M. M., Reynolds C. P. (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 24, 148–154 [DOI] [PubMed] [Google Scholar]
- 25. Teglund S., Toftgård R. (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim. Biophys. Acta 1805, 181–208 [DOI] [PubMed] [Google Scholar]
- 26. Lee S. J., Lindsey S., Graves B., Yoo S., Olson J. M., Langhans S. A. (2013) Sonic hedgehog-induced histone deacetylase activation is required for cerebellar granule precursor hyperplasia in medulloblastoma. PLoS One 8, e71455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robbins D. J., Fei D. L., Riobo N. A. (2012) The Hedgehog signal transduction network. Sci. Signal. 5, re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawson M. A., Prinjha R. K., Dittmann A., Giotopoulos G., Bantscheff M., Chan W. I., Robson S. C., Chung C. W., Hopf C., Savitski M. M., Huthmacher C., Gudgin E., Lugo D., Beinke S., Chapman T. D., Roberts E. J., Soden P. E., Auger K. R., Mirguet O., Doehner K., Delwel R., Burnett A. K., Jeffrey P., Drewes G., Lee K., Huntly B. J., Kouzarides T. (2011) Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478, 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaidos A., Caputo V., Gouvedenou K., Liu B., Marigo I., Chaudhry M. S., Rotolo A., Tough D. F., Smithers N. N., Bassil A. K., Chapman T. D., Harker N. R., Barbash O., Tummino P., Al-Mahdi N., Haynes A. C., Cutler L., Le B., Rahemtulla A., Roberts I., Kleijnen M., Witherington J. J., Parr N. J., Prinjha R. K., Karadimitris A. (2014) Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood 123, 697–705 [DOI] [PubMed] [Google Scholar]
- 30. Itzen F., Greifenberg A. K., Bösken C. A., Geyer M. (2014) Brd4 activates P-TEFb for RNA polymerase II CTD phosphorylation. Nucleic Acids Res. 42, 7577–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodrich L. V., Milenković L., Higgins K. M., Scott M. P. (1997) Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 [DOI] [PubMed] [Google Scholar]
- 32. Ward R. J., Lee L., Graham K., Satkunendran T., Yoshikawa K., Ling E., Harper L., Austin R., Nieuwenhuis E., Clarke I. D., Hui C. C., Dirks P. B. (2009) Multipotent CD15+ cancer stem cells in Patched-1-deficient mouse medulloblastoma. Cancer Res. 69, 4682–4690 [DOI] [PubMed] [Google Scholar]
- 33. Svärd J., Heby-Henricson K., Persson-Lek M., Rozell B., Lauth M., Bergström A., Ericson J., Toftgård R., Teglund S. (2006) Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell 10, 187–197 [DOI] [PubMed] [Google Scholar]
- 34. Chen M. H., Wilson C. W., Li Y. J., Law K. K., Lu C. S., Gacayan R., Zhang X., Hui C. C., Chuang P. T. (2009) Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 23, 1910–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang Y., Gholamin S., Schubert S., Willardson M. I., Lee A., Bandopadhayay P., Bergthold G., Masoud S., Nguyen B., Vue N., Balansay B., Yu F., Oh S., Woo P., Chen S., Ponnuswami A., Monje M., Atwood S. X., Whitson R. J., Mitra S., Cheshier S. H., Qi J., Beroukhim R., Tang J. Y., Wechsler-Reya R., Oro A. E., Link B. A., Bradner J. E., Cho Y. J. (2014) Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat. Med. 20, 732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz i Altaba A. (1999) Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 15, 418–425 [DOI] [PubMed] [Google Scholar]
- 37. Cheng Z., Gong Y., Ma Y., Lu K., Lu X., Pierce L. A., Thompson R. C., Muller S., Knapp S., Wang J. (2013) Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin. Cancer Res. 19, 1748–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]