Abstract
Background:
Basal cell carcinoma of the nose is common, with a potential of local recurrence and high-risk features.
Materials and Methods:
We provide a review on anatomy of the nose, tumour surgery and defect closure on the nose. We analysed our own patients with nasal BCC of a 24 months period.
Results:
We identified 321 patients with nasal BCC. There was a predominance of female patients of 1.2 to 1. The mean age was 74.8 years. Slow Mohs technique was employed for all tumours until 3D tumour-free margins were achieved. That resulted on average in 1.8 ± 0.7 Mohs stages. The most common histologic types were solitary (n = 182), morpheic (79), and micronodular (20), Perineural infiltration was evident in 56 tumours. Primary closure after mobilisation of soft tissue was possible in 105 BCCs. Advancement flaps were used in 91 tumours, rotation flaps in 47, transposition flaps in 34 tumours, and combined procedures in 6 cases. In 36 patients full-thickness skin grafting was performed. In two patients healing by second intention was preferred. Partial flap loss was seen in four patients (1.4%). All of them had significant underlying pathologies. None of the tumours treated showed a relapse during the observation time. However, this is a limitation of the present study since follow-up was on average only 10 months.
Conclusions:
BCCs of the nose are common. Only 3D-controlled micrographic surgery (Mohs or slow Mohs) guarantee a high rate of complete tumour removal and a very low risk of recurrence.
KEYWORDS: Basal cell carcinoma, defect closure, flaps, nose, slow Mohs
INTRODUCTION
Basal cell carcinoma (BCC) is the most common non-melanoma skin cancer. In Germany about 115,000 new cases are seen each year. The incidence has been calculated as 130 per 100,000 inhabitants and year.[1]
About 80% of all BCC occur on the face, of these tumours 25% to 30% are found on the nose. BCC is the most common non-melanoma skin cancer of this region.[2] The nose has a 2.5 times higher risk of recurrence of BCC after surgical excision.[3] Localization on the nose is also considered a feature of high-risk BCC due its anatomical peculiarities and problems in pre-surgical identification of tumour margins.[4] About one third of all incomplete BCC excisions are found on the nose.[5]
Nevertheless, surgery is the cornerstone of treatment of BCC on the nose and a wide variety of techniques have been developed to combine complete 3D tumour removal with good aesthetic and functional outcome. Mohs surgery leads to a low 5-year-recurrence rate.[6,7] In this review we will discuss useful methods for the dermatological surgeon to address the major challenges. Nevertheless there are patients with very advanced tumours who will benefit most from an interdisciplinary approach.[4,8]
Anatomical considerations
The nose is a midfacial organ with great functional and outstanding aesthetic importance. The outer nose can be subdivided into different parts [Figure 1]. From a lateral view the nose is separated from the glabella by the nasion (or nasal root) that develops into the nasal bridge. The shape of the upper part is defined by the nasal bone, the lower part by the nasal cartilage. The larger lateral cartilage and the smaller alar cartilage are paired. The lateral sidewall goes down to the nasolabial groove. Distally the supratip break demarcates the nose tip from the nasal bridge. The infratip break is localized near the soft triangle facets. It demarcates the nose tip from the columella. Columella and philtrum are the borders of the nasolabial angle.
Figure 1.
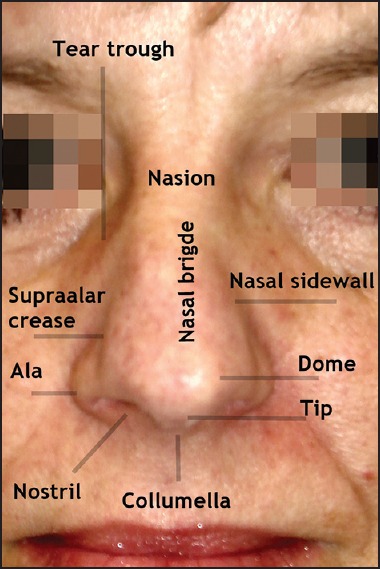
Gross anatomy of the nose
In the frontal view, the tear trough is the border to the medial lower lid. The borders of the alae nasi are defined by the supra-alar crease and the nostrils. Laterally, nasolabial and alarfacial groove mark the borderline to the cheeks. The ala nasi is devoid of cartilage.
The nose skeleton is covered by several small muscles. The procerus muscle attaches to the nasal root and the lateral part where the orbicularis oculi comes close. It inserts into the aponeurosis of the nasalis muscle. The levator labii superioris alaequae nasi goes along the nasofacial groove. On the distal part three delicate muscles are localized, that is, the transversus nasalis, the compressor narium minor and the dilatator nasi. Sensory nerve supply of the nasal bridge originates from ophthalmic branch of the trigeminal nerve (i.e., infratrochlear and anterior ethmoidal nerve). The other parts of the nose are innervated by the maxillary branches, that is, infraorbital nerve. Motoric innervation is by the nasal branches of the trigeminal nerve.
Arterial supply of the lateral parts of the nose originates from external carotid, ophthalmic and infraorbital arteries. Anterior ethmoidal and angular arteries are the major vessels of the nose. The venous drainage does not follow the arteries in parallel. Connections to the ophthalmic, anterior ethmoidal and facial vein drainage this area. Lymphatic drainage from the outer nose runs along the cheeks, the upper lip and the mouth corners. There are connections to submental lymph nodes, parotid and glabella.[9,10]
General principles in outer nose repair
Most of nasal skin is of the sebaceous type. Whenever possible, scar lines should be placed along relaxed skin tension lines. Aesthetic units of the nose need consideration although tumours do not respect their borders. Aging affects the nose anatomy. Characteristic symptoms are frown lines (although not necessarily connected to ageing), transverse crease on the nasal root, drooping of tip of nose, and deepened nasolabial folds. Skin diseases of elderly, like rosacea and rhinophyma can interfere with surgical techniques.[11]
The skin covering the bony parts is highly movable, while the skin over cartilage parts is thicker, tighter and bound to the cartilage. Healing by second ary intention of convex surfaces like the nose tip should be avoided since healing often is delayed and may lead to uneven scars.[12]
Basal cell carcinoma of the nose
BCC of the nose tip is common [Figure 2a]. Larger tumours will infiltrate and eventually destroy the neighbouring areas. Infiltration of the delicate muscles of the distal nasal part and later on the cartilaginous structures is characteristic for locally advanced tumours. BCCs of the lateral part of lateral sidewall do not necessarily respect the nasofacial groove. They may infiltrate the muscles including the orbicularis oculi in advanced stages. BCCs of the nasal root are less common than those of the bridge [Figure 2b and c]. Since inner canthus is in close proximity this may cause a particular challenge for defect closure.
Figure 2.
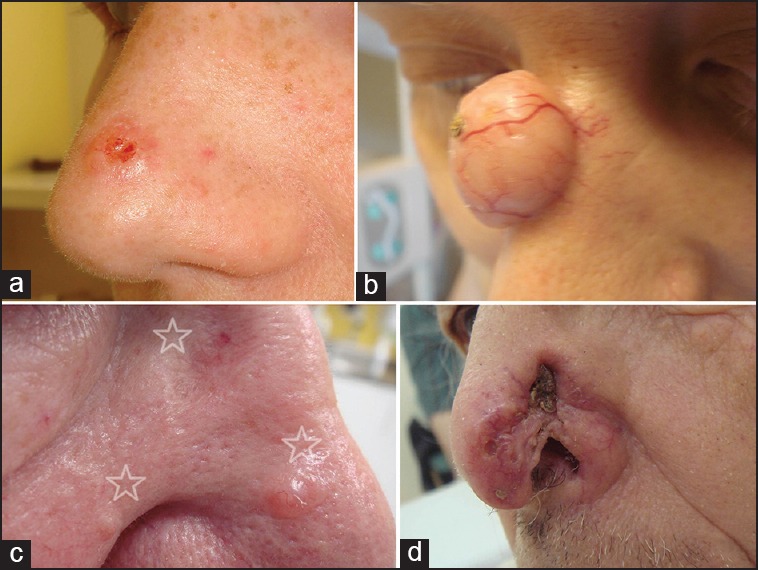
Clinical presentation of basal cell carcinoma (BCC) of the nose. (a) Small solid but ulcerated BCC of the nose tip. (b) Large adenoid-cystic BCC of the nasal root. (c) Patients with 3 (stars) lesions suspicious for BCC: Morpheic BCC, larger and smaller solid BCC (clockwise) (d) Ill-defined morphoeic BCC with partial destruction of nostril
The ala nasi is a common place for BCC. Larger tumours will involve the nostrils and/or alarfacial and nasofacial grooves [Figure 2d]. Tumours of the nasal bridge often extend to the lateral sidewall.
Even small tumours of the columella and tend to invade cartilagenous structures, mucous membranes, and subcutaneous tissue. Some patients present with multiple nasal BCC [Figure 2b].[1,4]
Primary closure
Primary closure is the simplest type of defect repair. Primary closure on the nose is possible for tumours with small tissue defects. It is most suitable at nasal root where skin is mobile. Another option for primary closure is the medial part of the nasal bridge after fusiform excision. Undermining of the edges is performed and the key suture is placed at the widest point of the elliptical defect.[13]
Advancement flaps
Rectangular advancement flaps are useful to cover medium-sized defects of nasal root and bridge. For more distal lesions there is an increasing risk of flap necrosis. It is recommended to limit the length of the flap to maximum four times its width. On its base, that is, the glabella, Burow's triangles are excised. This type of flap is also known as ventail flap since it resembles a helmet [Figure 3].
Figure 3.
Closure of a large distal nasal defect by a rectangular advancement flap. (a) Primary defect after slow Mohs surgery. (b) Preparation of the advancement flap. (c) Complete closure of the defect without modifications of nasal tip of alae
Another option for nasal bridge defects of medium size is the bilateral T-plasty (also known as A-T-plasty) where the lateral sidewalls are mobilised and a distal cut is along the supra-alar crease. In case of small defects on the lateral sidewall T-plasty is also possible with the bar placed along the nasofacial groove.[14]
For small- to medium-sized deep nasal tip defects an elegant approach is the sine wave flap figure. Incision of the flaps starts at the most inferolateral point of the defect and follows the curvature of inferior nasal tip concavity. It extends to the melolabial fold. Here the width of Burow's triangle must match with the width of the primary defect. While preparing the flap caution must be taken to preserve angular artery branches. Laxity of cheek skin is used for defect closure.[15]
Small- to medium-sized defects after Mohs surgery of the nasal sidewall above the supratip can be closed by the “east-west” flap figure. The basic principle is the creation of two triangles – A larger one on the sidewall apical to the primary defect, and a small triangle inferior medially along the columella. The flap is widely undermined in the submuscular plane. Tissue match is excellent. Care has to be taken to place the tension lines in such a way that no alar deformity results. By extending the horizontal incision even defects larger than 2 cm in diameter can be closed.[16] Modifications of the crescentic flap can be used for small defects of the alar sulcus as well.
For medium-sized and larger defects of the lateral sidewall the cheek advancement flap is a reliable method figure. The caudal part of the incision is placed in the melolabial fold. Redundant skin of the melolabial fold will be excised in a crescentic shape. If the surgical defect is larger on nasal sidewall and crosses the nasolabial groove then a combination of the cheek advancement flap with a glabella rotation flap (Rieger flap or its modifications) can be performed. This technique allows a one-stage closure with good aesthetics.[17]
The island pedicle flap (IFP) is designed as a V-Y tissue advancement. This flap is characterized by a robust central vascular pedicle that nourishes the flap about 50% of the size of the IFP. Best aesthetic outcome can be achieved when the flap has been harvested in the same aesthetic unit.[18]
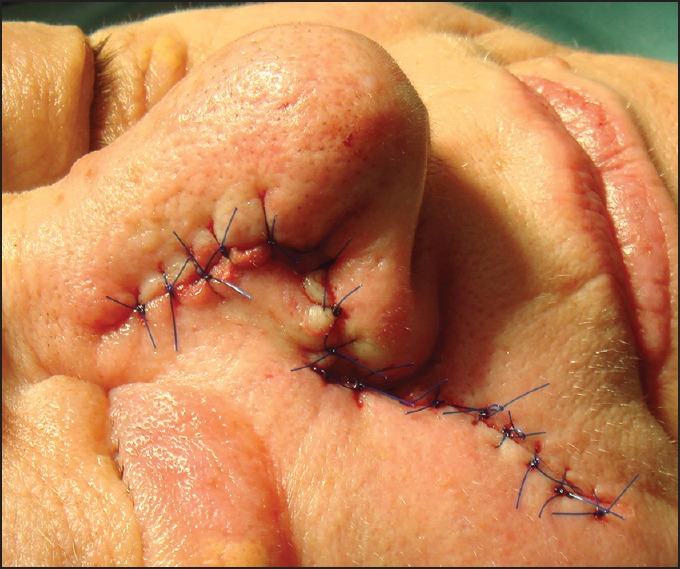
One unwanted effect of this special flap type is trapdooring. The width of the IFP should be slightly smaller than that of the surgical defect. This helps to prevent the elevation of the flap above neighbouring tissue.[19] The transposition IFP is a modification of the original technique to reconstruct lateral nasal ala [Figures 4 and 5].[20]
Figure 4.
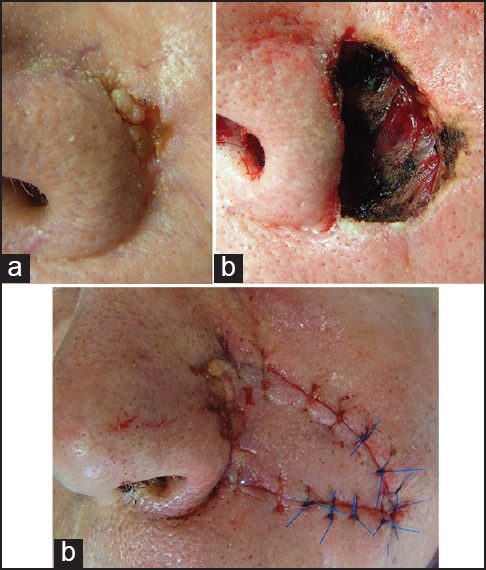
Para-alar island pedicle flap. (a) Basal cell carcinoma of the lateral ala. (b) Primary defect after slow Mohs. (c) Defect closure by island pedicle flap
Figure 5.
Alar defect closure by island pedicle flap. (a) Primary defect after slow Mohs. (b) Preparation of the flap. It is important to mobilise the flap at the alarfacial groove. (c) Defect closure
Rotation flaps
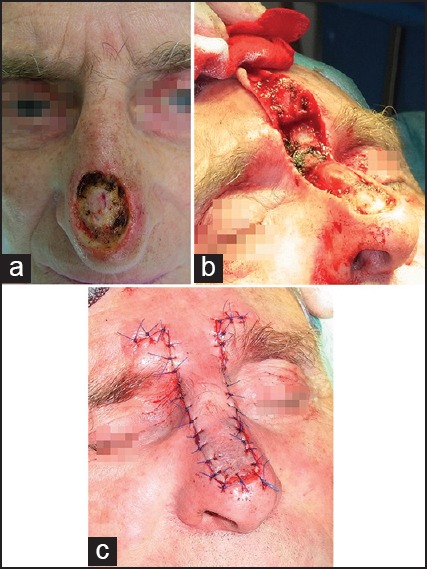
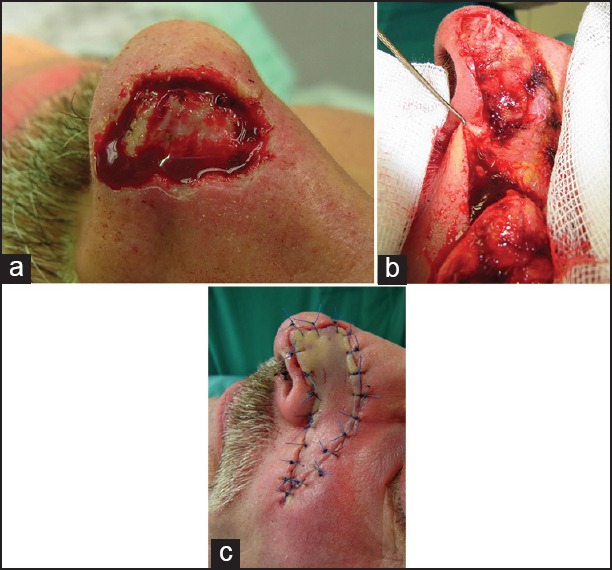
The axial dorsal rotation flap of Rieger and its numerous variations can be used for defect closure of medium-sized to large eccentric defects after BCC surgery of nasal bridge or defects on the nose tip.[21] The latter variant is known as distal nasal rotation flap. A back cut is extended to the glabella. Modifications can be used as well [Figure 6]. Proper flap design and mobilisation prevent the elevation of the nose tip. The larger the flap the more oedema will develop after surgery. We choose manual techniques to enhance the lymphatic pull into the lymphatic vessels of the neck and chest from day 1 after surgery. This prevents negative effects of oedema on vascular supply.
Figure 6.
Rotation flaps. (a) Nose tip defect after slow Mohs of about 2 cm in diameter. (b) Peparation of the Rieger flap. (c) Defect closure. (d) A modified unilateral dorsonasal rotation flap. The disfigurement of the right ale results from an earlier trauma
For closure of smaller defects on the lateral sidewall of the nose the perialar arc rotation flap can be used figure. Here a triangle is created superior to the defect and Burow's crescent is placed into the nasolabial fold. For closure the cheek is rotated superiomedially.[22]
A modification of the original technique uses the alar-facial junction for Burow's triangle–the perialar crescentic advancement flap. The primary movement is here in a horizontal line and the scar is concealed in the junction line.[23]
Medium-sized defects of the nasal sidewall can be repaired by medial cheek rotation flap, for larger defects rotational flaps with a more lateral extension are suitable [Figure 7]. Patients will experience a substantial lower lid oedema. The flap should be elevated within the loose adipose tissue of the medial cheek and just above the facial musculature. This ensures low risk of bleeding. The flap is rotated with minimal tension superomedially. One possible long-term adverse effect is lower lid ectropium.[24]
Figure 7.
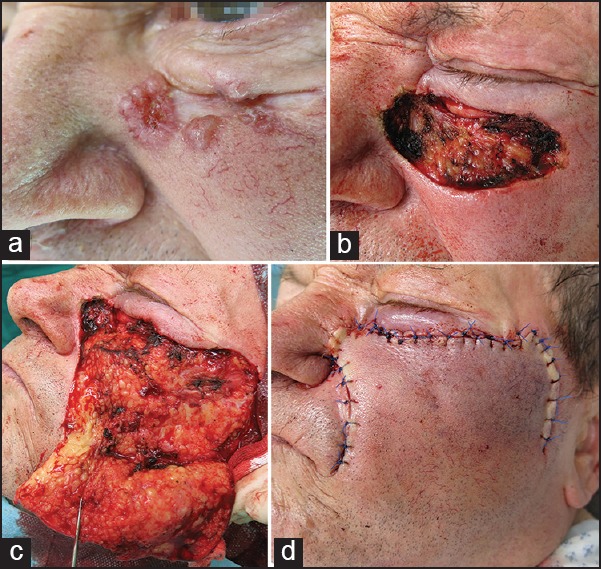
Medial cheek rotation flap. (a) A large ill-defined BCC with mixed solid and morpheic macromorphology. (b) Defect after slow Mohs. (d) Preparation of the cheek rotation flap. (d) Defect closure
For a small surgical wound of the alar region a superficial shark island flap is a good option with acceptable aesthetic outcome figure. Basically it represents an island flap with a pedicle. The superior arm is rotated by 90° in relation to the nasal defect to repair this area. Thereby an inverted cone of redundancy is built which results in the recreation of nasal sulcus.[25] This is the aesthetic advantage compared to the skin helix flap.[26]
Another option for small alar lesions is a spiral rotation flap created by incision along the alar-facial groove starting from the most superiolateral point of the primary defect. A small Burow's triangle is removed at the inferior position of the defect. The flap is mobilized just above the cartilage layer and advanced anteriorly into the primary defect. Sutures in all cases of rotation flaps should be placed along the natural borders of aesthetic units.[27]
Transposition flaps
The classical transposition flap was designed by Limberg. This is a rhombic flap and various modifications have been developed since. On the nose rhombic transposition flaps are used for small surgical defects on nasal bridge and sidewalls. Tension vectors should be directed away from lower eyelid and nasal ala, where distortion leads to aesthetic compromise.[28]
Banner type transposition flaps are created as finger-like flaps with a width equal to that of the primary defect. The flap is rotated around the pivot point to cover the defect. Dog ears need a removal. The usual angle of rotation is between 60° and 120°. This flap type is often employed at the inferior lateral sidewall and nasal ala [Figure 8].[29]
Figure 8.
Banner flap. The flap had been prepared from the nasolabial fold skin and was used to close a lateral sidewall defect
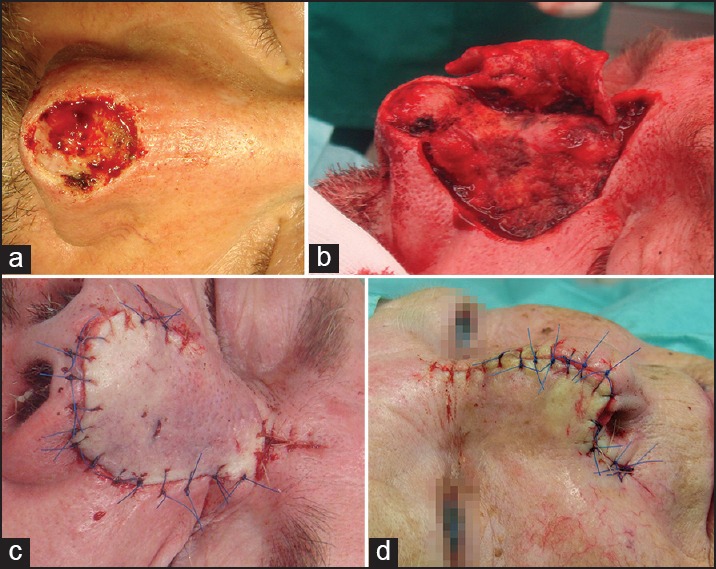
The bilobed flap is commonly used at the nasal sidewalls and nose tip. The primary lobe is created in the same size of the primary defect or up to 20% smaller in case of enough skin laxity. The second lobe is designed at a 90° angle to the pivot point of the flap. The major tension is created by the closure of the tertiary defect [Figure 9].[30]
Figure 9.
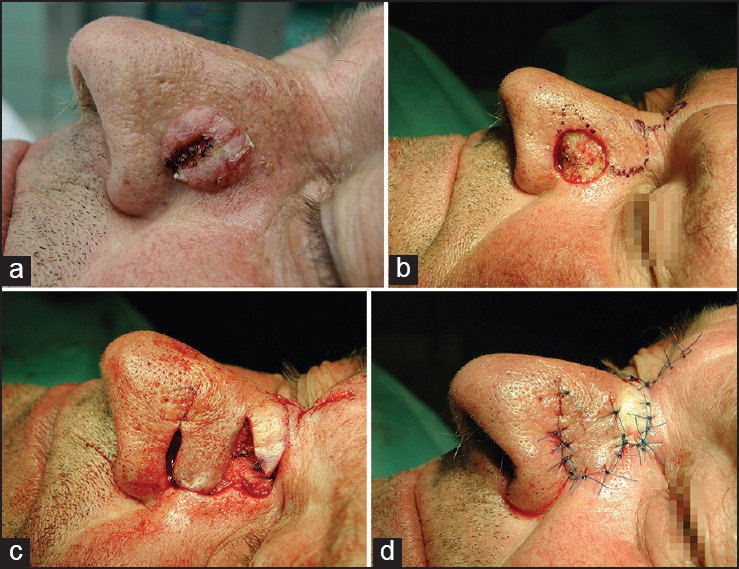
Bilobed flap. (a) Ulcerated solid basal cell carcinoma of the lateral sidewall. (b) Primary defect after slow Mohs and marks for flap preparation. (c) Preparation of the bilobed flap. (d) Defect closure
A variation is the trilobed flap. The bi- and trilobed flap can be performed laterally or medially. Trapdooring may be seen with bilobed and banner flaps.[31]
An alternative to the bilobed flap is the advancement and inferior rotation of the nasal sidewall (AIRNS) flap. This flap is best used for distal defects of the lateral nose. The width of the flap should be approximately vertical height of the primary defect, The elevation of the flap is performed in a subnasal plane.[32]
Staged interpolation flaps
The technique of staged interpolation flaps needs greater experience than all other procedure discussed above. Planning and execution have to be planned most exactly.
They key features of the stage interpolation flaps are the design of a vascular pedicle based on a nourishing artery, donor location distant from the defect, and at least two stages for completion (pedicle formation and closure, pedicle division, and revision).
The paramedian forehead flap (PFF) is used to repair mediodistal nasal defects. The flap design needs a sufficient height of the forehead to create a flap long enough to cover the nasal defect. The arterial supply is derived from the supratrochlear artery. The flap consists of skin musculature and vasculature. Dissection of the PFF is below the venter frontalis. The base of the pedicle should be 1 to 1.5 cm. Flaps mobilized down to the galea needs a meticulous haemostasis. Subperiostal release of the flap increases mobility. Before suturing the thickness of the flap needs to be adapted to the surgical defect. Sutures are placed in two layers. Donor site closure is performed with minimal tension. Residual defects can heal by second intention or a W-plasty is performed. About 3 weeks later the pedicle is thinned and after 6 weeks the PFF is detached from its base and sutured.[33,34]
For small- to medium-sized defects of nasal ala, infratip or columella the cheek-to-nose interpolation flap should be used. The vascular supply is based on tributaries of the angular artery. This flap needs cartilage grafting to the ala. The flap is harvested from skin and subcutis of melolabial fold. Mild oversizing of the flap exploits trapdooring for restoration of nasal ala convexity. The flap can be developed as myocutaneous or myosubcutaneous pedicle. Pedicle division needs at least 3 weeks. It is important to maintain the alar-facial sulcus for aesthetic outcome.[35]
Grafts
From an aesthetic point of view grafts are a second-line treatment for defects of the nose. However, for large defects after BCC surgery, full thickness skin grafting can be an option. Donor skin can be harvested from the pre- or postauricular skin that matches well with the outer nose. In case if larger grafts are needed, the supraclavicular region is a good donor site. The graft must be trimmed to remove subcutaneous adipose tissue. Basting or perimeter sutures anchor the graft at the recipient site. Bolsters or tie-over dressings prevent haematoma or seroma formation beneath the graft.
It takes several weeks for skin grafts to stabilize and match with the recipient site. Pigmentary changes may develop. Depressions may develop in the long-term followup [Figure 10].[36]
Figure 10.
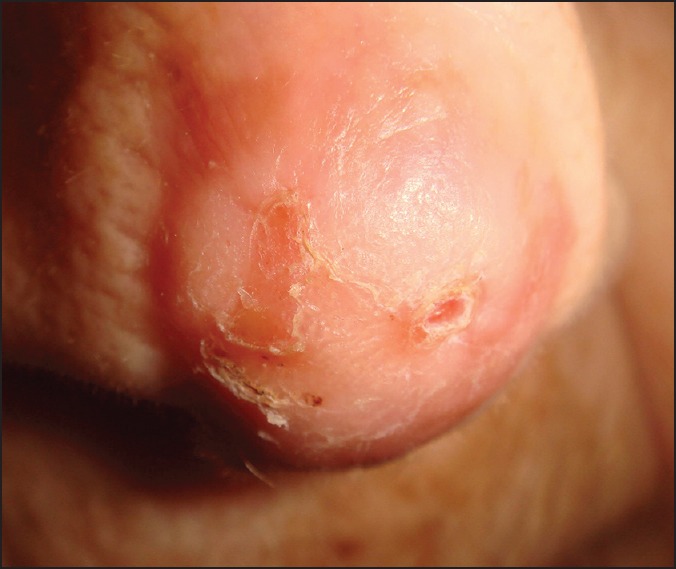
Basal cell carcinoma of the nose tip. The full-thickness skin grafting had been performed 20 years ago. Late adverse effects: dryness of skin and depression of the graft
Composite grafts with cartilage are used for repair of smaller alar rim defects. They can be harvested from the conchal bowl.[37] The combination of a composite auricular graft with a nasolabial flap can be used for full thickness alar defects up to 2 × 2.5 cm with good functional and aesthetic outcome.[38]
In case of exposed bone and/or cartilage and larger defects we use an artificial elastin-collagen matrix with skin grafting in the same session with good aesthetics. This technique has been named “sandwich technique.”[39]
Analysis of our data
We analysed our files of 2 years (January 2012 to December 2013). During these 24 months a total 321 patients with BCC of the nose were identified. The male-to-female ration was 0.85. The age of patients was 74.8 years ± 11.1 years (mean ± standard deviation, SD). The youngest patient was 38 year old, the oldest 95 year old [Figure 11].
Figure 11.
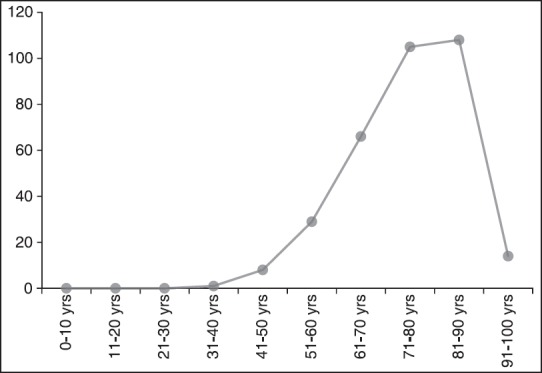
Age distribution of 321 patients with nasal basal cell carcinoma. Most patients were older than 70 years of age
All surgeries were performed under local anaesthesia. Perioperative prophylactic antibiotics were given. In most patients pre-operative cefuroxime 1 g was used. Patients with larger flaps and conditions predisposing to wound infection got antibiotics for 6 days.
Many patients with nasal BCC presented with additional BCCs on the face or other body parts. The average number of tumours was 1.40 ± 0.85. For nasal BCC a slow Mohs technique using formalin-fixed tissue specimen for three-dimensional histology was employed.[40] Incomplete primary excision was noted in 98 tumours (30.5%). Slow Mohs surgery was performed in all patients until 3D tumour-free margins were confirmed. That resulted on average in 1.8 ± 0.7 Mohs stages with a maximum of 10.
The most common histologic types were solitary (n = 182), morpheic (79), micronodular (20), adenoid (18), cystic (12) and metatypic (10). Perineural infiltration was observed in 56 tumours.
Primary closure after mobilisation of soft tissue was possible in 105 BCCs. The majority of these tumours were localized on the nasal bridge and lateral sidewalls.
Surgical repair of defects: Advancement flaps were used in 91 tumours with Rieger flaps in 26 cases. We used rotation flaps in particular for nasal bridge and alar defects (n = 47). Transposition flaps, most often bilobed and banner flaps were employed in 34 patients. In multistage Mohs leading to large defect and in patients older than 80 years, full-thickness skin grafts are an alternative. In 36 cases transplantation of full-thickness skin was performed. Combined flaps or flaps with grafting were used in six patients. Two patients denied a second surgery for closure of Mohs defects. Here, healing by second intention was preferred.
Partial flap loss was seen in four patients (1.4%). All of them had significant underlying pathologies [Table 1]. We recommend a 5 year follow-up of patients according to the German Guidelines for BCC.[41]
Table 1.
Causes of partial flap loss

None of the tumours treated showed a relapse during the observation time. However, this is a limitation of the present study since follow-up was on average only 10 months.
DISCUSSION
BCC of the nose is common. Surgery remains the cornerstone of treatment. Early diagnosis and Mohs surgery ensure a complete removal of these high-risk tumours. In the present study primary incomplete primary excisions were noted in 30.5% on the nose. This is in the range of reported 14% to 50% in the literature.[42]
The high percentage of patients older than 70 years of age and the percentage of morpheic tumours are contributing to the R1 resections. In all cases with positive tumour margins Mohs surgery was continued until a complete excision was confirmed by 3D histology. The recurrence rates of primary BCC for this approach are very low: between 0 to 2.5%.[6,7,43,44]
Analysis of factors that influence the type of defect repair after Mohs surgery argues for an impact of Mohs stages and experience of the surgeon. Most Mohs defects were closed by primary suturing in this study and that of Alam et al.[45] Flaps and grafts were seen increasingly after more than two Mohs stages.[45] This was the same in the present study.
Defect closure employs patient-oriented tailored techniques to obtain good functional and aesthetic outcome and to meet the patient's preferences and needs. Fortunately, there was not a case of bone infiltration or extensive ulceration in the present analysis. But for such patients an interdisciplinary approach is used at its best. For improvement of quality of life such patients will also benefit from supply with episthesis.[4,8]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wollina U, Tchernev G. Advanced basal cell carcinoma. Wien Med Wochenschr. 2013;163:347–53. doi: 10.1007/s10354-013-0193-5. [DOI] [PubMed] [Google Scholar]
- 2.Wollina U. Epithelial tumors of the outer nose. Indian J Dermatol. 2003;48:194–9. [Google Scholar]
- 3.Rogalski C, Kauer F, Simon JC, Paasch U. Meta-analysis of published data on incompletely excised basal cell carcinomas of the ear and nose with introduction of an innovative treatment strategy. J Dtsch Dermatol Ges. 2007;5:118–26. doi: 10.1111/j.1610-0387.2007.06197.x. [DOI] [PubMed] [Google Scholar]
- 4.Wollina U, Pabst F, Krönert C, Schorcht J, Haroske G, Klemm E, et al. High-risk basal cell carcinoma: An update. Expert Rev Dermatol. 2010;5:357–68. [Google Scholar]
- 5.Palmer VM, Wilson PR. Incompletely excised basal cell carcinoma: Residual tumor rates at Mohs re-excision. Dermatol Surg. 2013;39:706–18. doi: 10.1111/dsu.12113. [DOI] [PubMed] [Google Scholar]
- 6.Häfner HM, Breuninger H, Moehrle M, Trilling B, Krimmel M. 3D histology-guided surgery for basal cell carcinoma and squamous cell carcinoma: Recurrence rates and clinical outcome. Int J Oral Maxillofac Surg. 2011;40:943–8. doi: 10.1016/j.ijom.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452–7. doi: 10.1016/j.jaad.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 8.Więckiewicz W, Bieniek A, Więckiewicz M, Sroczyk L. Interdisciplinary treatment of BCC located on the nose-review of literature. Adv Clin Exp Med. 2013;22:289–93. [PubMed] [Google Scholar]
- 9.Lang J. Clinical Anatomy of the Nose, Nasal Cavity, and Paranasal Sinuses. New York, NY - Stuttgart: G. Thieme; 1989. pp. 6–30. [Google Scholar]
- 10.McCullouch TM, Van Daele DJ. Normal anatomy and physiology of the nose, the pharynx, and the larynx. In: Langmore SE, editor. Endoscopic Evaluation and Treatment of Swallowing Disorders. New York, NY: G. Thieme; 2001. pp. 7–36. [Google Scholar]
- 11.Wollina U. Rosacea and rhinophyma in the elderly. Clin Dermatol. 2011;29:61–8. doi: 10.1016/j.clindermatol.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Mott KJ, Clark DP, Stelljes LS. Regional variation in wound contraction of Mohs surgery defects allowed to heal by second intention. Dermatol Surg. 2003;29:712–22. doi: 10.1046/j.1524-4725.2003.29180.x. [DOI] [PubMed] [Google Scholar]
- 13.Mamelak AJ, Wang SQ, Goldberg LH. Linear closure for nasal defects after Mohs micrographic surgery. J Drugs Dermatol. 2009;8:23–8. [PubMed] [Google Scholar]
- 14.Svedman P. Advancement flaps for alar reconstruction. Ann Plast Surg. 1990;25:502–7. doi: 10.1097/00000637-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Hussain W. The “sine wave” flap for the repair of defects of the distal nose. Dermatol Surg. 2013;39:320–4. doi: 10.1111/dsu.12038. [DOI] [PubMed] [Google Scholar]
- 16.Geist DE, Maloney ME. The “east-west” advancement flap for nasal defects: Reexamined and extended. Dermatol Surg. 2012;38:1529–34. doi: 10.1111/j.1524-4725.2012.02453.x. [DOI] [PubMed] [Google Scholar]
- 17.Redondo P. Reconstruction of the anterior cheek, upper nasal ala, and lateral nasal sidewall. Dermatol Surg. 2010;36:123–7. doi: 10.1111/j.1524-4725.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 18.Braun M, Jr, Cook J. The island pedicle flap. Dermatol Surg. 2005;31:995–1005. doi: 10.1111/j.1524-4725.2005.31824. [DOI] [PubMed] [Google Scholar]
- 19.Otley CC, Roenigk RK. Surgical pearl: Preparing the defect for an island pedicle flap. J Am Acad Dermatol. 1997;36:257–8. doi: 10.1016/s0190-9622(97)70291-3. [DOI] [PubMed] [Google Scholar]
- 20.Hairston BR, Nguyen TH. Innovations in the island pedicle flap for cutaneous facial reconstruction. Dermatol Surg. 2003;29:378–85. doi: 10.1046/j.1524-4725.2003.29090.x. [DOI] [PubMed] [Google Scholar]
- 21.Rieger RA. A local flap for repair of nasal tip. Plast Reconstr Surg. 1967;40:147–9. doi: 10.1097/00006534-196708000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hussain W, Tan E, Salmon PJ. Inferiorly based crescentic “sliding” cheek flaps for the reconstruction of paranasal surgical defects. Dermatol Surg. 2012;38:249–55. doi: 10.1111/j.1524-4725.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- 23.Borchard KL, Gunson TH, Smith HR, Vinciullo C. Pushing the perialar: A modified perialar crescentic advancement flap for the reconstruction of large nasal sidewall defects. Dermatol Surg. 2013;39:956–9. doi: 10.1111/dsu.12198. [DOI] [PubMed] [Google Scholar]
- 24.Raschke GF, Rieger UM, Bader RD, Guentsch A, Schaefer O, Elstner S, et al. Cheek rotation flap reconstruction - An anthropometric appraisal of surgical outcomes. Clin Oral Investig. 2014;18:1251–7. doi: 10.1007/s00784-013-1075-3. [DOI] [PubMed] [Google Scholar]
- 25.André MC, Fraga A, Garcia CR, Pignatelli JG, Soares RO. Shark island pedicle flap for repairing of basal cell carcinoma localized in nasal ala-perialar region: A simple procedure. An Bras Dermatol. 2011;86(Suppl 1):S160–3. doi: 10.1590/s0365-05962011000700042. [DOI] [PubMed] [Google Scholar]
- 26.Ascari-Raccagni A, Dondas A, Righini MG, Trevisan G. A “skin helix” flap to correct circular skin loss on the nasal ala. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:11–4. [PubMed] [Google Scholar]
- 27.Higgins HW, 2nd, Lee KC, Rashid Z, Dufresne RG, Jr, Cruz A. An alternative approach to reconstruction of the lateral nose. Dermatol Surg. 2013;39:1270–3. doi: 10.1111/dsu.12224. [DOI] [PubMed] [Google Scholar]
- 28.Borges AF. The rhombic flap. Plast Reconstr Surg. 1981;67:458–66. doi: 10.1097/00006534-198104000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Masson JK, Mendelson BC. The banner flap. Am J Surg. 1977;134:419–23. doi: 10.1016/0002-9610(77)90421-4. [DOI] [PubMed] [Google Scholar]
- 30.Cook JL. A review of the bilobed flap's design with particular emphasis on the minimization of alar displacement. Dermatol Surg. 2000;26:354–62. doi: 10.1046/j.1524-4725.2000.99160.x. [DOI] [PubMed] [Google Scholar]
- 31.Skaria AM. The medial based bi- or trilobed flap for repair of distal alar defects. Dermatology. 2013;227:165–70. doi: 10.1159/000353921. [DOI] [PubMed] [Google Scholar]
- 32.Hafiji J, Salmon P, Hussain W. The AIRNS flap: An alternative to the bilobed flap for the repair of defects of the distal nose. J Am Acad Dermatol. 2012;67:712–6. doi: 10.1016/j.jaad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Menick FJ. A 10-year-experience in nasal reconstruction with the three-stage forehead flap. Plast Reconstr Surg. 2002;109:1839–61. doi: 10.1097/00006534-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimi A, Kalantar Motamedi MH, Nejadsarvari N, Shams Koushki E. Subcutaneous forehead island flap for nasal reconstruction. Iran Red Crescent Med J. 2012;14:271–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Fader DJ, Baker SR, Johnson TM. The staged cheek-to-nose interpolation flap for reconstruction of the nasal alar rim/lobule. J Am Acad Dermatol. 1997;37:614–9. doi: 10.1016/s0190-9622(97)70180-4. [DOI] [PubMed] [Google Scholar]
- 36.Gloster HM., Jr The use of full-thickness skin grafts to repair nonperforating nasal defects. J Am Acad Dermatol. 2000;42:1041–50. [PubMed] [Google Scholar]
- 37.Weisberg NK, Becker DS. Repair of nasal ala defects with conchal bowl composite grafts. Dermatol Surg. 2000;26:1047–51. doi: 10.1046/j.1524-4725.2000.0260111047.x. [DOI] [PubMed] [Google Scholar]
- 38.Qian C, Yaodong X, Xiaoming H, Shaochong F, Yiqing Z. Repair of full-thickness alar defects. Dermatol Surg. 2012;38:1639–44. doi: 10.1111/j.1524-4725.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 39.Wollina U. One-stage reconstruction of soft tissue defects with the sandwich technique: Collagen-elastin dermal template and skin grafts. J Cutan Aesthet Surg. 2011;4:176–82. doi: 10.4103/0974-2077.91248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Löser C, Rompel R, Breuninger H, Möhrle M, Häfner HM, Kunte C, et al. German Society for Dermatosurgery. Microscopically controlled surgery (MCS) J Dtsch Dermatol Ges. 2010;8:920–5. doi: 10.1111/j.1610-0387.2010.07314.x. [DOI] [PubMed] [Google Scholar]
- 41.Hauschild A, Breuninger H, Kaufmann R, Kortmann RD, Klein M, Werner J, et al. Brief S2k guidelines --Basal cell carcinoma of the skin. J Dtsch Dermatol Ges. 2013;11(Suppl 3):10. doi: 10.1111/ddg.12015_3. [DOI] [PubMed] [Google Scholar]
- 42.Malik V, Goh KS, Leong S, Tan A, Downey D, O’Donovan D. Risk and outcome analysis of 1832 consecutively excised basal cell carcinomas in a tertiary referral plastic surgery unit. J Plast Reconstr Aesthet Surg. 2010;63:2057–63. doi: 10.1016/j.bjps.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Mosterd K, Krekels GA, Nieman FH, Ostertag JU, Essers BA, Dirksen CD, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149–56. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 44.Smeets NW, Krekels GA, Ostertag JU, Essers BA, Dirksen CD, Nieman FH, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: Randomised controlled trial. Lancet. 2004;364:1766–72. doi: 10.1016/S0140-6736(04)17399-6. [DOI] [PubMed] [Google Scholar]
- 45.Alam M, Helenowksi IB, Cohen JL, Levy R, Liégeois N, Mafong EA, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: A cross-sectional study. Dermatol Surg. 2013;39:51–5. doi: 10.1111/dsu.12045. [DOI] [PubMed] [Google Scholar]


