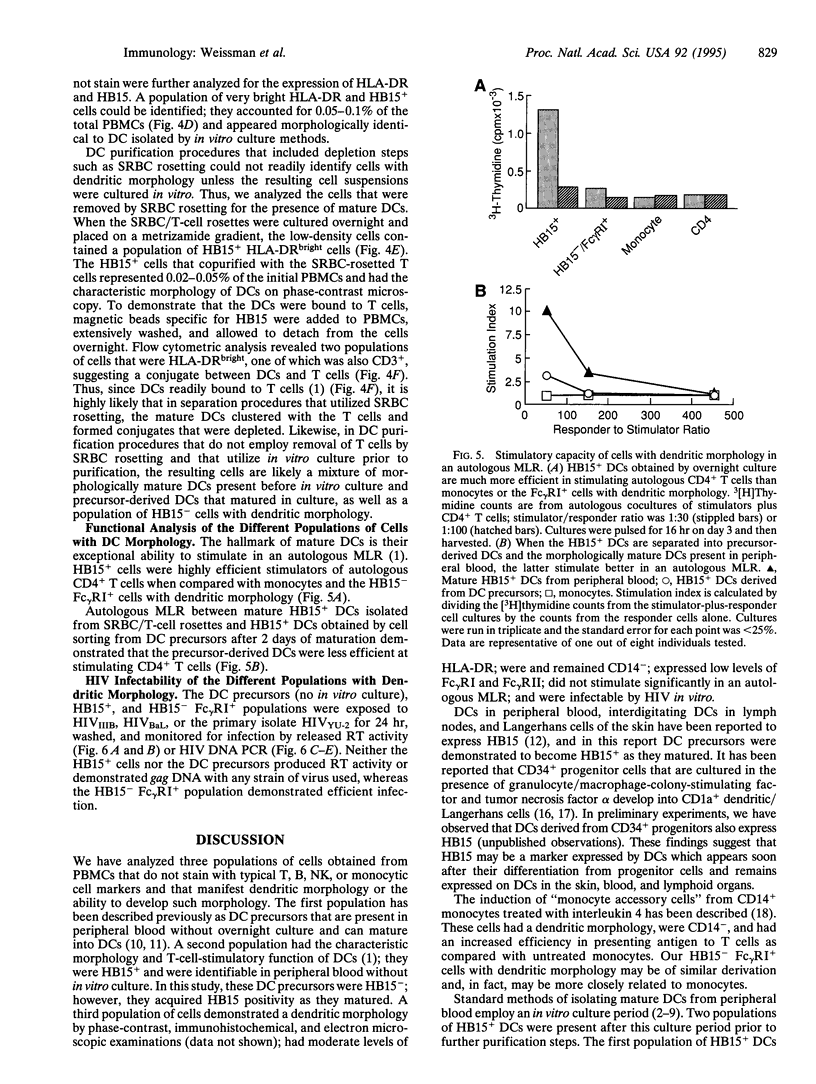
Abstract
Conflicting data have been reported with regard to the infectability, dysfunction, and depletion of dendritic cells (DCs) in human immunodeficiency virus (HIV) disease. These discrepancies could potentially be explained by the existence of multiple subsets of cells with dendritic morphology in peripheral blood. The isolation of DCs in humans is accomplished through negative selection until a morphologically pure population is obtained. Recently, DC precursors purified from peripheral blood by negative selection have been observed to develop into functionally and morphologically mature DCs. In this report we identify three populations of cells in peripheral blood that have or can develop a dendritic morphology. The first population, when allowed to mature in culture, develops a dendritic morphology and gains the expression of HB15, a marker of DCs in blood, thymus, skin, and lymphoid organs. The second population expresses HB15 and has the phenotypic and morphologic characteristics of mature DCs. The third population is morphologically very similar to mature DCs but does not share the same T-cell-stimulatory activity and is the only population that is infectable with HIV. Understanding the heterogeneity of cells of dendritic lineage and/or morphology in the peripheral blood will aid in understanding their role as antigen-presenting cells in general and as potential participants in the immunopathogenesis of HIV disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M. Antigen uptake and presentation by dendritic leukocytes. Semin Immunol. 1992 Aug;4(4):227–236. [PubMed] [Google Scholar]
- Cameron P. U., Forsum U., Teppler H., Granelli-Piperno A., Steinman R. M. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin Exp Immunol. 1992 May;88(2):226–236. doi: 10.1111/j.1365-2249.1992.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P. U., Freudenthal P. S., Barker J. M., Gezelter S., Inaba K., Steinman R. M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992 Jul 17;257(5068):383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992 Nov 19;360(6401):258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Chehimi J., Prakash K., Shanmugam V., Collman R., Jackson S. J., Bandyopadhyay S., Starr S. E. CD4-independent infection of human peripheral blood dendritic cells with isolates of human immunodeficiency virus type 1. J Gen Virol. 1993 Jul;74(Pt 7):1277–1285. doi: 10.1099/0022-1317-74-7-1277. [DOI] [PubMed] [Google Scholar]
- Karhumäki E., Viljanen M. E., Cottler-Fox M., Ranki A., Fox C. H., Krohn K. J. An improved enrichment method for functionally competent, highly purified peripheral blood dendritic cells and its application to HIV-infected blood samples. Clin Exp Immunol. 1993 Mar;91(3):482–488. doi: 10.1111/j.1365-2249.1993.tb05928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., Terwilliger E. F., Bos H. J., Kalland K. H., Poznansky M. C., Bacon O. M., Haseltine W. A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kappes J. C., Conway J. A., Price R. W., Shaw G. M., Hahn B. H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991 Aug;65(8):3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Gompels M., Pinching A. J., Patterson S., Knight S. C. Antigen-presentation by macrophages but not by dendritic cells in human immunodeficiency virus (HIV) infection. Immunology. 1992 Apr;75(4):576–581. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Lau R., Patterson S., Pinching A. J., Knight S. C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990 Sep;71(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R. M., Peng M., Cameron P. U., Gezelter S., Kopeloff I., Swiggard W. J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993 Sep 1;178(3):1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuk L. M., Polacino P. S., Agy M. B., Klaus S. J., Clark E. A. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994 Jul;1(4):317–325. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Ruppert J., Friedrichs D., Xu H., Peters J. H. IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency. Immunobiology. 1991 Aug;182(5):449–464. doi: 10.1016/S0171-2985(11)80209-3. [DOI] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Belilos E., Diamond B., Carsons S. E. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992 Sep;52(3):274–281. [PubMed] [Google Scholar]
- Stanley S. K., McCune J. M., Kaneshima H., Justement J. S., Sullivan M., Boone E., Baseler M., Adelsberger J., Bonyhadi M., Orenstein J. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993 Oct 1;178(4):1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Weissman D., Poli G., Bousseau A., Fauci A. S. A platelet-activating factor antagonist, RP 55778, inhibits cytokine-dependent induction of human immunodeficiency virus expression in chronically infected promonocytic cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2537–2541. doi: 10.1073/pnas.90.6.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992 Jul 15;149(2):735–742. [PubMed] [Google Scholar]