Background: Human memory CD4+ T cells mediate adaptive immune responses via rapidly producing effector cytokines.
Results: Memory CD4+ T cells highly express T-bet, which is closely correlated with IFN-γ production.
Conclusion: Pre-existence and nuclear mobilization of T-bet in memory CD4+ T cells contribute to regulating the rapid production of IFN-γ.
Significance: We declared a possible transcriptional mechanism for rapid production of cytokines by memory CD4+ T cells.
Keywords: Cell Biology, Cytokine, Immunology, T Helper Cells, Transcription Factor, Memory CD4+ T Cells, T-bet, Cytokines
Abstract
We found that after stimulation for a few hours, memory but not naive CD4+ T cells produced a large amount of IFN-γ; however, the mechanism of rapid response of memory CD4+ T cells remains undefined. We compared the expression of transcription factors in resting or activated naive and memory CD4+ T cells and found that T-bet, but not pSTAT-1 or pSTAT-4, was highly expressed in resting memory CD4+ T cells and that phenotypic characteristics of T-bet+CD4+ T cells were CD45RAlowCD62Llow CCR7low. After short-term stimulation, purified memory CD4+ T cells rapidly produced effector cytokines that were closely associated with the pre-existence of T-bet. By contrast, resting naive CD4+ T cells did not express T-bet, and they produced cytokines only after sustained stimulation. Our further studies indicated that T-bet was expressed in the nuclei of resting memory CD4+ T cells, which might have important implications for rapid IFN-γ production. Our results indicate that the pre-existence and nuclear mobilization of T-bet in resting memory CD4+ T cells might be a possible transcriptional mechanism for rapid production of cytokines by human memory CD4+ T cells.
Introduction
As the major part of immunological memory, memory CD4+ T cells play very important roles in mediating adaptive immune responses to a variety of pathogens and tumors and in autoimmunity. After short-term re-exposure to the same antigen, memory CD4+ T cells, but not naive CD4+ T cells, rapidly produce cytokines such as IFN-γ and TNF-α to mediate protective immune responses (1, 2). In the absence of pMHC II ligand, memory T cells are predominantly quiescent and capable of homeostatic proliferation and long-term survival (3). In humans, memory CD4+ T cells are divided into two major subsets based on the tissue localization and function: central memory T cells (TCM)2 and effector memory T cells (TEM) (1, 4). TCM constitutively express CD62L and CCR7, which are also characteristic of naive T cells and are required for cell extravasation and migration to T cell areas in the secondary lymphoid organs. TCM mostly locate in lymphoid organs and peripheral non-lymphoid tissues and broadly migrate between peripheral tissues, blood, and spleen. Compared with TCM and naive CD4+ T cells, TEM are CD45RO+CD62L−CCR7−. They primarily migrate to non-lymphoid sites of inflammation and produce a variety of effector cytokines within hours following antigenic stimulation.
Heterogeneous populations of effector CD4+ T cells are generated at the peak of the primary response. About 90% of the terminal effector cells die, and only less than 10% of effector T cells escape from apoptosis during the contraction phase to generate long-lived memory T cells (5). Adoptive transfer experiments in mice reveal that less differentiated IFN-γ− cells with long-term memory function are the source of TCM, and that further differentiated, short-lived IFN-γ+ cells with immediate effector function develop into TEM (6, 7). However, the origins and mechanisms resulting in the different functions and phenotypes of human TCM and TEM remain unknown.
Transcription factors and STATs are indispensable for determination of T helper cell fate and cytokine production (8). Although many studies have demonstrated the functions of T-bet, a transcription factor of T-box family, in the differentiation of mice effector and memory CD8+ T cells, T-bet and another T-box transcription factor EOMES cooperate to sustain memory CD8+ T cell homeostasis through expression of IL-2Rβ (9, 10). It has been reported that the ratio of T-bet to EOMES is high in effector CD8+ T cells and low in memory CD8+ T cells (11). Studies in mice showed that following LCMV infection, virus-specific effector and memory CD4+ T cells formed independently of T-bet and STAT-4 (12). The functions of transcription factors in human memory CD4+ T cells remain largely unknown. T-bet is the major transcription factor for the Th1-cell lineage commitment of CD4+ T cells because of its transactivation of the Th1 effector cytokine IFN-γ (13). Previous studies in a mouse model showed that IFN-γ production by Ag-stimulated memory CD4+ T cells occurred in the absence of significant nuclear T-bet expression or T-bet engagement on the IFN-γ promoter (14). In addition to T-bet, STAT-1 and STAT-4 also have important roles in regulating the differentiation of Th1 cells in the presence of IL-12 or IFN-γ. Activation of STAT-1 by IFN-γ is important for the induction of T-bet during Th1 differentiation in vitro (15, 16). STAT-4, mainly activated by Il-12, can directly induce IFN-γ production and expression of IL-12Rβ2 and T-bet during Th1 differentiation (17, 18). However, there is much debate about whether rapid IFN-γ secretion by memory CD4+ T cells is controlled at the transcriptional level, and the functions of T-bet and other transcription factors in the generation and formation of human memory CD4+ T cells remain largely unexamined and elusive.
With this information, we carried out studies to compare the levels and functions of Th1-defined transcription factors between naive and memory CD4+ T cells. We found that memory CD4+ T cells (CD3+CD4+CD45RO+CD45RA−) highly expressed T-bet without stimulation, but naive CD4+ T cells (CD3+CD4+CD45RO−CD45RA+) did not express T-bet. After short-term stimulation, T-bet expression was also up-regulated in naive CD4+ T cells, but only CD4+ T cells pre-expressing T-bet produced IFN-γ. To further analyze the translocation of T-bet in memory CD4+ T cells by Western blotting, we found that T-bet was expressed in both the cytoplasm and nucleus. These data suggest that pre-existing T-bet in the nuclei of memory CD4+ T cells might directly modulate the rapid responses of memory CD4+ T cells. These findings of T-bet-dependent regulation of memory immune responses have important implications for vaccine design, autoimmunity, and anti-tumor immunity.
EXPERIMENTAL PROCEDURES
Study Participants
Healthy volunteers comprised of half females and half males with ages from 19 to 45 years were recruited from Zhongshan School of Medicine, Sun Yat-sen University. Heparinized blood samples (10 IU ml−1 final) were collected from each donor under protocols approved by the Medical School Review Board at Sun Yat-sen University, China. Adequate written informed consent was obtained from all individuals involved in this study. Individuals that had been diagnosed with HIV, hepatitis B virus (HBV), or hepatitis C virus (HCV) infection or with a history of autoimmune diseases were excluded from the study.
Antibodies and Reagents
The following monoclonal antibodies were used for phenotypic, intracellular cytokine, and transcription factor analyses: phycoerythrin (PE) labeled-anti-IL-2, PE-anti-isotype IgG2b, PE-anti-pSTAT-1, PE-anti-pSTAT-4, PE-anti-CD45RO, PE-anti-CD45RA, kappa, PE-anti-isotype IgG2a, PE-anti-isotype IgG1, kappa, fluorescein isothiocyanate (FITC)-labeled anti-CD3, FITC-anti-CD4, FITC-anti-IFN-γ, FITC-anti-CD45RO, FITC-anti-CD45RA, PE-Cy7-labeled anti-TNF-α, PE-Cy7-anti-CCR7, allophycocyanin (APC)-labeled anti-CD3, APC-anti-CD62L, APC-anti-IFN-γ, PE-CF594-labeled anti-CD3, APC-Cy7-labeled anti-CD4, and peridin-chlorophyll protein (PerCP)-labeled anti-CD4 were purchased from BD Biosciences (San Jose, CA); PE-anti-T-bet was purchased from eBiosciences (San Diego, CA). For Western blotting, mouse anti-T-bet mAb was purchased from BD Biosciences. Mouse anti-β-actin and anti-mouse IgG-HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and mouse anti-GAPDH was purchased from Beyotime (Jiangsu, China). Rabbit anti-STAT-4, anti-STAT-1, anti-pSTAT-1, anti-nuclear matrix protein P84 (5E100), and anti-rabbit IgG-HRP were purchased from Cell Signaling Technology (Cambridge, MA). Rabbit anti-pSTAT-4 was purchased from R&D Systems (Minneapolis, MN). NE-PER Nuclear and Cytoplasmic Extraction Reagents was purchased from Thermo Scientific (Logan, Utah). Human ELISA kits for cytokines IFN-γ, IL-2, and TNF-α were purchased from BD Biosciences. PMA, ionomycin and Brefeldin A were purchased from Sigma-Aldrich.
Isolation and Preparation of Naive and Memory CD4+ T Cells
Briefly, PBMCs from heparinized blood were isolated by Ficoll-Hypaque (Hao Yang Biological Manufacture, Tianjin, China) gradient centrifugation within 24 h of blood drawing and washed twice in Hanks' balanced salt solution. CD4+ T cells were negatively isolated from PBMCs using human CD4+ T cell isolation kits II, and positive separations of naive and memory CD4+ T cells were based on CD45RA or CD45RO expression using anti-CD45RO or CD45RA-conjugated magnetic MACS microbeads and separated on a MACS magnet into CD45RAhighCD45ROlow (naive) and CD45RAlowCD45ROhigh (memory) CD4+ T cells, according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of naive and memory cells was >97%, as assessed by flow cytometry analysis. The cells were suspended at a final concentration of 2 × 106/ml in complete RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (Sijiqing, Hangzhou, China), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, and 50 μm 2-mercaptoethanol (all from Invitrogen).
ELISA
Cells were suspended in complete RPMI 1640 medium and cultured with immobilized anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml) in a round-bottom 96-well plate at a concentration of 2 × 106 cells/ml in triplicate and incubated for 0 to 72 h at 37 °C with 5%CO2. The supernatants were harvested, and concentrations of cytokines IFN-γ, IL-2, and TNF-α were detected by ELISA. The detection limits of IFN-γ, IL-2, and TNF-α assay kits were 4.7, 7.8, and 7.8 pg/ml, respectively.
Cell Surface and Intracellular Staining Analysis
PBMCs without stimulation from healthy donors were washed twice with PBS buffer containing 0.1% BSA and 0.05% sodium azide. For surface staining, cells were incubated with respective monoclonal antibodies at 4 °C in the dark for 30 min, washed twice, and fixed in 0.5% paraformaldehyde before acquisition. For the detection of intracellular cytokines, cells were incubated with (open histogram) or without (shaded histogram) PMA (20 ng/ml) plus ionomycin (1 μg/ml) for 6 h in the presence of Brefeldin A (10 μg/ml). After stimulation, cells were fixed in 4% paraformaldehyde, followed by permeabilization and stained for the intracellular cytokines in PBS buffer containing 0.1% saponin. For transcription factor staining, cells were stimulated with or without anti-CD3 and anti-CD28 for 0 to 72 h, rested overnight and fixed with BD cytofix buffer at 37 °C for 10 min, permeabilized with ice-cold 90% methanol at 4 °C for 30 min, and stained with specific transcription factor or isotype antibodies at 4 °C for 50 min in the dark. These cells were collected by BD FACS Calibur and Aria II (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (Treestar, San Carlos, CA).
Western Blotting
Naive and memory CD4+ T cells were isolated from purified CD4+ T cells by MACS separation as above, cells were either lysed directly or activated with anti-CD3 and anti-CD28 for 0 to 72 h in complete RPMI 1640 medium at 37 °C. Cytoplasmic and nuclear fraction isolations from resting memory cells were performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific) according to the manufacturer's instructions. Briefly, memory CD4+ T cells were washed with PBS, ice-cold CER I buffer was added to the tube, and the tube was incubated on ice for 10 min. An appropriate volume of ice-cold CER II buffer was added to the tube, which was vigorously vortexed, incubated on ice for 1 min, and centrifuged at high speed to harvest the supernatants as cytoplasmic fractions. NER buffer was added to the tube, which was vigorously vortexed to suspend the pellet and placed on ice. Vortexing was repeated every 10 min, for a total of 40 min, after which the tube was centrifuged at high speed again to harvest the nuclear fraction. Lysates were resolved on 10% SDS gels and immunoblotted with anti-β-actin, GAPDH, P84, T-bet, STAT-1, pSTAT-1, STAT-4, and pSTAT-4 mAbs as described above.
PCR
Purified memory CD4+ T cells were stimulated with immobilized anti-CD3 and soluble anti-CD28 for 12 h. Total RNA was extracted by StarSpin Animal RNA Mini Kit (GenStar Biosolutions, Beijing, China) according to the manufacturer's instructions. Reverse transcription of total RNA was performed at 37 °C using the ReactionReadyTM; First Strand cDNA Synthesis Kit (Invitrogen). Amplification of cDNA was conducted in a DNA thermal cycler (Biometra, Germany) at the following conditions: denaturation 45s at 94 °C, annealing 45s at 65 °C for GAPDH and IFN-γ, followed by 1 min of elongation at 72 °C. PCR rounds were repeated for 30 cycles each for both GAPDH and IFN-γ. The following sense and antisense primers for each molecule were used: IFN-γ forward: 5′-TGGCTTTTCAGCTCTGCATCGT-3′; reverse: 5′-TCCACACTCTTTTGGATGCTCTGGT-3′. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5′-GCATGGCCTTCCGTGTCC-3′; reverse: 5′-TGAGTGTGGCAGGGACTC-3′. The ratio of IFN-γ over GAPDH was calculated according to the relative intensities of the bands revealed under UV illumination with Bio-1D software (Vilber Lourmat, Marne la Vallee, France).
RESULTS
Kinetic Studies of Cytokine Secretion in Activated Purified Naive and Memory CD4+ T Cells
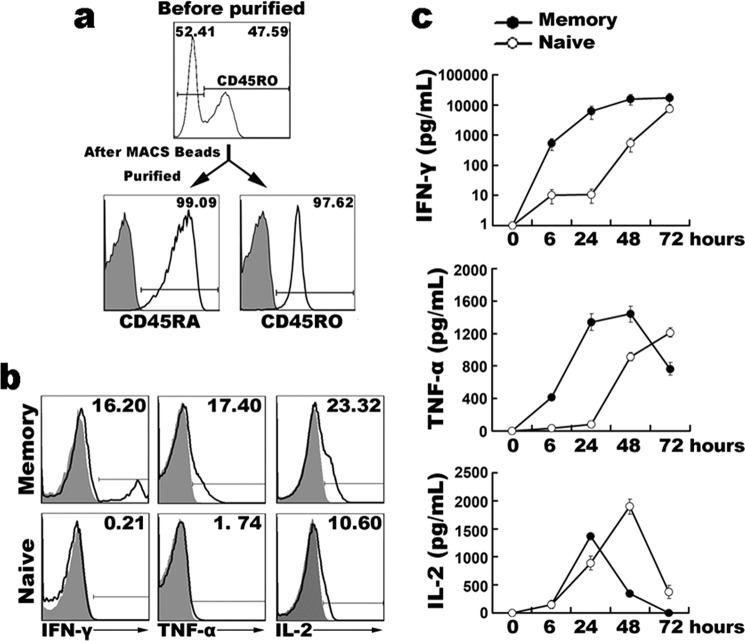
To compare the different functional properties of naive and memory CD4+ T cells upon antigenic exposure, we evaluated the kinetics of effector cytokine production by purified naive and memory cells after stimulation. Purified naive and memory CD4+ T cells were separated from whole CD4+ T cells by MACS microbeads, and the purity of naive or memory CD4+ T cells was more than 97% (Fig. 1a). Cells were stimulated with or without PMA plus ionomycin for 6 h in the presence of Brefeldin A, and the productions of IFN-γ, IL-2, and TNF-α were detected by intracellular cytokine staining (Fig. 1b). After short-term stimulation, memory CD4+ T cells rapidly produced cytokines. By contrast, the production of cytokines in naive CD4+ T cells was negligible. Although IL-2 was positively produced by stimulated naive T cells, the frequency of memory CD4+ T cells producing IL-2 was >2-fold more than naive T cells.
FIGURE 1.
Kinetics of cytokine secretion by activated naive and memory CD4+ T cells. Naive and memory CD4+ T cells were isolated from CD4+ T cells by MACS microbeads, and the purity of naive or memory CD4+ T cells was more than 97% as determined by flow cytometry (a). Naive and memory CD4+ T cells were stimulated with (open histogram) or without (shaded histogram) PMA and ionomycin in the presence of BFA for 6 h. Cells were stained by intracellular staining and analyzed by FACS (b). Purified naive and memory CD4+ T cells were stimulated with immobilized anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 0 to 72 h, and the concentrations of cytokines IFN-γ, IL-2, and TNF-α were detected by ELISA (c). Data are representative of three separate experiments with similar results.
To further confirm the results, we detected the concentrations of cytokines in the culture supernatants by ELISA. Purified cells were stimulated with immobilized anti-CD3 plus soluble anti-CD28 for 0 to 72 h, supernatants were harvested at different time points, and the concentrations of IFN-γ, IL-2, and TNF-α were measured by ELISA (Fig. 1c). Consistent with the flow cytometry results, the levels of IFN-γ, IL-2, and TNF-α produced by memory cells were significantly higher than naive cells, especially after short-term stimulation. As the time of stimulation increased, the levels of cytokines in supernatants were increased. Stimulation for 6 to 24 h resulted in the quick up-regulation of IFN-γ production in memory cells, whereas naive CD4+ T cells exhibited negligible IFN-γ production. The time of peak IL-2 or TNF-α production in naive CD4+ T cells was later than in memory cells. Memory cells exhibited peak IL-2 expression after 24 h and naive T cells exhibited peak expression after 48 h; the peak TNF-α expression occurred at 48 h of stimulation for memory cells and after 72 h for naive cells. Moreover, the levels of TNF-α in memory cells were distinctly higher than in naive T cells. Collectively, these data indicated that memory CD4+ T cells could more rapidly respond to stimulation compared with naive CD4+ T cells, and that enhanced kinetics and magnitude of effector cytokine secretion was a distinguishing feature of memory CD4+ T cells.
Expression of Several Th1 Transcription Factors and IFN-γ in Resting and Activated Naive and Memory CD4+ T Cells
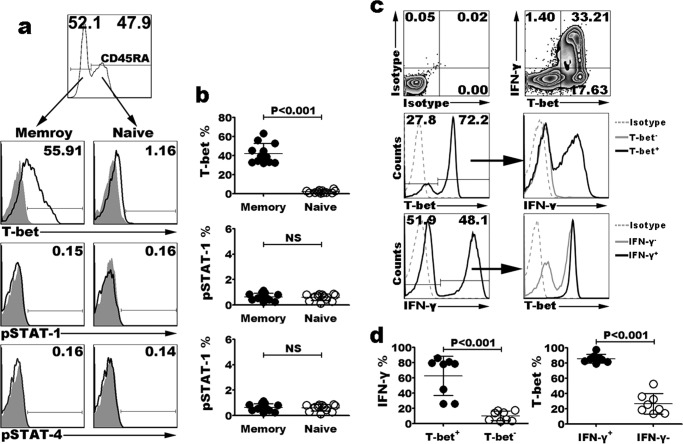
We hypothesized that rapid production of effector cytokines by memory T cells could be due to elevated production of transcription factors, which were required for cytokine mRNA transcription. Interestingly, we had found that after stimulation with antigen, IFN-γ-producing CD4+ T cells completely co-expressed T-bet (Fig. 2c). To confirm the possible transcriptional mechanism responsible for the more rapid production of effector cytokines by memory, as compared with naive CD4+ T cells, we detected some Th1-defined transcription factors in resting naive and memory CD4+ T cells (Fig. 2a). PBMCs without stimulation were surface stained with fluorescently conjugated anti-CD3, CD4 and CD45RA mAbs, and intracellular stained with anti-transcription factors T-bet, pSTAT-1, and pSTAT-4 mAbs. T-bet was expressed by 42.17% of memory (CD4+CD45RA−) T cells (mean value) but by less than 2% of naive (CD4+CD45RA+) T cells (Fig. 2b, p < 0.001). However, the percentages of phosphorylated STAT-1 and STAT-4 were not significantly different between memory and naive T cells (Fig. 2b, p = 0.179; p = 0.857). We also analyzed the expression of other T helper cell-defining transcription factors Bcl-6 (Tfh cells) (19), GATA-3 (Th2 cells) (20), and RORγt (Th17 cells) (21, 22), and there were no significant differences between resting naive and memory CD4+ T cells (data not shown). We further analyzed the correlation between T-bet and IFN-γ in activated CD4+ T cells after stimulation with PMA plus ionomycin for 6 h, cells expressing T-bet were more likely to be IFN-γ producers, and cells producing IFN-γ had a higher T-bet expression than did cells that failed to produce cytokine (Fig. 2, c and d). From the statistical results, we hypothesize that the rapid production of Th1 effector cytokines by short-term activated memory cells is strongly related to the high expression of transcription factor, T-bet. However, the rapid production of Tfh (IL-21), Th2 (IL-4), and Th17 (IL-17) effector cytokines by short-term activated memory cells needs to be further studied.
FIGURE 2.
Expression of Th1 transcription factors and IFN-γ in resting naive and memory CD4+ T cells. Gated on CD3+CD4+ T cells of resting PBMCs, the expression of T-bet, pSTAT-1, and pSTAT-4 in resting naive and memory T cells was analyzed by FACS; resting memory CD4+ T cells greatly expressed higher T-bet when compared with naive CD4+ T cells. In contrast, pSTAT-1 and pSTAT-4 were not obviously different between memory and naive cells (a). Statistical data shown are the results from twelve independent experiments (b). PBMCs were stimulated with PMA and ionomycin in the presence of BFA for 6 h. Co-expression of T-bet and IFN-γ was analyzed by FACS (c). Statistical data shown are the results from eight independent experiments (d). ns, not significant.
Kinetic Studies of the Expression of Transcription Factors in Naive and Memory CD4+ T Cells
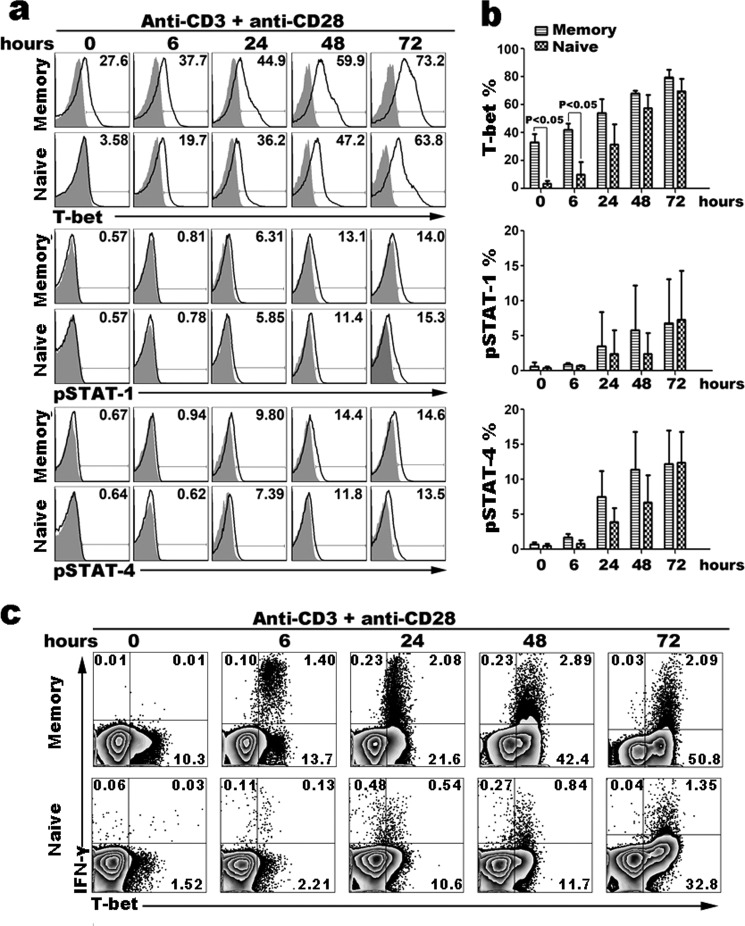
To further determine the functions of transcription factors in cytokine production, we analyzed the kinetic expression of transcription factors in purified resting and activated naive and memory CD4+ T cells by intracellular staining (Fig. 3a). Purified naive and memory CD4+ T cells were cultured with or without immobilized anti-CD3 and soluble anti-CD28 for 0 to 72 h, and the percentages of transcription factor-producing CD4+ T cells were analyzed by flow cytometry. T-bet expression in purified resting or activated memory CD4+ T cells was higher than naive T cells, particularly prior to or at 6 h following stimulation (Fig. 3b. 0 h, p = 0.0014; 6 h, p = 0.0059). Maximal T-bet up-regulation in naive CD4+ T cells happened after 24 h of stimulation. Anti-CD3 plus anti-CD28 stimulation of CD4+ T cells for 48 h resulted in maximal up-regulation of phosphorylated STAT-1 and STAT-4 in both naive and memory CD4+ T cells, and the frequency of pSTAT-1+CD4+ or pSTAT-4+CD4+ T cells was not statistically significantly different before 24 h stimulation (Fig. 3b). We also determined that higher production of IFN-γ was correlated with T-bet expression in activated memory CD4+ T cells as stimulation-time extended (Fig. 3c). These results showed that resting memory CD4+ T cells positively expressed high levels of transcription factor T-bet compared with naive T cells, but did not significantly differ in expression of pSTAT-1 or pSTAT-4.
FIGURE 3.
Kinetics of transcription factor expression in activated naive and memory CD4+ T cells. Naive and memory CD4+ T cells were stimulated with immobilized anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 0 to 72 h. The kinetics of transcription factor expression in activated cells were analyzed by FACS (a). By intracellular staining analysis, at early time of stimulation (before 6 h), there was significantly higher T-bet expression in memory CD4+ T cells compared with naive CD4+ T cells, but the expression of pSTAT-1 and pSTAT-4 (b) was not significantly different between activated naive and memory CD4+ T cells. BFA was added 6 h before cells were harvested at different time points, and IFN-γ and T-bet were detected by FACS (c). Statistical data shown are for the results from three independent experiments.
To confirm these results, we examined the signaling pathway by Western blotting (Fig. 4). Consistent with the flow cytometry results, we observed higher positive T-bet expression in memory CD4+ T cells, especially in resting cells and early at 6 h post-stimulation (Fig. 4a). Both resting and activated naive and memory cells produced STAT-1 and STAT-4, the phosphorylation of which occurred after 24–48 h stimulation, beyond the time point for rapid cytokine production. To compare the early difference of T-bet expression in naive and memory CD4+ T cells, we assessed the kinetics of early expression in activated purified naive and memory CD4+ T cells (Fig. 4, b and c). There was up-regulation of T-bet expression after stimulation in both naive and memory CD4+ T cells, and early T-bet expression following stimulation was higher in memory versus naïve cells, as measured by Western blotting. We hypothesize that for the rapid production of Th1 effector cytokines early after stimulation, pre-existing T-bet in memory CD4+ T cells plays an important role, but not by the pathway of STAT-1 or STAT-4 phosphorylation.
FIGURE 4.
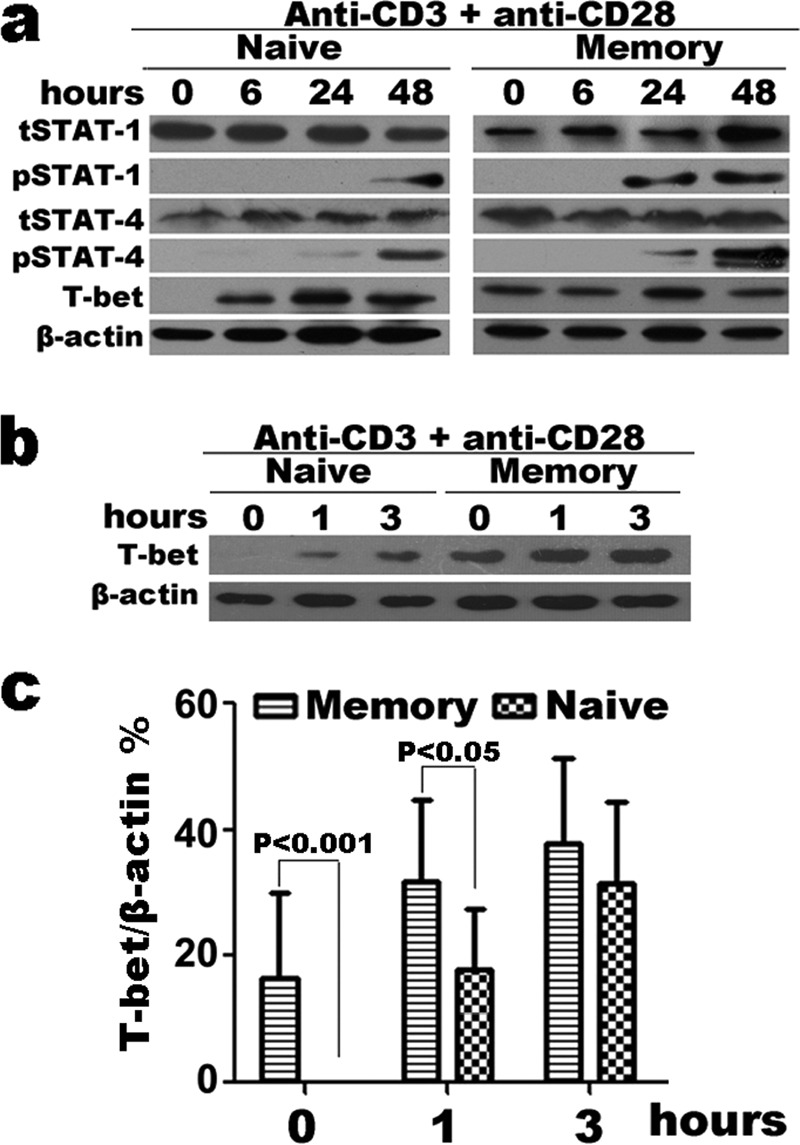
Expression of transcription factor T-bet in activated naive and memory CD4+ T cells. Purified naive and memory CD4+ T cells were stimulated with immobilized anti-CD3 and anti-CD28 for 0 to 72 h; cells were harvested and lysed directly. Lysates were resolved on 10% SDS gels and immunoblotted with anti-β-actin, and anti-T-bet, anti-STAT-4, anti-STAT-1, anti-pSTAT-4, and anti-pSTAT-1 antibodies following Western blotting protocols. There was distinct expression of T-bet in both resting and activated memory CD4+ T cells (a). The expression of T-bet in activated naive and memory CD4+ T cells (0 to 3 h) (b) and the ratio of T-bet to β-actin were quantified by densitometry (c). Statistical data shown are for the results from three independent experiments.
Expression of T-bet and Production of IFN-γ in Naive, Effector, TEM, and TCM CD4+ T Cells
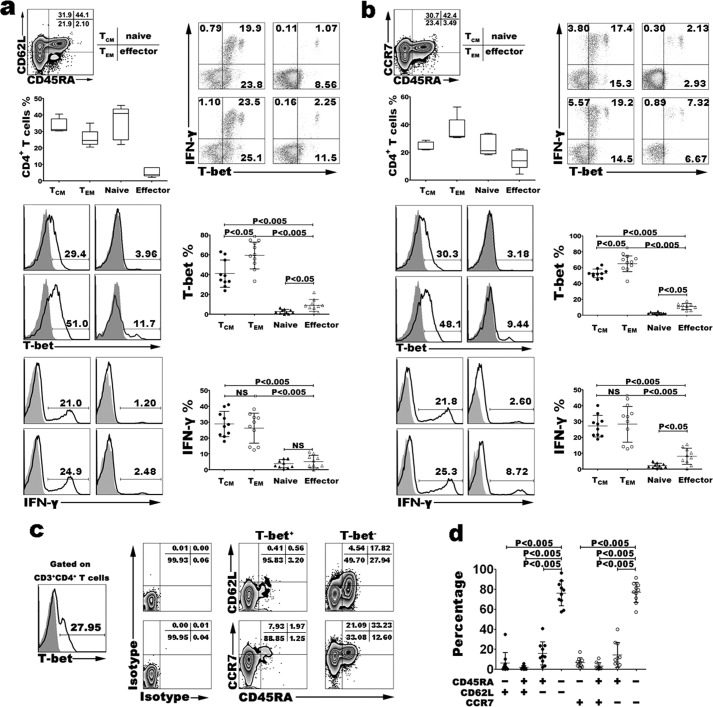
We had proved that resting or short term-stimulated memory CD4+ T cells highly expressed T-bet based on surface marker CD45RO or CD45RA to identify naive and memory CD4+ T cells. To further observe the phenotype of T-bet+CD4+ T cells, we analyzed which subsets of memory T cells expressed T-bet. By flow cytometry, the frequency of T-bet+ T cells in naive, effector, TEM and TCM CD4+ T cells of healthy donors' PBMCs without stimulation was assessed. First, we gated on CD3+CD4+ T cells and distinguished naive, effector, TEM and TCM cells by surface markers CD45RA and CD62L or CD45RA and CCR7 (Fig. 5, a and b). Based on surface markers CD45RA and CD62L, the percentages of naive (CD45RA+CD62L+), effector (CD45RA+CD62L−), TEM (CD45RA−CD62L−), and TCM (CD45RA−CD62L+) cells were 35.60 ± 4.56%, 5.18 ± 1.22%, 25.74 ± 1.95%, and 33.49 ± 1.95% (Fig. 5a). Based on surface markers CD45RA and CCR7, the percentages of naive (CD45RA+CCR7+), effector (CD45RA+CCR7−), TEM (CD45RA−CCR7−), and TCM (CD45RA−CCR7+) cells were 24.99 ± 3.33%, 15.05 ± 3.22%, 36.00 ± 4.22%, and 24.21 ± 1.36% (Fig. 5b). T-bet was almost entirely expressed in memory T cells. Average 64.32% of CD45RA−CD62L− and 62.18% of CD45RA−CCR7− memory T cells positively expressed T-bet, average 47.38% of CD45RA−CD62L+ and 53.80% CD45RA−CCR7+ memory T cells positively expressed T-bet. On the other hand, there were few T-bet-expressing cells among naive T cells (CD45RA+CD62L+CD4+ T cells: 4.33 ± 0.74 or CD45RA+CCR7+CD4+ T cells: 2.44 ± 0.61), and only a low percentage of effector T cells expressed T-bet (CD45RA+CD62L−CD4+ T cells: 12.42 ± 2.75, CD45RA+CCR7−CD4+ T cells: 11.34 ± 2.37). We also analyzed the correlation of T-bet and IFN-γ in different cell subsets after short-term stimulation with PMA plus ionomycin, results of flow cytometry showed that most of IFN-γ+CD4+ T cells in TEM and TCM expressed T-bet (Fig. 5, a and b). Further analyses found that T-bet+CD4+ T cells exhibited the characteristic phenotype of TEM cells, CD62LlowCCR7lowCD45RAlow (Fig. 5, c and d). Above all, the results showed that memory CD+ T cells highly expressed T-bet, and that T-bet+CD4+ T cells were almost all CD45RA−CD45RO+CCR7+/−CD62L+/−.
FIGURE 5.
Expression of T-bet in resting naive, effector, TEM, and TCM CD4+ T cells. PBMCs with or without stimulation were stained with surface marker mAbs including anti-CD3, anti-CD4, anti-CD45RA, anti-CD62L, and anti-CCR7 for 30 min at 4 °C, and fixed in fixation buffer for 10 min at 37 °C, permeabilized in ice-cold 90% methanol for 30 min, and labeled with anti-IFN-γ, anti-T-bet (open histogram), or anti-IgG1κ (shaded histogram) mAbs for 50 min at 4 °C. Gated on CD3+ CD4+ T cells, expression of T-bet in resting naive, effector, TEM, and TCM CD4+ T cells distinguished by surface markers CD45RA and CD62L was analyzed (a). The same results were observed based on the surface markers CCR7 and CD45RA (b). Gated on T-bet+ or T-bet− CD4+ T cells, expression of CD45RA, CD62L, and CCR7 was analyzed (c and d). Statistical data are shown for the results from ten independent experiments.
T-bet Expression and Nuclear Mobilization in Resting Memory CD4+ T Cells
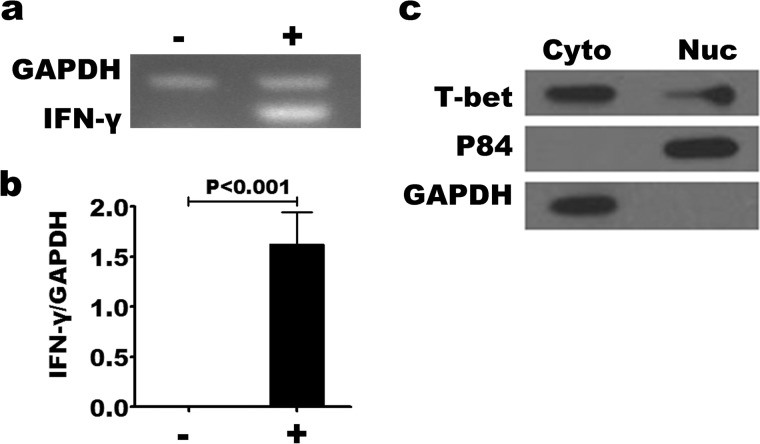
Memory CD4+ T cells highly expressed T-bet. We hypothesized that the high level of T-bet expression made an important contribution to rapid cytokine transcription by memory T cells. Consistent with many studies, we found that the enhanced kinetics and magnitude of cytokine production by memory T cells were controlled at the level of mRNA, and that preformed mRNA was not notably present in memory CD4+ T cells (Fig. 6, a and b). Therefore, we speculated that pre-existing T-bet controlled the transcription of cytokine mRNA in nuclei. To confirm this speculation, we isolated cytoplasmic and nuclear fractions from resting purified CD4+CD45RO+CD45RA− T cells by using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit; and the expression of T-bet, cytoplasmic protein GAPDH and nuclear martrix protein P84 in fractions were detected by Western blotting. We found that there was T-bet expression both in cytoplasmic and nuclear fractions (Fig. 6c). We down-regulated the expression of T-bet by different inhibitors in the program of Th1 cells differentiation. The results showed that the expression of IFN-γ by T-betlowCD4+ T cells was reduced (data not shown). These data indicated that T-bet was the master transcription factor to regulate the differentiation and function of Th1 cells. However, there is high pre-existing T-bet in the nuclei of memory CD4+ T cells, which might make a key contribution to the generation or maintenance of memory Th1 cells; we hypothesize that pre-existing T-bet in nuclei, but not T-bet that is up-regulated following activation, plays a more important role at the early time point for rapid memory T cell recall. The mechanism by which T-bet regulates the rapid production of cytokines will need to be further studied.
FIGURE 6.
T-bet expression and nuclear mobilization in resting memory CD4+ T cells. Memory CD4+ T cells were isolated to >95% purity by MACS separation. Cytoplasmic and nuclear fractions from resting memory CD4+ T cells were isolated according to the manufacturer's instructions. Memory CD4+ T cells were activated with or without immobilized anti-CD3 and anti-CD28 for 12 h, and the levels of IFN-γ and GAPDH mRNA were determined by PCR (a). The ratio of T-bet to GAPDH was quantified by densitometry (b). Means are shown for the results from three independent experiments. T-bet was expressed in the cytoplasm and nuclei of resting memory CD4+ T cells (c).
DISCUSSION
Immunological memory T cells exhibit enhanced functional properties compared with naive T cells in their rapid production of effector cytokines leading to efficacious secondary immune responses. Studies in mouse models have shown that low level stimulation can modulate memory CD4+ T cell function and survival at the recall level, and that a novel biochemical and transcriptional signature is imparted to memory CD4+ T cells enabling efficacious responses (14, 23, 24). In this study, we present data showing that human memory CD4+ T cells without any stimulation express a high level of T-bet compared with naive CD4+ T cells. In further studies, we find that TEM express the highest level of T-bet, and that T-bet is mobilized in both the cytoplasm and nuclei. Our results indicate a possible mechanism by which pre-existing T-bet contributes to generating and maintaining the characteristic ability of human memory CD4+ T cells to rapidly produce effector cytokines.
In contrast to naive CD4+ T cells, which can only produce effector cytokines such as IFN-γ after sustained antigenic stimulation over days, memory CD4+ T cells can produce large amounts of IFN-γ after TCR stimulation within a few hours (3). We show that memory CD4+ T cells are characterized by rapidly producing effector cytokines such as IFN-γ, IL-2, and TNF-α after short-term stimulation with anti-CD3 plus anti-CD28 or PMA plus ionomycin. ELISA results showed that peak production of cytokines by purified memory CD4+ T cells occur much earlier than by naive cells. T-bet is necessary and sufficient for Th1 differentiation and IFN-γ production resulting from stimulation of naive CD4+ T cells and is the main controlling factor for IFN-γ production in CD4+ T cells, NK, and NKT cells (15, 25). Therefore, we hypothesized that pre-expression or up-regulation of T-bet might control rapid production of IFN-γ by memory CD4+ T cells. Our data showed that approximately half of resting CD4+CD45RA− T cells (memory T cells) and only less than 2% of CD4+CD45RA+ T cells (naive T cells) expressed T-bet, moreover, all of IFN-γ-producing CD4+ T cells co-expressed T-bet. The data indicate that rapid memory CD4+ T cell recall is strongly regulated by transcription factor T-bet, which might be different from memory CD8+ T cells in human (9). We also analyzed some other key transcription factors which control the differentiation of Th1 or the production of IFN-γ. Activation of STAT-1 or STAT-4 by IFN-γ and IL-12 is important for the induction of T-bet during in vitro Th1 differentiation, and directly induces production of IFN-γ and expression of IL-12Rβ2 (17, 26). However, in the unstimulated state or short-term stimulated with antigens or TCR signaling neither memory nor naive CD4+ T cells expressed pSTAT-1 and pSTAT-4, and the expression of total STAT-1 or STAT-4 was not significantly different in naïve and memory cells. We demonstrate that the rapid production of Th1 effector cytokines by short-term activated memory cells has an inalienable relationship with high pre-expression of T-bet in human resting memory CD4+ T cells.
Some memory CD4+ T cells express T-bet, which indicates that a possible transcriptional mechanism may be responsible for rapid effector cytokine production. By intracellular flow cytometry and Western blotting, we detected the kinetics of the expression of transcription factors in activated naive and memory CD4+ T cells at different times of stimulation. Although activated naive and memory CD4+ T cells up-regulated expression of T-bet, pSTAT-1, and pSTAT-4 as the time of stimulation increased, only T-bet up-regulation in memory CD4+ T cells was significantly greater than that in naive CD4+ T cells at activated stage. We further evaluated T-bet expression at times earlier than 6 h, the results showed that T-bet expression in purified naive CD4+ T cells happened at 1 h, but there was not noteworthy effector cytokine production by naive CD4+ T cells until 48 h. We demonstrate that pre-existing T-bet plays a remarkable role in controlling the rapid production of IFN-γ, independent of increased expression or up-regulation of T-bet.
Based on the surface markers CCR7 and CD62L, memory CD4+ T cells of PBMCs are divided into two subpopulations, TEM and TCM. TEM have lost the expression of CCR7 and CD62L, and display characteristic sets of chemokine receptors and adhesion molecules that are required for homing to inflamed tissues (1, 5). When compared with TCM, TEM are characterized by rapid effector function. We observed that TEM had the highest levels of T-bet expression as compared with TCM, naive and effector cells, however, there was no significant statistical difference in expression of T-bet in TCM and TEM. Our results establish that TCM and TEM highly express T-bet, and highest expression of T-bet in TEM has a close relationship with the rapid production of effector cytokines, especially IFN-γ. T-bet is required for establishment of the Th1 gene-expression profile by inducing the transcripts of Th1 signature genes (Ifng), and repressing the transcripts of other T helper cells genes (Il4, Il17, and Il21) (27–30). Our further studies found that T-bet had been translocated into the nuclei of resting memory CD4+ T cells, although the levels in nuclei were lower than in cytoplasm by Western blot analysis. Thus we hypothesize that pre-mobilization of T-bet into nuclei might play more important roles at early time points for rapid responses of memory T cells. However, the mechanism by which pre-existing T-bet regulates the rapid production of cytokines by memory CD4+ T cells and maintains the characteristics of memory CD4+ T cells needs to be further studied.
Acknowledgments
We thank Dr. Maurice K. Gately of NJ who read and edited the manuscript, and Dr. J. Li of Sun Yat-sen University of China who provided P84 mAb.
This study was supported by grants from Guangdong Recruitment Program of Creative Research Groups (2009010058) and National Basic Research Program of China Grant 2013CB531500 (973).
- TCM
- central memory T cell
- ECM
- effector memory T cell
- PE
- phycoerythrin
- IFN
- interferon
- FACS
- fluorescence activated cell sorting
- BFA
- Brefeldin A
- TNF
- tumor necrosis factor
- FITC
- fluorescein isothiocyanate.
REFERENCES
- 1. Sallusto F., Geginat J., Lanzavecchia A. (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 [DOI] [PubMed] [Google Scholar]
- 2. van Leeuwen E. M., Sprent J., Surh C. D. (2009) Generation and maintenance of memory CD4(+) T Cells. Curr. Opin Immunol. 21, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogers P. R., Dubey C., Swain S. L. (2000) Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 4. Kallies A. (2008) Distinct regulation of effector and memory T-cell differentiation. Immunol. Cell Biol. 86, 325–332 [DOI] [PubMed] [Google Scholar]
- 5. Pepper M., Jenkins M. K. (2011) Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 12, 467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu C. Y., Kirman J. R., Rotte M. J., Davey D. F., Perfetto S. P., Rhee E. G., Freidag B. L., Hill B. J., Douek D. C., Seder R. A. (2002) Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3, 852–858 [DOI] [PubMed] [Google Scholar]
- 7. Dooms H., Abbas A. K. (2002) Life and death in effector T cells. Nat. Immunol. 3, 797–798 [DOI] [PubMed] [Google Scholar]
- 8. Zhu J., Yamane H., Paul W. E. (2010) Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Intlekofer A. M., Takemoto N., Wherry E. J., Longworth S. A., Northrup J. T., Palanivel V. R., Mullen A. C., Gasink C. R., Kaech S. M., Miller J. D., Gapin L., Ryan K., Russ A. P., Lindsten T., Orange J. S., Goldrath A. W., Ahmed R., Reiner S. L. (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 [DOI] [PubMed] [Google Scholar]
- 10. Joshi N. S., Cui W., Chandele A., Lee H. K., Urso D. R., Hagman J., Gapin L., Kaech S. M. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joshi N. S., Cui W., Dominguez C. X., Chen J. H., Hand T. W., Kaech S. M. (2011) Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J. Immunol. 187, 4068–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mollo S. B., Ingram J. T., Kress R. L., Zajac A. J., Harrington L. E. (2014) Virus-specific CD4 and CD8 T cell responses in the absence of Th1-associated transcription factors. J. Leukoc. Biol. 95, 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szabo S. J., Kim S. T., Costa G. L., Zhang X., Fathman C. G., Glimcher L. H. (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 [DOI] [PubMed] [Google Scholar]
- 14. Lai W., Yu M., Huang M. N., Okoye F., Keegan A. D., Farber D. L. (2011) Transcriptional control of rapid recall by memory CD4 T cells. J. Immunol. 187, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lighvani A. A., Frucht D. M., Jankovic D., Yamane H., Aliberti J., Hissong B. D., Nguyen B. V., Gadina M., Sher A., Paul W. E., O'Shea J. J. (2001) T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. U.S.A. 98, 15137–15142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Afkarian M., Sedy J. R., Yang J., Jacobson N. G., Cereb N., Yang S. Y., Murphy T. L., Murphy K. M. (2002) T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 3, 549–557 [DOI] [PubMed] [Google Scholar]
- 17. Kaplan M. H., Sun Y. L., Hoey T., Grusby M. J. (1996) Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 382, 174–177 [DOI] [PubMed] [Google Scholar]
- 18. Usui T., Preiss J. C., Kanno Y., Yao Z. J., Bream J. H., O'Shea J. J., Strober W. (2006) T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 203, 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nurieva R. I., Chung Y., Martinez G. J., Yang X. O., Tanaka S., Matskevitch T. D., Wang Y. H., Dong C. (2009) Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu J., Yamane H., Cote-Sierra J., Guo L., Paul W. E. (2006) GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 16, 3–10 [DOI] [PubMed] [Google Scholar]
- 21. McGeachy M. J. (2013) Th17 memory cells: live long and proliferate. J. Leukoc Biol. 94, 921–926 [DOI] [PubMed] [Google Scholar]
- 22. Ivanov II., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. (2006) The orphan nuclear receptor RORγ directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 23. Patke D. S., Farber D. L. (2005) Modulation of memory CD4 T cell function and survival potential by altering the strength of the recall stimulus. J. Immunol. 174, 5433–5443 [DOI] [PubMed] [Google Scholar]
- 24. Chandok M. R., Okoye F. I., Ndejembi M. P., Farber D. L. (2007) A biochemical signature for rapid recall of memory CD4 T cells. J. Immunol. 79, 3689–3698 [DOI] [PubMed] [Google Scholar]
- 25. Matsuda J. L., George T. C., Hagman J., Gapin L. (2007) Temporal dissection of T-bet functions. J. Immunol. 178, 3457–3465 [DOI] [PubMed] [Google Scholar]
- 26. Frucht D. M., Aringer M., Galon J., Danning C., Brown M., Fan S., Centola M., Wu C. Y., Yamada N., El Gabalawy H., O'Shea J. J. (2000) Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J. Immunol. 164, 4659–4664 [DOI] [PubMed] [Google Scholar]
- 27. Schulz E. G., Mariani L., Radbruch A., Höfer T. (2009) Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-γ and interleukin-12. Immunity 30, 673–683 [DOI] [PubMed] [Google Scholar]
- 28. Hwang E. S., Szabo S. J., Schwartzberg P. L., Glimcher L. H. (2005) T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307, 430–433 [DOI] [PubMed] [Google Scholar]
- 29. Lazarevic V., Chen X., Shim J. H., Hwang E. S., Jang E., Bolm A. N., Oukka M., Kuchroo V. K., Glimcher L. H. (2011) T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat. Immunol. 12, 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oestreich K. J., Huang A. C., Weinmann A. S. (2011) The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 208, 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]