Abstract
Chronic heart failure (CHF) is highly prevalent in older individuals and a major cause of morbidity, mortality, hospitalizations and disability. Cardiac rehabilitation (CR) exercise training and CHF self-care counseling have each been shown to improve clinical status and clinical outcomes in CHF. Systematic reviews and meta-analyses of CR exercise training alone (without counseling) have demonstrated consistent improvements in CHF symptoms in addition to reductions of cardiac mortality and hospitalizations, although individual trials have been less conclusive of the latter two findings. The largest single trial, HF-ACTION, showed a reduction in the adjusted risk for the combined end point of all-cause mortality or hospitalization (HR: 0.89, 95% CI: 0.81-0.99; P=0.03). Quality of life and mental depression also improved. CHF-related counseling whether provided in isolation or in combination with CR exercise training improves clinical outcomes and reduces CHF-related hospitalizations We review current evidence on the benefits and risks of CR and self-care counseling in patients with CHF, provide recommendations for patient selection for third party payers, and discuss the role of CR in promoting self-care and behavioral changes.
Keywords: Chronic Heart Failure, Cardiac Rehabilitation, Exercise Training, Self-Care, Counseling
Introduction
Cardiac Rehabilitation for CHF
Cardiac rehabilitation (CR)/secondary prevention programs are recognized as integral to the comprehensive care of patients with chronic heart failure (CHF) (1, 2). Effective CR for CHF incorporates both supervised exercise training and comprehensive disease–related self-care counseling. Programs that consist of exercise training alone are not considered CR (3). Exercise training and CHF disease-related self-care counseling are both recommended by the AHA and the ACC as useful and effective in CHF at the class I level (2). CR, which combines exercise training and self-care is recommended by the ACC at the class IIa level (2).
CHF affects > 6.5 million Americans and > 650,000 new cases are diagnosed each year (4). Moreover, the prevalence and incidence of CHF are increasing, largely due to the aging of the population. CHF is the leading cause of hospitalization in the Medicare age group, accounting for > 1 million admissions annually, and it is also a major source of diminished functional capacity, impaired quality of life, disability and mortality (4). Despite major advances in CHF therapies, most patients continue to experience exercise intolerance due to intrinsic abnormalities of cardiac function coupled with maladaptive changes in skeletal muscles, the vasculature, and pulmonary circulation. Additionally, the magnitude of the exercise intolerance, as measured by peak oxygen uptake (VO2), is strongly and independently associated with prognosis in patients with CHF (5). While CHF was once considered a contraindication to exercise, numerous studies demonstrate that regular exercise is safe and associated with a multitude of benefits in appropriately selected patients. This review will delineate the role of structured CR, including exercise training and self-care counseling, in patients with CHF and makes recommendations for selection of appropriate patients for coverage of a CR benefit by third party payers.
Exercise Training Studies in Chronic Heart Failure
Effects on Exercise Capacity
Exercise training is recommended in the therapeutic approach to the stable CHF patient, supported by the ACC, the AHA and the HFSA at a Class 1or 2 level (2,6). Endurance-type exercise training favorably affects peak VO2, central hemodynamic function, autonomic function, peripheral vascular and muscle function, and exercise capacity in CHF (Table 1) (7). These adaptations result in an exercise training effect that allows individuals to exercise to higher peak workloads or to the same submaximal workload at a lower heart rate and perceived effort (8). Daily activities are performed with less dyspnea and fatigue. While training protocols vary, most CHF trials employ moderate-vigorous intensity exercise (50-60% peak VO2) yielding improvements of 13-31% in peak exercise capacity (Figure 1). One study of lower intensity training (40-50% peak VO2) demonstrated a training effect after 8-12 weeks (9). A newer training technique termed high intensity interval training (HIT) may yield greater improvements in peak VO2 (up to 46%) than moderate intensity continuous training in patients with systolic CHF (10) (See section on exercise prescription for more details). A meta-analysis of 57 studies that involved patients with reduced ejection fraction and that directly measured peak VO2, reported an average 17% improvement in peak VO2 (11). This is identical to the improvement in fitness seen in CR for patients with coronary artery disease (CAD) (12). Of more than 2 dozen single-site, randomized exercise training studies, 8 were conducted with >70% of subjects taking angiotensin-converting enzyme inhibitors and β-adrenergic blockers. The unweighted median increase in peak VO2 was 2.1 mL/kg/min (15%), while the unweighted median change among non-exercising controls was 0.1 mL/kg/min (1%) (Figure 1) (13).
Table 1. Beneficial Effects of Exercise in Chronic Heart Failure.
| Aerobic Training | Increased exercise capacity |
| Lower heart rate response to submaximal exercise | |
| Improved diastolic function | |
| Improved endothelial function | |
| Increased skeletal muscle oxidative capacity | |
| Enhanced vagal tone and lower sympathetic tone | |
| Reduced inflammatory cytokines | |
| Lower all-cause mortality or hospitalization | |
| Improved quality of life | |
| Resistance Training | Increased muscle strength and endurance |
Adapted from Downing and Balady 2011 (7).
Figure 1.
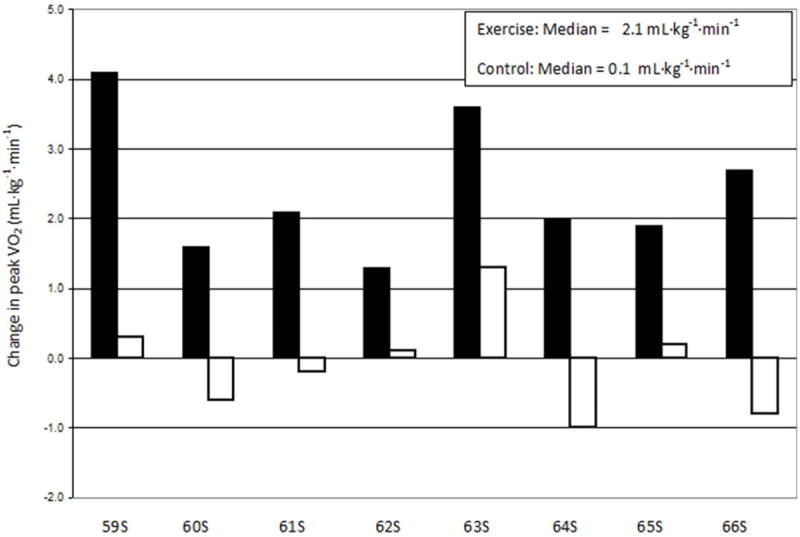
Reported changes in peak VO2 in aerobic exercise-trained subjects from 8 single site, randomized clinical trials in patients with CHF (13). (Filled bars represent exercise-trained subjects; open bars represent control subjects).
Adapted from Keteyian 2011 (13)
Heart failure with preserved ejection fraction (HFPEF) occurs in approximately 50% or more of CHF patients and the proportion is higher among women and the very elderly (14,15). Despite its prevalence, due to its more recent recognition as a clinical entity (16), there are considerably fewer data on the role of physical training in HFPEF than in systolic CHF. However, 7 controlled trials (5 randomized, 1 multi-center) of physical training, in HFPEF patients, (17-24)(Table 2) demonstrate that physical training is a safe and effective intervention to improve symptoms, increase aerobic capacity and endurance, and generally improves self-reported quality of life as well. Resting diastolic left ventricular function was found to be improved following exercise training in some studies (19,20) but not others (18,21). Improvements in peripheral, non-cardiac factors, particularly skeletal muscle, are major contributors to the training-related improvement in exercise capacity in older HFPEF patients (21,23,25). This is not dissimilar to observations made in HFREF patients, and highlights the potential for CR to improve not only cardiac function, but also arterial and skeletal muscle function (26).
Table 2. Controlled Exercise Intervention Trials in HFPEF patients.
| Study | Group/Sample size | Age, yrs | EF % | Mode | Freq, d/wk | Intensity | Duration, min | Length of program, wks | Main findings |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Gary 2006 (17) | ET (n=15) | 67 | 54 | Walk | 3 | 40-60% | 30 | 12 | ↑ 6MWD, QOL |
| CNT (n=13) | 69 | 57 | |||||||
|
| |||||||||
| Kitzman 2010 (18) | ET (n=24) | 70 | 61 | Walk, Cycle | 3 | 40-70% HRR | 60 | 16 | ↑ peak VO2, ventilation threshold, 6MWD, and physical QOL |
| CNT (n=22) | 69 | 60 | |||||||
|
| |||||||||
| Edelmann 2011 (19) | ET (n=44) | 64 | 67 | Cycle, RT(UE/L E) | 2-3 | HR = 50-70% peak VO2 | 20-40 | 12 | ↑ peak VO2, 6MWD, physical function, ↓ rest LAV, E/e’, and procollagen type I |
| CNT n=(20) | 65 | 66 | 2 | 60-65% 1RM | 15 REPS | wks 5-12 | |||
|
| |||||||||
| Alves 2012 (20) | ET (n=20) | GP | GP | Cycle, Treadmill | 3 | 5-7 intervals (3 to 5 min duration) at 70-75% HRmax with 1 min active recovery at 45-55% HRmax | 15 to 35 | 24 | ↑ peak MET, ↑ rest LVEF, E/A ratio, ↓ DT |
| CNT (n=11) | 63 | 56 | |||||||
|
| |||||||||
| Kitzman 2013 (21) | ET (32) | 70 | 58 | Walking, Cycle, Arm ergometry | 3 | 40-79% HRR | 60 | 16 | ↑ peak VO2, ↑ QOL, No change FMD, arterial stiffness |
| CNT (31) | 56 | ||||||||
|
| |||||||||
| Smart 2012 (22) | ET (N=15) | 64 | 57 | Cycle Ergometry | 3 | 60-70% peak VO2 | 30 | 16 | ↑ peak VO2, ↓VE/VCO2, No change systolic or diastolic LV function |
| CNT (N=15) | |||||||||
|
| |||||||||
| Fujimoto 2012 (23) | ET (N=7) | 73 | 76 | Walking, cycling | 3 | 70-80% Max HR | 25-40 | 52 | No change peak VO2, arterial stiffness, LV compliance and volumes. |
| CNT (N=13) | |||||||||
(CNT, control; d, days; DT, deceleration time; E/A, early to late mitral inflow velocity; ET, exercise training; GP, data provided is for mean of whole group; HFpEF, heart failure and preserved ejection fraction; HRmax; maximal heart rate; HRR, heart rate reserve; LAV, left atrial volume; LE, lower extremity; LVEF, left ventricular ejection fraction; MET, metabolic rate; min, minutes; peak VO2, peak oxygen uptake; REPS, repetitions, RT, resistance training; UE, upper extremity; wk, weeks; ys, years; 1RM, one-repetition maximum 6MWD, distance walked in 6 minutes, FMD, flow-mediated vasodilatation).
Exercise Training Alone on Morbidity and Mortality
The ExTraMATCH meta-analysis of 9 datasets included 801 systolic CHF patients and demonstrated a significant 35% reduction in mortality in trained patients versus controls during a mean follow-up of 705 days (27). A more recent Cochrane Review of 19 trials (3647 participants) showed no difference in pooled mortality at < 1 year follow-up but a non-significant trend to lower mortality among trials with follow-up of >1year (28). A significant 28% reduction in hospitalization rate at one year was demonstrated with exercise.
An analysis of > 600,000 patients from the Medicare database addressed the effects of CR exercise training on mortality. Subgroup analyses of patients with CHF showed a 15% lower mortality in CHF patients who participated in CR compared to carefully matched CHF patients who did not participate (29).
HF-ACTION was a multicenter, randomized-controlled trial designed to measure the effects of exercise training alone (without CHF self-care counseling) on clinical outcomes in medically optimized, stable systolic CHF patients (LVEF ≤ 35%) (30,31). The composite primary endpoint was all-cause mortality or all-cause hospitalization. Study subjects had NYHA class II-IV symptoms (n = 2,331, 63% Class II, 36% class III) and were randomized to 36 sessions of supervised, moderate intensity training (60-70% heart rate reserve) followed by home-based training or to usual care. At a median follow-up of 30 months, there was a 7% reduction in the primary combined end point (p =0.13). After adjustment for pre-specified predictors of mortality (duration of exercise test; LVEF; Beck Depression Inventory II score; history of atrial fibrillation), there was a statistically significant difference in the primary endpoint (HR 0.89; P = 0.03). The median improvement in peak VO2 in the exercise training group was only 4%. This less than expected training effect reflected a low adherence rate to exercise in the training group, as only 30% of subjects attained the targeted minutes per week. Furthermore, 8% of control patients exercised regularly. These factors might have attenuated differences in clinical outcomes between the study groups. In a subsequent analysis that investigated the relationship between volume of exercise and clinical events, patients who exercised as prescribed experienced a >30% reduction in mortality or hospitalization (32). In HF-ACTION, exercise-training induced increases in peak VO2 were closely correlated with a better prognosis (33). For every 6% increase in peak VO2 (+0.9 ml/kg/min) there was an associated 5% lower risk of the primary endpoint (P<0.001) and an associated 8% lower risk of combined cardiovascular mortality and CHF hospitalizations (P<0.001). Exercise training at 3 to 5 MET hours/week was associated with 37% and 64% reductions in adjusted risks for death/hospitalization (p=0.03) and cardiovascular death/heart failure hospitalization (p=0.001) (25). Thus, for exercise to improve long-term outcomes, adherence to the exercise prescription is necessary (32,33). In the clinical setting, it is notable that third party payers would reimburse only for CR sessions attended which, as noted above, is linked with improved clinical outcomes.
Concerning any potential interaction between minority status, exercise training, and clinical outcomes, a secondary analysis of the HF-ACTION trial (34) found no interaction effect for all-cause mortality / hospitalization (P = 0.66), all-cause mortality alone (P = 0.68), or cardiovascular mortality/HF hospitalization (P = 0.75). This suggested that the favorable effects ascribed to exercise training and clinical outcomes occurred irrespective of race (31). Similarly, concerning gender, exercise training and mortality, no interaction effect was observed when comparing women (versus men, P= 0.27) (27). HF-ACTION showed a similar finding for sex, exercise training and the combined end-point of all-cause mortality/hospitalization (P value for interaction = 0.17) (31), also suggesting that the favorable exercise training-induced improvement in this clinical outcome occurred irrespective of gender. In The European Heart Failure Training Group study the authors reported an observed increase in peak VO2 among women (+ 2.7 mL/kg/min) that paralleled the increase observed among men (43).
Finally, a study of 123 patients with CHF randomized to supervised exercise training vs. control status found that after 10 years of training, the training group had higher quality of life (P<0.05), fewer hospital readmissions (P<0.001) and a lower cardiac mortality (P<0.001) than the control group (35). Thus, clinical benefits of exercise training in systolic CHF can be expected to be long lasting. Effects of exercise training on prognosis may relate to its effects on autonomic function. Neurohumeral excitation and increased sympathetic nerve activity, both characteristic of CHF, are associated with long-term mortality and these abnormalities are reduced by exercise training (36-38).
Resistance Training in CHF
Studies of resistance training among systolic CHF patients have demonstrated clinical benefits, including improved muscle strength and endurance with a lower VO2 at submaximal workloads (39,40). Strength training also plays an important role in decreasing functional disability and improving physical function in CHF patients (41). Strength training has no deleterious effects on LV function or structure in CHF patients (42). Resistance training was included as an adjunctive modality to standard aerobic exercise training in one trial of HFPEF (19) but has not been studied as a primary training modality.
Exercise Training Effects on Functional Class, Quality of Life and Mental Depression in CHF
The European Heart Failure Training Group reported on 134 patients with CHF and observed that NYHA class improved with aerobic training by 0.5 class compared to controls (P<0.01), and the benefit was proportional to improvement in peak VO2 (43). In HF-ACTION, 30% of subjects in the exercise group improved by 1 full NYHA class or more (30).
The effect of exercise training on health status and quality of life in CHF has been studied using the KCCQ. In HF-ACTION, 3 months of exercise training led to a greater improvement in the KCCQ total score than in controls (5.2 vs. 3.3 points P<0.001), an increase that was maintained throughout 30 months follow-up (30). Greater improvements in the exercise arm were seen for physical limitations, symptoms and quality of life. A 2010 Cochrane Review of 6 exercise studies (700 patients) that utilized the MLWHF questionnaire showed a clinically relevant improvement in quality of life of more than 10 units after exercise training (28). In the HF-ACTION study, exercise training resulted in lower depression scores at 3 months and 12 months (both P<0.05) (44). Improved quality of life has generally been observed with exercise training in HFPEF patients as well (24).
Cardiac Rehabilitation for Patients with CHF and Cardiac Devices
Many patients with CHF have ICDs, some with the capability of cardiac resynchronization therapy. Three randomized-controlled trials have shown that structured exercise training leads to further improvements in exercise capacity and quality of life beyond cardiac resynchronization therapy alone (45-47). In HF-ACTION, 490/2331 patients had ICDs and only 1 patient experienced an exercise-induced discharge (31).
Safety of Exercise Training in Heart Failure
Large meta-analyses show no evidence of adverse events from exercise training for CHF (11,27,28). HF- ACTION (31) provides the largest single study where the safety of exercise in patients with stable heart failure with reduced ejection fraction was carefully assessed. Exercise training was well tolerated and safe (48). No serious adverse training related events have been reported in 6 trials of training in HFPEF patients (approximately 250 patients), which generally included older patients than in systolic CHF trials and a larger percentage of women (24).
Exercise Prescription for CHF
Pre-training Exercise Testing
Exercise testing is recommended prior to enrollment in an exercise program to screen for patients at high risk for adverse events and to assist in the determination of an exercise training intensity range. Such testing is safe, with a non-fatal major event rate of 1 per 2,000 tests (49). Exercise testing can be performed with simultaneous measurement of expired gases since both peak VO2 and the relationship between ventilation and carbon dioxide production (VE-VCO2 slope) provide useful prognostic and prescriptive information (50).
Exercise Prescription
Exercise protocols used in clinical trials primarily included aerobic-type activities such as walking, stationary cycling or rowing (11,27,31). The volume of exercise performed each week is progressively adjusted over time. For most patients, the prescribed volume of exercise approximates 3 to 7 MET-hours per week (13). Common approaches to titration of exercise intensity involve progressively increasing effort until it falls within a training range between 55 - 80% of heart rate reserve (11,49) or 70-85% of maximal heart rate at a Borg perceived exertion between 12 and 14. The duration and frequency of effort should be up-titrated before intensity is increased. The target duration should be ≥30 minutes per session 4 days per week (13). Once patients demonstrate a tolerance of aerobic training levels, resistance training activities are added. Such training should occur 2-3 times per week and focus on the major muscle groups using 1-2 sets of 10-12 repetitions per set (39,40). The intensity of resistance training is increased progressively.
Over the past decade, several groups have studied HIT to improve exercise intolerance (10). Wisloff showed that HIT (four intermittent 4-minute intervals at up to 95% of peak heart rate) improved peak VO2 by 46% in patients with CHF and was associated with favorable remodeling of the left ventricle compared to a 14% increase in peak VO2 with moderate intensity continuous training(10). Additionally, improvements in brachial artery flow-mediated dilation (endothelial function) were greater with HIT. A recent meta-analysis by Haykowsky and co-workers included 7 randomized-controlled trials of HIT and showed greater improvements in exercise tolerance with HIT versus traditional moderate intensity continuous training, although no effect was observed on resting LV ejection fraction (51). One recent report (52) suggests that HITis safe, however, further work is needed to elucidate its use in older patients and women, as well as describe the equivalency of clinical benefits between HIT and moderate intensity continuous training.
Optimizing Adherence with Exercise Training
The greatest difficulties with improving adherence with exercise lie in three areas: 1) failure to address adherence barriers prior to exercise initiation, 2) application of strategies known to improve adherence, and 3) methods to ensure that adherence is addressed in clinical practice. Factors amenable to interventions include treating anxiety and depression, improving motivation, seeking social support, and managing logistical problems of transportation and time. Strategies shown to improve exercise adherence in CHF include goal-setting, developing exercise prescriptions, problem-solving, feedback, positive reinforcement, motivational interviewing and group interaction (53,54).
CR Self-Care Counseling in Heart Failure
Effective self-care is fundamental to maintaining physiological stability and improving health outcomes in patients with CHF (55,56). Most readmissions for CHF exacerbations are attributable, at least in part, to poor self-care, including non-adherence to medications and diet and failure to act upon escalating symptoms (55). As with other chronic conditions (e.g. diabetes), and in accordance with the Chronic Care Model (57), optimal CHF self-care requires a knowledgeable patient who is actively engaged in maintaining his or her health through self-monitoring of symptoms and adherence to prescribed treatment, including medications and behavioral recommendations. Table 3 summarizes key elements of self-care in patients with HF (56).
Table 3. Self-Care Behaviors for Patients with Heart Failure.
|
Adapted from the American Heart Association’s scientific statement on promoting self-care in persons with heart failure (46).
Multiple clinical trials, observational studies, and meta-analyses have documented that CHF disease management programs that emphasize self-care are associated with reductions in hospitalizations and mortality (58,59). Moreover, participation in self-care has been associated with better quality of life and functional status, as well as reduced symptom burden and, in some studies, decreased health care costs (60,61). In a review of 19 CHF self-care intervention studies from 2000-2010 involving 3,166 patients, Baranson found that the majority of studies reported significantly higher levels of knowledge and self-care behaviors, including higher use of regular weighing, adherence to diuretic prescriptions, restriction of dietary sodium, and smoking cessation (62). Based on the large body of evidence demonstrating the benefits of CHF disease management programs and self-care interventions, the 2013 ACCF/AHA HF guidelines provide a Class I recommendation for patients to receive specific education to facilitate heart failure self care (Level of Evidence B) (2). Nonetheless, there is a critical need to augment traditional patient education and counseling strategies for self-care management and maintenance and the CR setting is an obvious place to institute such strategies (62).
The benefits of a nurse-directed comprehensive CHF management program administered in a CR setting were demonstrated in a prospective randomized clinical trial involving 105 CHF patients, mean age 72 years, 62% male (63). Patients randomized to the intervention group received 12 weeks of exercise training and care coordination through self-care management with individualized goals and adherence strategies. Control group patients received education and routine care by their regular physicians. At 12 months follow-up, patients in the intervention group experienced significant reductions in all-cause hospital readmissions (44% vs. 69%, p=0.01), cardiac readmissions (24 vs. 55%, p=0.001), and all-cause mortality (7% vs. 21%, p= 0.03). Intervention patients also showed improvements in six minute walk distance and were more likely to be on evidence-based therapies at 12 months.
As demonstrated by Davidson et al (63), CR programs offer an ideal venue for supporting CHF self-care, as patients are seen on a regular basis by a multidisciplinary team of nurses, exercise physiologists, dieticians, physical therapists and physicians. CR personnel are well positioned and fully qualified to provide ongoing education and support for incorporating self-care behaviors into the patients’ daily routines. In addition, CR personnel can assist in managing complex medical problems, coordinating care with other providers, identifying medication side effects, and facilitating early recognition and management of worsening symptoms, thereby reducing readmissions. Self-care tools such as the Self-Care of Heart Failure Index (SCHFI) (64,65), the European Heart Failure Self-Care Behavior Scale (66) and the Heart Failure Activity Checklist (67) can be utilized to evaluate the impact of self-care interventions. In summary, CR programs can facilitate management of CHF patients through a combination of exercise training, education, and promotion and monitoring of self-management skills.
Selection of Appropriate Patients for Cardiac Rehabilitation
Ambulatory patients with AHA/ACC stage C stable CHF with stable NYHA class II or III symptoms (dyspnea and fatigue) despite guideline-directed medical therapy should be considered for supervised CR.
Rationale for Patient Selection Criteria
Most studies of exercise training for individuals with CHF were carried out in individuals with stable NYHA class II and III symptoms thus class IV patients are presently excluded. All patients should be clinically stable prior to embarking on CR, thus the above recommendations build in a required period of at least one month of clinical stability after an index hospitalization.
Contraindications to CR exercise training also include uncontrolled diabetes or hypertension, moderate to severe aortic stenosis, hypertrophic obstructive cardiomyopathy, significant ischemia at < 2 metabolic equivalents (METs) of work, any co-morbidity that prevents exercise participation, or acute myocarditis or other acute cardiomyopathy until stabilized.
Summary and Conclusions
CR exercise training and CHF self-care counseling confer significant clinical benefits to individuals with stable CHF; exercise capacity is increased, clinical symptoms are markedly improved, quality of life is enhanced, and the risk for future clinical events is decreased. Based upon this evidence, third party payers should provide medical insurance coverage for supervised CR for appropriately selected patients with stable CHF.
Abbreviations
- CHF
Chronic heart failure
- CR
Cardiac rehabilitation
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- HFREF
Heart failure with reduced ejection fraction
- HFPEF
Heart failure with preserved ejection fraction
- Peak VO2
Peak aerobic capacity
- ACC
American College of Cardiology
- AHA
American Heart Association
- NYHA
New York Heart Association
- HFSA
Heart Failure Society of America
- HIT
Higher Intensity Interval Training
- HF-ACTION
Heart Failure: A controlled trial investigating outcomes of exercise training
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- MLWHF
Minnesota Living with Heart Failure Questionnaire
- ICD
Implantable Cardioverter-Defibrillator
- CAD
Coronary artery disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Philip A. Ades, University of Vermont College of Medicine, Burlington, VT.
Steven J. Keteyian, Henry Ford Hospital, Detroit, MI.
Gary J Balady, Boston University Medical Center, Boston, MA.
Nancy Houston-Miller, Stanford University School of Medicine.
Dalane W. Kitzman, Wake Forest School of Medicine, Winston-Salem, NC.
Donna M. Mancini, Columbia University College of Medicine, New York, NY.
Michael W. Rich, Washington University School of Medicine, St Louis, MO.
References
- 1.Balady GJ, Williams MA, Ades PA, et al. Components Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee. Circulation. 2007;115:2675–82. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 Jun 5; doi: 10.1016/j.jacc.2013.05.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Wenger NK, Froelicher ES, Smith LK, et al. Clinical Practice Guideline No 17. Rockville MD: US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research and the National Heart, Lung and Blood Institute, AHCPR Publication No.96-0672; Oct, 1995. Cardiac Rehabilitation. [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transplant. 2004;23:1414–1422. doi: 10.1016/j.healun.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Guideline. J Card Failure. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Downing J, Balady GJ. The role of exercise training in heart failure. J Am Coll Cardiol. 2011;58:561–9. doi: 10.1016/j.jacc.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher GF, Ades PA, Kligfield P, et al. Exercise Standards for Testing and Training A Scientific Statement from the American Heart Association. Circulation. 2013 doi: 10.1161/CIR.0b013e31829b5b44. CIR.0b013e31829b5b44 Published online before print July 22, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 10.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 11.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Ades PA, Savage PD, Brawner CA, et al. Aerobic capacity in patients entering cardiac rehabilitation. Circulation. 2006;113:2706–12. doi: 10.1161/CIRCULATIONAHA.105.606624. [DOI] [PubMed] [Google Scholar]
- 13.Keteyian SJ. Exercise training in congestive heart failure: risks and benefits. Prog Cardiovasc Dis. 2011;53:419–28. doi: 10.1016/j.pcad.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW. Understanding results of trials in heart failure with preserved ejection fraction: remembering forgotten lessons and enduring principles. J Am Coll Cardiol. 2011;57:1687–1689. doi: 10.1016/j.jacc.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM. Understanding “diastolic” heart failure. N Engl J Med. 2004;350:1930–1931. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- 17.Gary R. Exercise self-efficacy in older women with diastolic heart failure: results of a walking program and education intervention. J Gerontol Nurs. 2006;32:31–39. doi: 10.3928/00989134-20060701-05. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomizedcontrolled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelmann F, Gelbrich G, Dungen HD, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Alves AJ, Ribeiro F, Goldhammer E, et al. Exercise Training Improves Diastolic Function in Heart Failure Patients. Med Sci Sports Exerc. 2012;44:776–85. doi: 10.1249/MSS.0b013e31823cd16a. [DOI] [PubMed] [Google Scholar]
- 21.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of Endurance Exercise Training on Endothelial function and Arterial Stiffness in Older Patients with Heart Failure and Preserved Ejection Fraction: A Randomized Controlled, Single-Blind Trial. J Am Coll Cardiol. 2013 May 8; doi: 10.1016/j.jacc.2013.04.033. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smart NA, Haluska L, Jeffries S, Leung D. Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18:295–301. doi: 10.1111/j.1751-7133.2012.00295.x. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor RS, Davies EJ, Dalal HM, et al. Effects of exercise training for heart failure with preserved ejection fraction: A systematic review and meta-analysis of comparative studies. International Journal of Cardiology. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 25.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keteyian SJ. Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: Findings Awaiting Discovery. Journal of the American College of Cardiology. 2013 May 8; doi: 10.1016/j.jacc.2013.01.098. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piepoli MF, Davos C, Francis DP, Coats AJ ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003331.pub3. CD003331. [DOI] [PubMed] [Google Scholar]
- 29.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 30.Flynn KE, Piña IL, Whellan DJ, et al. HF-ACTION Investigators. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keteyian SJ, Leifer ES, Houston-Miller N, et al. for the HF-ACTION Investigators. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60:1899–905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swank AM, Horton J, Fleg JL, et al. Modest Increase in Peak VO2 is Related to Better Clinical Outcomes in Chronic Heart Failure Patients: Results from Heart Failure and a Controlled Trial to Investigate Outcomes of Exercise Training (HF-ACTION) Circ Heart Fail. 2012;5:579–85. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mentz RJ, Bittner V, Schulte PJ, et al. for the HF-ACTION Investigators. Race, Exercise Training and Outcomes in Chronic Heart Failure: Findings from HF-ACTION. American Heart Journal. doi: 10.1016/j.ahj.2013.06.002. Accepted for Publication July 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. 2012 Oct 16;60(16):1521–8. doi: 10.1016/j.jacc.2012.06.036. Epub 2012 Sep 19. [DOI] [PubMed] [Google Scholar]
- 36.Negrao CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev. 2008;13:51–60. doi: 10.1007/s10741-007-9057-7. [DOI] [PubMed] [Google Scholar]
- 37.Barretto AC, Santos AC, Munhoz R, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135:302–7. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 38.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–60. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 39.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update : A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 40.Selig SE, Carey MF, Menzies DG, et al. Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength endurance, heart rate variability, and forearm blood flow. J Card Fail. 2004;10:21–30. doi: 10.1016/s1071-9164(03)00583-9. [DOI] [PubMed] [Google Scholar]
- 41.Savage PA, Shaw AO, Miller MS, et al. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc. 2011;43:1379–86. doi: 10.1249/MSS.0b013e31820eeea1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levinger I, Bronks R, Cody DV, et al. The effect of resistance training on left ventricular function and structure of patients with chronic heart failure. Int J Cardiol. 2005;105:159–63. doi: 10.1016/j.ijcard.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 43.European Heart Failure Training Group. Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. Eur Heart J. 1998;19:466–75. doi: 10.1053/euhj.1997.0736. [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308:465–74. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol. 2009;53:2332–9. doi: 10.1016/j.jacc.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 46.Conraads VM, Vanderheyden M, Paelinck B, et al. The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur J Cardiovasc Prev Rehabil. 2007;14:99–106. doi: 10.1097/HJR.0b013e32801164b3. [DOI] [PubMed] [Google Scholar]
- 47.Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil. 2006;13:818–25. doi: 10.1097/01.hjr.0000230104.93771.7d. [DOI] [PubMed] [Google Scholar]
- 48.McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev. 2008;13:3–11. doi: 10.1007/s10741-007-9052-z. [DOI] [PubMed] [Google Scholar]
- 49.Keteyian SJ, Isaac D, Thadani U, et al. HF-ACTION Investigators. Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J. 2009;158(4 Suppl):S72–7. doi: 10.1016/j.ahj.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 51.Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. 2013;111:1466–9. doi: 10.1016/j.amjcard.2013.01.303. [DOI] [PubMed] [Google Scholar]
- 52.Rognmo Ø, Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation. 2012;126:1436–40. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 53.Barbour K, Houston Miller N. Adherence to exercise training in heart failure: a review. Heart Fail Rev. 2008;13:81–89. doi: 10.1007/s10741-007-9054-x. [DOI] [PubMed] [Google Scholar]
- 54.Tierney S, Mamas M, Woods S, et al. What strategies are effective for exercise adherence in heart failure? A systematic review of controlled trials. Heart Fail Rev. 2012;17:107–113. doi: 10.1007/s10741-011-9252-4. [DOI] [PubMed] [Google Scholar]
- 55.Moser DK, Dickson V, Jaarsma T, Lee C, Stromberg A, Riegel B. Role of self-care in the patient with heart failure. Curr Cardiol Rep. 2012;14:265–275. doi: 10.1007/s11886-012-0267-9. [DOI] [PubMed] [Google Scholar]
- 56.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 57.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 58.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 59.McAlister FA, Stewart S, Ferrua S, McMurray JV. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized controlled trials. J Am Coll Cardol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 60.Lee CS, Moser DK, Lennie TA, Riegel B. Event-free survival in adults with heart failure who engage in self-care management. Heart&Lung. 2011;40:12–20. doi: 10.1016/j.hrtlng.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jovicic A, Holroyd-Leduc J, Straus S. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baranson S, Zimmerman L, Young L. An integrative review of interventions promoting self-care of patients with heart failure. Journal of Clinical Nursing. 2011;21:448–475. doi: 10.1111/j.1365-2702.2011.03907.x. [DOI] [PubMed] [Google Scholar]
- 63.Davidson PM, Cockburn J, Newton PJ, et al. Can a heart-failure specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil. 2010;17:393–402. doi: 10.1097/HJR.0b013e328334ea56. [DOI] [PubMed] [Google Scholar]
- 64.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10:350–60. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24:485–97. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaarsma T, Stromberg A, Martensson J, Dracup K. Development and testing of the European Heart Failure Self-Care Behaviour Scale. Eur J Heart Fail. 2003;5:363–370. doi: 10.1016/s1388-9842(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 67.Sears SF, Woodrow L, Cutitta K, Ford J, Shea JB, Cahill J. A patient’s guide to living confidently with chronic heart failure. Circulation. 2013 Apr 2;127(13):e525–8. doi: 10.1161/CIRCULATIONAHA.112.000734. [DOI] [PubMed] [Google Scholar]


